Abstract
Generation of collagen dipeptides and deposition of orally administered prolylhydroxyproline (Pro-Hyp) in local inflammatory sites were examined in mice with hapten (2,4-dinitrofluorobenzene)-induced dermatitis in the ear. Pro-Hyp content in the hapten-treated ear was significantly higher in the chronic phase of contact dermatitis than the vehicle control. In contrast, hydroxyprolylglycine contents remained at lower levels in all cases compared to Pro-Hyp. Four hours after the ingestion of [13C5,15N]Pro and [13C5,15N]Pro-Hyp, labeled-Pro-Hyp and Pro, respectively, appeared only in the ear with dermatitis. Thus, Pro-Hyp is generated and degraded as part of the rapid synthesis and degradation of collagen in the ear with dermatitis. In addition to the endogenously generated Pro-Hyp, the orally administered Pro-Hyp was deposited in the ears.
[1] Pro-Hyp is generated and degraded as part of the rapid synthesis and degradation of collagen in the ear with dermatitis. [2] The orally administered Pro-Hyp was deposited in the ears.
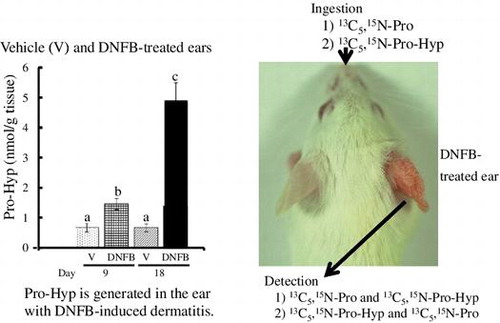
Collagen is one of the main protein components in the extracellular matrix and is the most abundant protein in the body comprising about one-third of the total protein.1) Heat-denatured collagen extracted from skin, bone, or fish scale is referred to as gelatin. Collagen peptide, prepared by limited digestion of gelatin with proteases, is widely used as a food supplement to improve conditions pertaining to the skin and joints. Recent placebo-controlled double-blind trials have demonstrated that ingestion of collagen peptide significantly improves skin and joint conditions.Citation2–4) Preclinical studies using animal models have also demonstrated that ingestion of collagen peptide or gelatin thickens collagen fibrils,Citation5,6) promotes healing of pressure ulcersCitation7) and bone fracture,Citation8) and increases bone mineral density.Citation9,10)
Presence of food-derived collagen oligopeptides in human peripheral blood after the ingestion of collagen peptide has been studied.Citation11–14) Prolylhydroxyproline (Pro-Hyp) has been identified as major constituents of food-derived collagen peptide in human blood.Citation11,12,14) Pro-Hyp has been demonstrated to stimulate the growth of primary cultured mouse skin fibroblasts on collagen,Citation15) enhance the production of hyaluronic acid by human skin fibroblasts,Citation16) modulate lipid metabolism in adipocytes,Citation17) and suppress mineralization in chondrocytes.Citation18) Another collagen-derived dipeptide, hydroxyprolylglycine (Hyp-Gly), was also reported to stimulate growth of skin fibroblasts on collagen.Citation13) These studies suggest that the beneficial effects of ingestion of collagen peptide depend, at least in part, on the biological activities of these collagen oligopeptides.
Free and peptide forms of Hyp have been detected in the urine of growing children,Citation19) patients with bone tumors,Citation20) and rheumatoid arthritis patients.Citation21) These studies indicate that collagen peptides are generated by extensive degradation of extracellular matrix and under systemic inflammation. In the restricted site with inflammation or damage, Pro-Hyp may be locally generated, which can stimulate fibroblast growth for tissue repair and reconstruction of the extracellular matrix. However, local production of collagen oligopeptides at the damaged site has not been reported.
The objectives of the present study were to detect the local generation of Pro-Hyp and Hyp-Gly in the restricted site with inflammation and to detect the deposition of orally administered Pro-Hyp using mice with hapten-induced contact dermatitis in the ear.
Materials and methods
Chemicals
Pro-Hyp and Hyp-Gly were obtained from Bachem (Bubendorf, Switzerland). [13C5,15N]Pro-Hyp and [13C5,15N]Pro were obtained from Anygen, (Jeollanam-do, Korea) and Cambridge Isotope Laboratories (Tewksbury, MA, USA), respectively. 2,4-Dinitrofluorobenzene (DNFB) was obtained from Sigma–Aldrich (St. Louis, MO, USA). All reagents were of analytical grade or better.
Animal experiments
All animal experiments in this study were approved by the Ethics Committee of Nippi (Tokyo, Japan) and were carried out in the animal facilities of Nippi. Specific pathogen-free female BALB/cAJcl mice (age, 7 weeks) were purchased from CLEA Japan (Tokyo, Japan) and maintained at 23 ± 5 °C on a 12-h/12-h light–dark cycle throughout the experimental period. The mice were fed a standard diet (MF; Oriental Yeast, Tokyo, Japan). One day before the determination of collagen dipeptide contents (for Pro-Hyp and Hyp-Gly), the diet was changed to collagen-free AIN-93M (Oriental Yeast). Mice were given tap water ad libitum.
Induction of allergic contact dermatitis was performed by the method described by Kusubata et al.Citation22) Briefly, after 1 week of acclimatization, the mice were treated with 10 μL of 0.2% DNFB in acetone every 3 days to both dorsal and ventral sides of the right ear for 9 or 18 days to induce allergic contact dermatitis. Control mice received the treatment with acetone. Ear thickness was measured using a dial thickness gauge (Ozaki MFG, Tokyo, Japan) to evaluate development of edema by contact dermatitis.Citation23)
To estimate the absorption of Pro-Hyp into blood, Pro-Hyp was orally administered. The diet was changed from MF to AIN-93M (collagen-free) in the morning on the day prior to Pro-Hyp administration, and the mice were fasted overnight. Pro-Hyp was dissolved in water at a concentration of 1 mg/mL and orally administered to mice via gavage (400 μg·400 μL−1·20 g body weight−1). This dose was employed on the basis of our previous human trial (collagen peptide 0.2 g kg body weight−1 day−1)Citation5,6) and the frequency of Pro-Hyp motif in collagen (10%). After Pro-Hyp administration, the mice were allowed to ingest AIN-93M ad libitum. Blood was collected 0, 0.5, 1, 2, and 4 h after administration.
To detect the deposition of orally administered Pro-Hyp in the ear, stable isotope labeled [13C5,15N]Pro-Hyp or [13C5,15N]Pro was administered to mice receiving DNFB application on the right ear. The diet was changed from MF to AIN-93M on day 17 as described above, and mice were fasted overnight. On day 18, 400 μg·400 μL–1 20 g body weight of the labeled Pro-Hyp or Pro in water was administered to mice via gavage. Ears were collected 4 h after the administration.
Preparation of plasma and ear samples
Mice were anesthetized with diethyl ether and blood samples were collected from the left ventricle by using a heparinized needle and syringe. After centrifugation at 12,000 rpm for 20 min at 4 °C, plasma was collected and stored at −80 °C until use. Plasma samples were mixed with three volumes of ethanol. The resultant precipitate was removed by centrifugation. The supernatant was diluted in 20 volumes of 0.1% formic acid and subjected to liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis.
The ears were excised and stored in 10 volumes of 70% ethanol at −80 °C until use. They were cut into small pieces by using scissors and homogenized with a BioMasher 2 (Nippi, Tokyo, Japan) in 70% ethanol. After centrifugation at 12,000 rpm for 20 min at 4 °C, the supernatant was collected and dried using a centrifugal concentrator. The dried material was dissolved in 0.4 mL of 0.1% formic acid and centrifuged at 12,000 rpm for 20 min at 4 °C. The supernatant was filtrated through a 0.45-μm membrane filter and subjected to LC-MS/MS analysis.
LC-MS/MS analysis
Multiple reaction monitoring was carried out to determine the amount of each compound by using 3200 QTRAP (AB Sciex, Framingham, MA, USA) equipped with HPLC Agilent1200 (Agilent, Santa Clara, CA, USA), monitoring the transition of m/z 235–75 (13C5,15N-Pro-Hyp), m/z 229–70 (Pro-Hyp), m/z 189–86 (Hyp-Gly), m/z 132–68 (Hyp), m/z 122–75 (13C5,15N-Pro), and m/z 116–70 (Pro). HPLC was performed according to Yoshida et al.Citation24) with minor modifications. The ion source was set with values of curtain gas of 15.0 psi, collision gas of 5 psi, ion spray voltage of 3000 V, temperature of 600 °C, ion source gas 1 of 80 psi, and ion source gas 2 of 80 psi. The peak area was calculated using Analyst 1.5 (AB Sciex, Framingham, MA, USA).
Statistical analysis
Statistical differences illustrated in Fig. were detected using the Tukey–Kramer test, and the difference was considered to be significant when p < 0.05. Statistical differences illustrated in Fig. were assessed using the paired t-test, and p < 0.05 was considered significant.
Results
Endogenous generation of Pro-Hyp in the ear with dermatitis
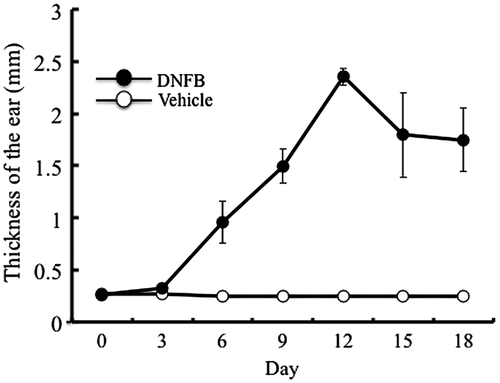
Temporal changes in the thickness of DNFB- and vehicle-treated ears are shown in Fig. . Treatment with the vehicle did not induce thickening of the ear. In contrast, repeated treatment with DNFB induced a marked increase in ear thickness in the acute phase (days 3–12). In the chronic phase (after day 15), the ear thickness decreased slightly and remained constant thereafter as reported previously.Citation22) Thus, allergic contact dermatitis was induced in the right ear. The mice in this experiment were fed collagen-free diet and subjected to detection of Pro-Hyp and Hyp-Gly in the ears.
Fig. 1. Induction of contact dermatitis.
Notes: Contact dermatitis was induced by applying DNFB or vehicle every 3 days to the right ear of mice and the temporal changes of ear thickness were measured. The results are presented as mean ± SD (n = 6).
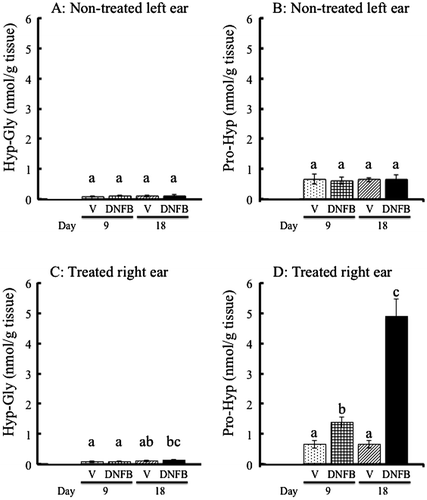
As shown in Fig. (A) and (C), there was no significant difference in Hyp-Gly content between the DNFB- and vehicle-treated groups in the acute (day 9) and chronic phases (day 18). Higher contents of Pro-Hyp compared to Hyp-Gly were detected even in the non-treated left ears (Fig. (A) and (B)). Pro-Hyp content in the DNFB-treated right ear increased to approximately 2-fold in the acute phase (day 9) and 7-fold in the chronic phase (day 18) compared to the vehicle-treated right ear (Fig. (D)).
Fig. 2. Contents of Hyp-Gly and Pro-Hyp in mouse ear.
Notes: (A, B): Hyp-Gly (A) and Pro-Hyp (B) in the non-treated left ear of mice treated with vehicle (V) or DNFB on the right ear for 9 or 18 days. (C, D): Hyp-Gly (C) and Pro-Hyp (D) in the right ear treated with vehicle (V) or DNFB for 9 or 18 days. The results are presented as mean ± SD (n = 6). Values in the same figure not sharing a common letter above the bar are significantly different from each another, p < 0.05.
Metabolism of orally administered Pro-Hyp and Pro in the ear
To evaluate the deposition of orally administered Pro-Hyp in ears, Pro-Hyp levels in the plasma after ingestion of Pro-Hyp at 400 μg/20 g body weight were first examined. As shown in Fig. , plasma Pro-Hyp levels increased sharply 0.5 h after administration and returned to the initial levels 4 h after administration. Therefore, deposition of orally administered Pro-Hyp and Pro in the ear with the dermatitis was examined 4 h after oral administration. As shown in Fig. (A) and (B), no significant amounts of the labeled Pro-Hyp and Pro were detected in the ears from either side in mice receiving water. Labeled Pro-Hyp (Fig. (A)) and Pro (Fig. (B)) were detected in the both DNFB-treated right ears and non-treated left ears after the administration of labeled Pro-Hyp and Pro, respectively. On the other hand, the labeled Pro-Hyp (Fig. (A)) and Pro (Fig. (B)) were specifically detected in the DNFB-treated right ears after the administration of labeled Pro and Pro-Hyp, respectively.
Fig. 3. Pro-Hyp in plasma after ingestion of Pro-Hyp.
Notes: Pro-Hyp was orally administered at a dose of 400 μg/20 g body weight and measured in the plasma. The results are presented as mean ± SD (n = 3).
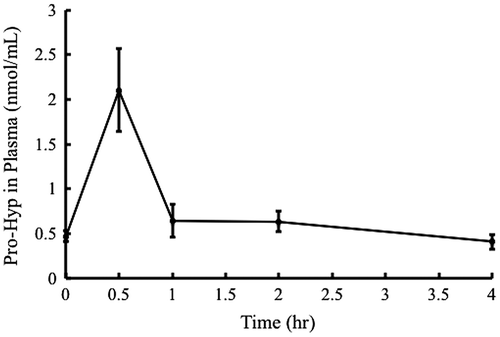
Fig. 4. Contents of [13C5,15N]Pro-Hyp and [13C5,15N]Pro in the ears of mice with contact dermatitis.
Notes: The right ear of mice was treated with DNFB (+) and the left ear was non-treated (−) for 18 days. On day 18, the mice were orally administered water, [13C5,15N]Pro-Hyp or [13C5,15N]Pro, and the contents of the labeled Pro-Hyp (A) and Pro (B) in each ear were determined 4 h after ingestion (n = 4). *p < 0.05.
![Fig. 4. Contents of [13C5,15N]Pro-Hyp and [13C5,15N]Pro in the ears of mice with contact dermatitis.Notes: The right ear of mice was treated with DNFB (+) and the left ear was non-treated (−) for 18 days. On day 18, the mice were orally administered water, [13C5,15N]Pro-Hyp or [13C5,15N]Pro, and the contents of the labeled Pro-Hyp (A) and Pro (B) in each ear were determined 4 h after ingestion (n = 4). *p < 0.05.](/cms/asset/2158be5e-0b7a-48ba-9d5b-00b59deca31b/tbbb_a_1027653_f0004_b.gif)
Discussion
Under bone metastasis of cancer and chronic and systemic inflammatory disorders such as rheumatoid arthritis, increase in free Hyp and Hyp-containing peptides, including Pro-Hyp, in blood and urine have been reported.Citation20,21) In such cases, extensive degradation of collagen occurs and the resultant collagen peptides can be detected in blood and urine. However, increase in these collagen oligopeptides in blood and urine from the animals and human suffering from local and restricted inflammation has not been reported. The present study demonstrates the local generation of Pro-Hyp in the right ear with dermatitis without affecting Pro-Hyp level in the normal left ear of the same animal. Hyp-Gly appears in human peripheral blood at a relatively high concentration after ingestion of collagen peptide.Citation13) However, no significant increase of Hyp-Gly was observed in the mouse ears both with and without contact dermatitis, which might be explained by the reduced production of Hyp-Gly from collagen by mouse proteases and peptidases or rapid metabolism of Hyp-Gly.
After ingestion of the stable isotope-labeled Pro-Hyp, the labeled Pro was detected only in the ear with dermatitis, indicating that prolidase, which splits dipeptides that contain carboxyl-terminal proline or hydroxyproline, is activated in the ear with the dermatitis. The activation and increase of prolidase in rat under inflammatory conditions have been reported.Citation25) The present study also demonstrates that the stable isotope-labeled Pro is incorporated into the Pro-Hyp as early as within 4 h, but only in the ear with the dermatitis. This indicates that orally administered Pro is incorporated into collagen molecule and that Pro-Hyp is generated from the newly synthesized collagen within 4 h. The prolidase can, therefore, provide Pro for collagen synthesis. As part of this very rapid synthesis and degradation of collagen in the ear with dermatitis, collagen peptides are simultaneously generated and degraded, which results in specific and local increase of Pro-Hyp in the ear with dermatitis.
It has been reported that Pro-Hyp increases the number of fibroblasts migrated from the explanted mouse skin and that it enhances the proliferation of mouse skin fibroblasts on collagen gel; these have been considered as wound healing models.Citation15) Therefore, Pro-Hyp that was locally generated at the inflammatory site can trigger fibroblast growth for local tissue reconstruction.
Pro-Hyp appears as a major food-derived collagen peptide in human blood after the ingestion of collagen peptide.Citation11) The present study also indicates that an increase in Pro-Hyp in mouse plasma after the ingestion of 0.02 g/kg Pro-Hyp, which is equivalent to 0.2 g/kg body weight of collagen peptide. The maximum level of Pro-Hyp in the plasma is approximately 2 μM, which is considerably lower compared to that in human plasma (approximately 20 μM) after the ingestion of similar dose of collagen peptide (10 g/serving).Citation11) The plasma Pro-Hyp level returned to the initial level 4 h after the administration, after the orally administered Pro-Hyp was cleared from the plasma. However, the labeled Pro-Hyp remained in the ears both with and without dermatitis, which indicates that the orally administered Pro-Hyp deposits in the ear. Therefore, oral administration of collagen peptide can affect the Pro-Hyp level in the inflammatory site and cooperatively act with the endogenously generated Pro-Hyp on fibroblasts and other cells in the inflammatory site for the reconstruction of the extracellular matrix. In fact, oral administration of collagen peptide enhances wound healing of pressure ulcer in animal model possibly due to enhanced growth of fibroblast.Citation26) On the other hand, effect of administration of collagen peptide on immune response itself remains to be solved.
As shown in Fig. , swelling occurred in the ear with dermatitis. Therefore, accumulation of Pro-Hyp-containing fluid in the ear with the dermatitis is expected. However, there is no significant difference in the level of labeled Pro-Hyp between the ear with and without dermatitis 4 h after the administration, which is partially explained by the specific degradation of Pro-Hyp by prolidase in the ear with dermatitis as discussed above. In addition, Kawaguchi et al. demonstrated that orally administered [14C] Pro-Hyp deposits in a peptide form in rat tissues and is rapidly converted into unidentified metabolites that were or were not susceptible to HCl hydrolysis.Citation27) It is possible that Pro-Hyp is rapidly converted into these unidentified metabolites with some biological activities in the ear with dermatitis compared to the normal ear. To address these problems at the molecular level, it is necessary to identify the metabolites. Currently, further studies to identify the metabolites of Pro-Hyp and Hyp-Gly in animal tissues and in cultured fibroblast are underway.
Acknowledgments
The authors are grateful to Dr O. Hayashida for his valuable discussions.
Additional information
Funding
References
- Eastoe JE. Composition of collagen and allied proteins. In: Ramachandran GN, editor. Treaties on collagen. London and New York: Academic Press; 1967. pp. 1–72.
- Clark KL, Sebastianelli W, Flechsenhar KR, Aukermann DF, Meza F, Millard RL, Deitch JR, Sherbondy PS, Albert A. 24-week study on the use of collagen hydrolysate as a dietary supplement in athletes with activity-related joint pain. Curr. Med. Res. Opin. 2008;24:1485–1496.10.1185/030079908X291967
- Proksch E, Segger D, Degwert J, Schunck M, Zague V, Oesser S. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol. Physiol. 2014;27:47–55.10.1159/000351376
- Proksch E, Schunck M, Zague V, Segger D, Degwert J, Oesser S. Oral intake of specific bioactive collagen peptides reduces skin wrinkles and increases dermal matrix synthesis. Skin Pharmacol. Physiol. 2014;27:113–119.10.1159/000355523
- Matsuda N, Koyama Y, Hosaka Y, Ueda H, Watanabe T, Araya T, Irie S, Takehana K. Effects of ingestion of collagen peptide on collagen fibrils and glycosaminoglycans in the dermis. J. Nutr. Sci. Vitaminol. 2006;52:211–215.10.3177/jnsv.52.211
- Minaguchi J, Koyama Y, Meguri N, Hosaka Y, Ueda H, Kusubata M, Hirota A, Irie S, Mafune N, Takehana K. Effects of ingestion of collagen peptide on collagen fibrils and glycosaminoglycans in Achilles tendon. J. Nutr. Sci. Vitaminol. 2005;51:169–174.10.3177/jnsv.51.169
- Nakao K, Kusubata M, Hara K, Igarashi M, Yamazaki N, Koyama Y. Effects of collagen peptide ingestion on healing of skin wound in a rat model of pressure ulcer. Jpn. Pharmacol. Ther. 2013;41:587–596.
- Tsuruoka N, Yamato R, Sakai Y, Yoshitake Y, Yonekura H. Promotion by collagen tripeptide of type I collagen gene expression in human osteoblastic cells and fracture healing of rat femur. Biosci. Biotechnol. Biochem. 2007;71:2680–2687.10.1271/bbb.70287
- Koyama Y, Hirota A, Mori H, Takahara H, Kuwaba K, Kusubata M, Matsubara Y, Kasugai S, Itoh M, Irie S. Ingestion of gelatin has differential effect on bone mineral density and body weight in protein undernutrition. J. Nutr. Sci. Vitaminol. 2001;47:84–86.10.3177/jnsv.47.84
- Wu J, Fujioka M, Sugimoto K, Mu G, Ishimi Y. Assessment of effectiveness of oral administration of collagen peptide on bone metabolism in growing and mature rats. J. Bone Miner. Metab. 2004;22:547–553.10.1007/s00774-004-0522-2
- Iwai K, Hasegawa T, Taguchi Y, Morimatsu F, Sato K, Nakamura Y, Higashi A, Kido Y, Nakabo Y, Ohtsuki K. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J. Agric. Food Chem. 2005;53:6531–6536.10.1021/jf050206p
- Ichikawa S, Morifuji M, Ohara H, Matsumoto H, Takeuchi Y, Sato K. Hydroxyproline-containing dipeptides and tripeptides quantified at high concentration in human blood after oral administration of gelatin hydrolysate. Int. J. Food Sci. Nutri. 2010;61:52–60.10.3109/09637480903257711
- Shigemura Y, Akaba S, Kawashima E, Park EY, Nakamura Y, Sato K. Identification of a novel food-derived collagen peptide, hydroxyprolyl-glycine, in human peripheral blood by pre-column derivatisation with phenyl isothiocyanate. Food Chem. 2011;129:1019–1024.10.1016/j.foodchem.2011.05.066
- Ohara H, Matsumoto H, Ito K, Iwai K, Sato K. Comparison of quantity and structures of hydroxyproline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources. J. Agric. Food Chem. 2007;55:1532–1535.10.1021/jf062834s
- Shigemura Y, Iwai K, Morimatsu F, Iwamoto T, Mori T, Oda C, Taira T, Park EY, Nakamura Y, Sato K. Effect of prolyl-hydroxyproline (Pro-Hyp), a food-derived collagen peptide in human blood, on growth of fibroblasts from mouse skin. J. Agric. Food Chem. 2009;57:444–449.10.1021/jf802785h
- Ohara H, Ichikawa S, Matsumoto H, Akiyama M, Fujimoto N, Kobayashi T, Tajima S. Collagen-derived dipeptide, proline-hydroxyproline, stimulates cell proliferation and hyaluronic acid synthesis in cultured human dermal fibroblasts. J. Dermatol. 2010;37:330–338.10.1111/jde.2010.37.issue-4
- Minaguchi J, Tometsuka C, Koyama Y, Kusubata M, Nagayasu A, Sawaya S, Shiga T, Shima H, Hara T, Takehana K. Effects of collagen-derived oligopeptide prolylhydroxyproline on differentiation of mouse 3T3-L1 preadipocytes. Food Sci. Technol. Res. 2012;18:593–599.10.3136/fstr.18.593
- Nakatani S, Mano H, Sampei C, Shimizu J, Wada M. Chondroprotective effect of the bioactive peptide prolyl-hydroxyproline in mouse articular cartilage in vitro and in vivo. Osteoarthritis Cartilage. 2009;17:1620–1627.10.1016/j.joca.2009.07.001
- Ziff M, Kibrick A, Dresner E, Gribetz HJ. Excretion of hydroxyproline in patients with rheumatic and non-rheumatic diseases. J. Clin. Invest. 1956;35:579–587.10.1172/JCI103311
- Hosley HF, Taft EG, Olson KB, Gates S, Beebe RT. Hydroxyproline excretion in malignant neoplastic disease. Arch. Intern. Med. 1966;118:565–571.10.1001/archinte.1966.00290180041008
- Bienenstock H, Kibrick AC. Urinary excretion of prolylhydroxyproline in rheumatic diseases. Ann. Rheum. Dis. 1969;28:28–30.10.1136/ard.28.1.28
- Kusubata M, Hirota A, Ebihara T, Kuwaba K, Matsubara Y, Sasaki T, Kusakabe M, Tsukada T, Irie S, Koyama Y. Spatiotemporal changes of fibronectin, tenascin-C, fibulin-1, and fibulin-2 in the skin during the development of chronic contact dermatitis. J. Invest. Dermatol. 1999;113:906–912.10.1046/j.1523-1747.1999.00802.x
- Gorbachev AV, Fairchild RL. Induction and regulation of T-cell priming for contact hypersensitivity. Crit. Rev. Immunol. 2001;21:451–472.
- Yoshida H, Mizukoshi T, Hirayama K, Miyano H. Comprehensive analytical method for the determination of hydrophilic metabolites by high-performance liquid chromatography and mass spectrometry. J. Agric. Food Chem. 2007;55:551–560.10.1021/jf061955p
- Imai K, Nagatsu T, Yajima T, Maeda N, Kumegawa M, Kato T. Developmental changes in the activities of prolinase and prolidase in rat salivary glands, and the effect of thyroxine administration. Mol. Cell. Biochem. 1982;42:31–36.
- Nakao K, Kusubata M, Hara K, Igarashi M, Yamazaki N, Koyama Y. Effects of collagen peptide ingestion on healing of skin wound in a rat model of pressure ulcer. Jpn. Pharmacol. Ther. 2013;41:587–595.
- Kawaguchi T, Nanbu PN, Kurokawa M. Distribution of prolylhydroxyproline and its metabolites after oral administration in rats. Biol. Pharm. Bull. 2012;35:422–427.10.1248/bpb.35.422

