Abstract
Lipid peroxidation products react with cellular molecules, such as DNA bases, to form covalent adducts, which are associated with aging and disease processes. Since lipid peroxidation is a complex process and occurs in multiple stages, there might be yet unknown reaction pathways. Here, we analyzed comprehensively 2′-deoxyguanosine (dG) adducts with oxidized arachidonic acid using liquid chromatography–tandem mass spectrometry and found the formation of 7-(2-oxo-hexyl)-etheno-dG as one of the major unidentified adducts. The formation of this adduct was reproduced in the reaction of dG with 2-octenal and predominantly with 4-oxo-2-octenal (OOE). We also found that other 2-alkenals (with five or more carbons) generate corresponding 4-oxo-2-alkenal-type adducts. Importantly, it was found that transition metals enhanced the oxidation of C4-position of 2-octenal, leading to the formation of OOE-dG adduct. These findings demonstrated a new pathway for the formation of 4-oxo-2-alkenals during lipid peroxidation and might provide a mechanism for metal-catalyzed genotoxicity.
Graphical abstract
4-Oxo-2-alkenals, which are highly reactive to nucleosides, are generated through metal-catalyzed oxidation of corresponding 2-alkenals during lipid peroxidation.
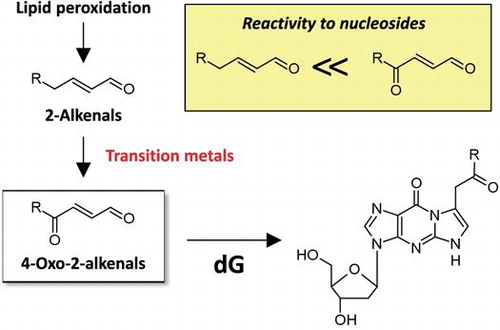
Lipid peroxidation, one of the results from oxidative stress, is considered to be involved in various diseases, such as cancer, arteriosclerosis, and neurodegenerative diseases.Citation1–3) Polyunsaturated fatty acids, such as arachidonic acid (AA) and linoleic acid, essential components of cellular membranes, are the major targets for lipid peroxidation. Lipid hydroperoxides are generated as primary products and degraded to a variety of reactive electrophiles as secondary products.Citation4) Among numerous lipid peroxidation-derived degradation products, malondialdehyde, acrolein, crotonaldehyde, 4-hydroxy-2-nonenal (HNE), 4-hydroxy-2-hexenal and 4-oxo-2-nonenal (ONE) have been well studied in their reactivity to cellular components, such as proteins and DNA.Citation5,6) Especially, HNE and 4-hydroxy-2-hexenal are considered as specific markers for peroxidation of ω-6 and ω-3 polyunsaturated fatty acids, respectively.Citation7,8) On the other hand, we speculated that many other products could also be associated with lipid peroxidation-related diseases, because the processes of lipid peroxidation are complex and are strongly influenced by oxidative conditions.
Lipid peroxidation products readily react with DNA bases and form etheno (ε)- and propano-DNA base adducts through Schiff base formation, Michael-type addition reactions, epoxide-opening, and/or retro-aldol reactions.Citation9–11) These adducts are suggested to play roles in mutations, carcinogenesis, aging, and other diseases.Citation12,13) Recently, some researchers have reported the detection of various lipid peroxidation-derived DNA adducts comprehensively using liquid chromatography–tandem mass spectrometry (LC-MS/MS), called adductome approach,Citation14,15) and exhibited the relationship between the amount/pattern of DNA adducts and the risk of several diseases. However, the elucidation of overall structures and reaction mechanisms of lipid peroxidation-derived DNA adducts have not yet been completed.
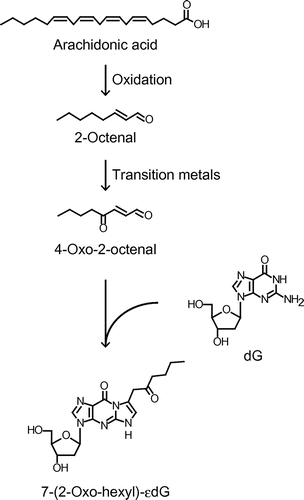
In the present study, we analyzed 2′-deoxyguanosine (dG) adducts derived from the reaction with oxidized AA by comprehensive analysis using LC-MS/MS and found the formation of 7-(2-oxo-hexyl)-εdG, 4-oxo-2-octenal (OOE)-derived dG adduct, as one of the major unidentified adducts. We unexpectedly found that OOE and other 4-oxo-2-alkenals could be formed through the autoxidation of the corresponding 2-alkenals during metal-catalyzed lipid peroxidation. Considering the fact that 4-oxo-2-alkenals are highly reactive toward nucleobases as compared with the corresponding 2-alkenals,Citation16) our study indicates a new mechanism for the metal-catalyzed genotoxicity associated with lipid peroxidation.
Materials and methods
Materials
2′-Deoxyguanosine[15N5] was purchased from Silantes GmbH (Munich, Germany). Nuclease P1 was obtained from Wako Pure Chemical Industries (Osaka, Japan). Alkaline phosphatase (calf intestine) was purchased from TOYOBO (Osaka, Japan). Semicarbazide hydrochloride was obtained from NACALAI TESQUE, INC. (Kyoto, Japan). Calf thymus DNA was purchased from Sigma-Aldrich Japan Corporation (Tokyo, Japan). 15-Hydroperoxyeicosatetraenoic acid (15-HPETE) was obtained from Cayman Chemical Company (Ann Arbor, MI., USA).
LC-MS/MS analysis of dG adducts
AA (10 mM) was incubated with dG (1 mM) in 0.1 M phosphate buffer (pH 7.4) containing FeSO4 (0.1 mM) and ascorbic acid (1 mM) at 37 °C for 3 days. Comprehensive analysis of dG adducts derived from oxidized AA was performed by LC-MS/MS (API 2000, AB Sciex, Framingham, MA., USA) in a positive ion mode using Scherzo SM-C18 (2 × 75 mm, Imtakt, Kyoto, Japan). The gradient elution program using solvent A (water) and solvent B (acetonitrile) containing 0.1% formic acid was as follows: 0–2 min, 2% solvent B; 2–10 min, linear gradient to 60% solvent B at a flow rate of 0.2 mL/min. The multiple reaction monitoring (MRM) of each dG adduct was set at [M + H]+ → [M + H−116]+ for the loss of deoxyribose moiety over the range from m/z 292 to 508. 15-HPETE (2 mM) was also incubated with dG (2 mM) in 0.1 M phosphate buffer (pH 7.4) at 37 °C for 3 days. The quantitation of OOE-dG was performed by stable-isotope dilution LC-MS/MS analysis as described below.
Each 2-alkenal (crotonaldehyde, 2-pentenal, 2-hexenal, 2-hepenal, 2-octenal, and 2-nonenal; 20 mM) was incubated with dG (2 mM) in 0.1 M phosphate buffer (pH 7.4) at 37 °C for 3 days. The possible MRM transition for each 7-(2-oxo-alkyl)-εdG was also monitored at [M + H]+ → [M + H−116]+.
Identification of 7-(2-oxo-hexyl)-εdG
2-Octenal (20 mM) was incubated with dG (2 mM) in 0.1 M phosphate buffer (pH 7.4) at 37 °C for 6 days. The reaction mixture was concentrated and desalted by adding excess methanol. The methanolic solution was evaporated to dryness, and the residues were dissolved in 30% acetonitrile and then analyzed by reversed-phase high-performance liquid chromatography (HPLC) using a Develosil C30-UG-5 (8 × 250 mm, Nomura Chemical, Japan). The gradient elution program using solvent A (water) and solvent B (acetonitrile) was as follows: 0–5 min, 5% solvent B; 5–35 min, linear gradient to 60% solvent B at a flow rate of 2 mL/min. Peaks were collected and analyzed by LC-MS/MS as described above. The peak with m/z 390 was purified and dissolved in CD3OD for the 1H-nuclear magnetic resonance (NMR) analysis (400 MHz, Bruker AVANCE 400).
Synthesis of OOE
Pyridine (10 mL) and N-bromosuccinimide (1.9 g) were dissolved in tetrahydrofuran–acetone–water (5: 4: 2) in an ice bath. 2-Butylfuran (2.6 mL) was then added gradually under stirring. The solution was mixed for 1 h in the ice bath and a further hour at room temperature and then poured into water/dichloromethane (1:1). The organic layer was separated, dried with anhydrous sodium sulfate and evaporated in vacuo. The residue was applied onto a silica-gel column and OOE was eluted with ethyl acetate/hexane (3:1) (285.7 mg, 12.4%).
Preparation of 7-(2-oxo-hexyl)-εdG and 7-(2-oxo-hexyl)-εdG[15N5]
For preparing 7-(2-oxo-hexyl)-εdG (OOE-dG) or stable isotope-labeled OOE-dG, OOE (10 mM) was incubated with dG or dG[15N5] (2 mM) in 0.1 M phosphate buffer (pH 7.4) at 37 °C for 3 days. Each adduct was purified using reversed-phase HPLC as described above.
Preparation of 2-octenal-modified calf thymus DNA
Calf thymus DNA (300 μg) in 0.1 M phosphate buffered saline (300 μL, pH 7.4) was treated with 2-octenal (10 mM) at 37 °C for 3 days. After adding 30 μL of sodium acetate (3 M, pH 5.2), the DNA was precipitated by adding 825 μL of ice-cold ethanol followed by centrifugation at 20,000 g for 10 min. The DNA pellet was washed by 70% ethanol, centrifuged, and then the supernatant was removed. The pellet was washed again by ice-cold ethanol. The DNA pellet was dried and then dissolved in 500 μL of water.
Quantitation of 7-(2-oxo-hexyl)-εdG in the modified calf thymus DNA
Collected DNA in 500 μL of water was mixed with 10 μL of sodium acetate (3 M, pH 5.2) and 30 units of nuclease P1. After incubation at 37 °C for 12 h, 50 μL of Tris-HCl (1 M, pH 8.0) and 20 units of alkaline phosphatase were added, and the mixtures were further incubated at 37 °C for 12 h. Stable isotope-labeled OOE-dG[15N5] (5 pmol) was then added to the hydrolysates. The enzymes in the hydrolysates were filtrated using Nanosep, 3 K (Pall Life Sciences, Port Washington, USA). The 10 μL of the filtrates was used for quantitation of unmodified dG. The remaining filtrates were applied to solid-phase extraction using Sep-Pak C18 cartridge (Waters). The cartridges were washed by methanol and equilibrated with 10% methanol. After applying the hydrolysates, the cartridges were washed with 5 mL of 10% methanol to remove unmodified nucleosides and relatively hydrophobic lipid-nucleoside adducts were then eluted with 8 mL of 100% methanol. The methanolic fractions were evaporated to dryness in vacuo, and the residues were dissolved in 0.1 mL of 40% acetonitrile.
Unmodified nucleosides were analyzed by a reversed-phase HPLC using a TSKgel ODS-80Ts (4.6 × 150 mm, TOSOH). The gradient elution program using solvent A (50 mM sodium acetate containing 3% methanol, pH 5.2) and solvent B (50% methanol) was as follows: 0–25 min, 100% solvent A; 25–35 min, linear gradient to 20% solvent B; 35–45 min, isocratic hold at a flow rate of 0.8 mL/min.
DNA adducts were analyzed by LC-MS/MS (Agilent, G6410B, Santa Clara, CA, USA) in a positive ion mode using TSKgel Super-ODS (2 × 100 mm, TOSOH). The gradient elution program using solvent A (water) and solvent B (acetonitrile) containing 0.1% formic acid was as follows: 5% solvent B at first; 0–10 min, linear gradient to 80% solvent B; 10–15 min, isocratic hold at a flow rate of 0.2 mL/min. The MRM transitions for OOE-dG and the stable isotope-labeled internal standard were monitored at m/z 390.1 → 274.1 and 395.1 → 279.1, respectively.
Semicarbazide derivatization
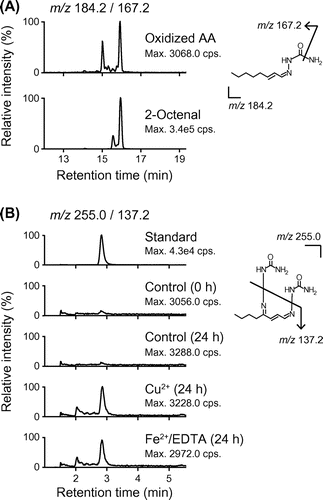
AA (20 mM) was incubated in 0.1 M phosphate buffer (pH 7.4) containing FeSO4 (0.1 mM) and ascorbic acid (1 mM) at 37 °C for 2 days. 2-Octenal (10 mM) was incubated in 0.1 M phosphate buffer (pH 7.4) containing CuSO4 (10 μM) or FeSO4 (0.1 mM)/ethylenediaminetetraacetic acid (EDTA, 0.1 mM) at 37 °C for 24 h. Each reaction mixture or authentic OOE (1 mM) was mixed with ninefold of semicarbazide (5 mM in methanol) and incubated at 40 °C for 6 h. The semicarbazone derivatives were separated using TSKgel ODS-100 V (2 × 100 mm, TOSOH) in an isocratic elution with a mobile phase consisting of 25% aqueous acetonitrile containing 0.1% formic acid at a flow rate of 0.2 mL/min and detected by API 3200 system (AB Sciex, Framingham, MA., USA). The MRM transitions for the semicarbazone derivatives of 2-octenal and OOE were monitored in a positive ion mode at m/z 184.2 → 167.2 and 255.0 → 137.2, respectively.
Results
Identification of 7-(2-oxo-hexyl)-εdG in the reaction of dG with oxidized arachidonic acid
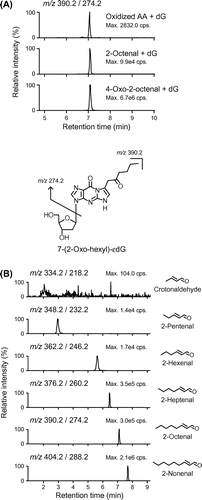
To investigate the oxidized AA-derived dG adducts, the reaction mixture of AA (10 mM) and dG (1 mM) in Fe2+-mediated oxidation system was analyzed by LC-MS/MS in a positive ion mode. 109 MRM transitions with the loss of deoxyribose, [M + H]+ → [M + H−116]+, were monitored over the range from m/z 292 to 508. As well as many already identified adducts, such as εdG (m/z 292 → 176), acrolein-dG (m/z 324 → 208) and 7-(2-oxo-heptyl)-εdG (ONE-dG, m/z 404 → 288), an unexpected adduct was detected at m/z 390 → 274 as one of the major unidentified adducts. Based on the molecular ion, we speculated that the aldehyde(s) with 8-carbon chain could react with dG to form this adduct. To test this hypothesis, we analyzed the reaction mixture of dG with 2-octenal, an α,β-unsaturated aldehyde with 8-carbon chain. As shown in Fig. (A), not only expected Michael adducts at m/z 394 → 278 (data not shown), this adduct (m/z 390 → 274) was also successfully detected upon reaction with 2-octenal. To determine the structure, we purified this adduct and characterized by 1H-NMR (Supplementary information). Surprisingly, the 1H-NMR signals were similar to those of previously reported ONE- and 4-oxo-2-pentenal-derived dG adducts.Citation16–18) We then identified this adduct to be 7-(2-oxo-hexyl)-εdG (OOE-dG), which is presumed to be formed upon reaction with OOE. Indeed, this adduct was predominantly formed in the reaction of dG with authentic OOE (Fig. (A)). Incubation of dG (2 mM) with an isomer of AA hydroperoxides, 15-HPETE (2 mM) in a phosphate buffer, also resulted in the formation of OOE-dG (data not shown), showing that the degradation product(s) of an AA hydroperoxide could contribute to the formation of OOE-dG. Stable-isotope dilution assay demonstrated that the concentrations of OOE-dG after the incubation for 3 days were 51.9 ± 7.3 nM, estimating that equimolar 15-HPETE generated approximately 26 adducts per 106 dG.
Fig. 1. LC-MS/MS analysis of 7-(2-oxo-hexyl)-εdG (OOE-dG) and 2-alkenal-derived 7-(2-oxo-alkyl)-εdG adducts.
Formation of 7-(2-oxo-alkyl)-εdG adducts from 2-alkenals
To further investigate whether other 2-alkenals, as well as 2-octenal, could also form 7-(2-oxo-alkyl)-εdG adducts, the reaction mixtures of dG and 2-alkenals with different carbon chain lengths (from C-4 to C-9) were analyzed by LC-MS/MS. As shown in Fig. (B), -alkenals with five or more carbons generated their corresponding 7-(2-oxo-alkyl)-εdG adducts, suggesting that these 2-alkenals could be oxidized into corresponding 4-oxo-2-alkenals. Except for the adduct from 2-hexenal, which is thought to be formed during oxidation of ω-3 polyunsaturated fatty acids, other 7-(2-oxo-alkyl)-εdG adducts were also detected in the reaction of oxidized AA and dG (data not shown). The result that crotonaldehyde-derived 4-oxo adduct (7-ethanal-εdG) was scarcely detected might be due to less susceptibility of the methyl carbon at C4 of crotonaldehyde to oxidation.
Fig. 2. Effects of transition metals on the formation of OOE-dG.
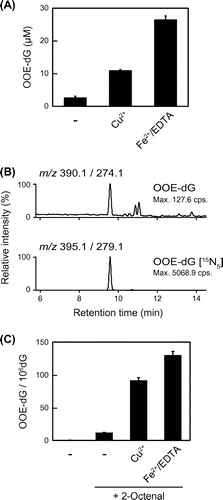
Effects of transition metals on the formation of 7-(2-oxo-hexyl)-εdG
Since the lipid peroxidation process is enhanced by transition metals, such as iron and copper, we examined the effects of Cu2+ and Fe2+/EDTA, a stable complex of iron, on the formation of OOE-dG in the reaction of dG with 2-octenal. As shown in Fig. (A), the formation of OOE-dG was notably enhanced by addition of Cu2+ and Fe2+/EDTA. The results showed that 10 mM of 2-octenal converted into approximately 2.7–26.6 μM of OOE-dG. Next, to examine whether 2-octenal-derived OOE-dG formation and its enhancement by transition metals could be observed, not only in nucleosides, but also in calf thymus DNA (ctDNA), the enzymatic hydrolysates of 2-octenal-modified ctDNA were analyzed by LC-MS/MS. We noted that small amounts of OOE-dG were detected in the native (untreated) ctDNA (Fig. (B)), suggesting that this adduct could endogenously be formed in calf. As compared with native ctDNA, the increased amounts of OOE-dG were detected upon treatment with 2-octenal. Stable-isotope dilution assay revealed that 0.20 or 11.86 adducts were formed per 106 dG in the native or 2-octenal-modified ctDNA, respectively. Furthermore, the adduct levels in 2-octenal-modified ctDNA were significantly enhanced into 91.57 or 130.1 adducts in the presence of Cu2+ or Fe2+/EDTA, respectively (Fig. (C)). These results indicated that the formation of OOE-dG, a possible endogenous DNA lesion, is affected by transition metals, which could oxidize 2-octenal into highly reactive OOE.
Formation of 4-oxo-2-octenal from 2-octenal
Finally, to reveal the formation of 2-octenal and its oxidation products, we analyzed semicarbazone derivatives of aldehydes derived from oxidative degradation of AA by LC-MS/MS.Citation19) As shown in Fig. (A), we successfully detected 2-octenal in the oxidized AA, whereas, probably due to instability of 4-oxo-2-alkenals, semicarbazone derivative of OOE could not be detected in this condition. In turn, to examine the conversion of 2-octenal to OOE, 2-octenal was incubated in the presence or absence of Cu2+ or Fe2+/EDTA and then derivatized for LC-MS/MS analysis. As shown in Fig. (B), semicarbazone derivative of OOE was detected from 2-octenal, especially incubated in the presence of transition metals. On the other hand, we confirmed the amount of OOE-dG was not increased by transition metals in the reaction of dG with OOE (data not shown), suggesting that transition metals induced the oxidation of 2-octenal, but not the reactivity of OOE. These results suggest that 2-octenal could be formed during peroxidation of AA and further oxidized into OOE in the presence of transition metals to form OOE-dG adduct.
Fig. 3. LC-MS/MS analysis of semicarbazone derivatives of 2-octenal and OOE.
Discussion
In the present study, we identified an unidentified dG adduct, 7-(2-oxo-hexyl)-εdG (OOE-dG), generated in the reaction of dG with oxidized AA. It has been shown that this type of adducts, 7-(2-oxo-alkyl)-εdG, is formed upon reaction with corresponding 4-oxo-2-alkenals. Hecht et al. first reported this-type of adducts, identifying 7-(2-oxo-propyl)-εdG in the reaction of dG with 4-oxo-2-pentenal, a hydrolysis product of α-hydroxylated metabolite of a carcinogen N-nitrosopiperidine.Citation17) It has also been reported that 7-(2-oxo-heptyl)-εdG was formed from ONE, a product of lipid peroxidation,Citation18) and 7-(2-oxo-butyl)-εdG from 4-oxo-2-hexenal, reported as a mutagen derived from lipid peroxidation.Citation20) The reaction mechanism for the formation of these adducts was reported to be initiated by nucleophilic addition of N2 of dG to the aldehydic carbon of 4-oxo-2-alkenals followed by Michael-type addition reaction between C2 of 4-oxo-2-alkenals and N1 of dG, resulting in the generation of etheno ring.Citation18) Until now, it has been understood that 2-alkenals mainly generate propano-adducts through Michael-type addition. Otherwise, peroxide-mediated epoxidation of 2-alkenalsCitation21) leads to the formation of different types of DNA adducts, etheno-adducts, through epoxide-opening and/or retro-aldol reactions.Citation9,10) Following these reports, we detected Michael-type adducts (m/z 394 → 278) and 2,3-epoxyoctanal-derived adducts (m/z 392 → 276, m/z 410 → 294) in the reaction of dG with 2-octenal by LC-MS/MS (data not shown). However, we unexpectedly found that OOE-dG was formed as one of the major products in the reaction of dG with 2-octenal (Fig. (A)). We also confirmed that other 2-alkenals (with at least five carbon atoms) also generated corresponding 7-(2-oxo-alkyl)-εdG adducts (Fig. (B)), suggesting that 4-oxo-2-alkenals could be formed from the autoxidation of 2-alkenals. Indeed, we confirmed by LC-MS/MS that OOE was produced during incubation of 2-octenal in the presence of transition metals (Fig. (B)). Thus, our results suggest a new pathway for the formation of 2-alkenal-derived DNA adducts, in which 2-alkenals (with five or more carbons) could be oxidized at C4-position into 4-oxo-2-alkenals and then react with DNA bases.
Several reports have previously suggested that ONE was a major lipid peroxidation product derived from ω-6 polyunsaturated fatty acids.Citation22) In addition, we have previously reported that ONE is highly reactive with nucleobases as compared with other types of aldehydes.Citation16) Indeed, ONE-dG adduct was detected as one of the major adducts in our comprehensive analysis (data not shown). These observations show that ONE is a major genotoxic lipid peroxidation product. It has been reported 4-hydroperoxy-2-nonenal, a major homolytic degradation product of hydroperoxy ω-6 polyunsaturated fatty acids, undergoes metal-catalyzed degradation into ONE and HNE.Citation22,23) Similarly, the formation of OOE could be mediated through 4-hydroperoxy-2-octenal. The detection of 4-hydroperoxy- and 4-hydroxy-2-octenal, although we did not demonstrate within this study, might clarify the mechanism for the formation of OOE during oxidative degradation of AA.
To understand the contribution of lipid peroxidation products to oxidative modifications in vivo, it is important to estimate the concentrations of lipid peroxidation products in tissues and biological fluids. For example, it has been reported that the concentrations of HNE, calculated in the range of 0.1–0.5 μM in rat liver, were significantly increased fivefold under vitamin E deficiency in vivo and that they could also be accumulated up to around 5 mM in peroxidized microsomal membranes in vitro.Citation24) The concentrations of 2-octenal and OOE formed during oxidation of AA have not yet been calculated within this study, because we could not establish stable-isotope dilution assays for 2-octenal and OOE. Lipid peroxidation processes in vivo include both enzymatic and non-enzymatic reactions and are continuously initiated and/or terminated. Furthermore, some lipid peroxidation products, such as lipid hydroperoxides and 4-oxo-2-alkenals, are relatively unstable under physiological conditions. Therefore, it sometimes might be difficult to estimate the exact amounts of lipid peroxidation products in vivo. On the other hand, we here measured the amounts of OOE-dG adduct in the model reactions of 2-octenal with dG or ctDNA (Fig. ) and of an AA hydroperoxide isomer with dG. Thus, estimating stable lipid-DNA adducts, such as 4-oxo-2-alkenal-derived dG adducts, will reflect the occurrence/extent of lipid peroxidation and of metal-catalyzed oxidative stress in vivo.
Our data show that the amount of OOE-dG in the reaction of dG with 2-octenal was significantly up-regulated in the presence of Cu2+ or Fe2+/EDTA (Fig. (B)) and that the increased formation of OOE was observed during incubation of 2-octenal in the presence of transition metals (Fig. (B)). In addition, we confirmed that transition metals did not affect the formation of OOE-dG adduct in the reaction of dG with OOE (data not shown), suggesting that the transition metals enhanced the oxidation of 2-octenal, but not the reactivity between dG and OOE. It has been reported in human epidemiological study that the higher concentrations of serum iron and copper increased the risk of dying from cancer.Citation25) It has also been reported in human and animal studies that transition metals, especially iron, are associated with oxidative tissue damage and following carcinogenesis.Citation26–28) Furthermore, it has been reported that copper-dependent formation of εDNA adducts was involved in liver carcinogenesis.Citation29) These reports suggest that higher concentrations or accumulation of transition metals enhance the production of reactive oxygen species to induce tissue damage or diseases directly or indirectly through lipid peroxidation. Moreover, it has been shown that iron chelates, such as ferric nitrilotriacetate complex, have high catalytic activity,Citation30) supporting the effects of Fe2+/EDTA in our experiments. Therefore, it is considered that up-regulated oxidative conditions by transition metals enhance the production of highly reactive 4-oxo-2-alkenals from 2-alkenals and then result in increased formation of 7-(2-oxo-alkyl)-εdG. Thus, our observation might provide one mechanism for metal-associated diseases, such as iron or copper overload. The potential sources and amounts of transition metals that will convert 2-alkenals into 4-oxo-2-alkenals in vivo are still unknown.
Although the quantitative detection of OOE-dG in vivo has not yet been demonstrated, we successfully detected trace amounts of this adduct in native/untreated ctDNA (Fig. (B)), strongly suggesting that OOE-derived DNA damage could occur in vivo. Thus, OOE- and other 4-oxo-2-alkenal-derived dG adducts might be biomarkers of metal-catalyzed lipid peroxidation and genotoxicity, and therefore establishment of analytical procedures for these adducts in biological fluids, such as plasma and urine, is expected in the near future. In conclusion, we here demonstrated a novel mechanism for the formation of lipid peroxidation-derived dG adducts, in which 2-alkenals are oxidized by transition metals into 4-oxo-2-alkenals to form corresponding 7-(2-oxo-alkyl)-εdG (Fig. ). In addition to classic Michael addition and epoxyaldehyde-derived reactions, we proposed a new pathway for the formation of dG adducts originated from 2-alkenals. Thus, our evidence would provide a pathophysiological basis for metal-catalyzed genotoxicity associated with carcinogenesis and other chronic diseases.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2016.1191334.
Authors Contribution
E Nuka and Y Kawai conceived and designed the experiments, the results, and wrote the manuscript. E Nuka and S Tomono performed the experiments and analyzed the data. A Ishisaka, Y Kato, and N Miyoshi instructed the LC-MS/MS analysis technique.
TBBB_1191334_Supplementary_material.pdf
Download PDF (1.7 MB)Notes
Abbreviations: AA, arachidonic acid; HNE, 4-hydroxy-2-nonenal; ONE, 4-oxo-2-nonenal; LC-MS/MS, liquid chromatography–tandem mass spectrometry; dG, 2′-deoxyguanosine; OOE, 4-oxo-2-octenal; 15-HPETE, 15-hydroperoxyeicosatetraenoic acid; MRM, multiple reaction monitoring; HPLC, high-performance liquid chromatography; NMR, nuclear magnetic resonance; εdG, 1,N2-etheno-dG; OOE-dG, 7-(2-oxo-hexyl)-εdG; EDTA, ethylenediaminetetraacetic acid.
References
- Sander CS, Hamm F, Elsner P, et al. Oxidative stress in malignant melanoma and non-melanoma skin cancer. Br. J. Dermatol. 2003;148:913–922.10.1046/j.1365-2133.2003.05303.x
- Gonenc A, Ozkan Y, Torun M, et al. Plasma malondialdehyde (MDA) levels in breast and lung cancer patients. J. Clin. Pharm. Ther. 2001;26:141–144.10.1046/j.1365-2710.2001.00334.x
- Shichiri M. The role of lipid peroxidation in neurological disorders. J. Clin. Biochem. Nutr. 2014;54:151–160.10.3164/jcbn.14-10
- Kawai Y, Takeda S, Terao J. Lipidomic analysis for lipid peroxidation-derived aldehydes using gas chromatography-mass spectrometry. Chem. Res. Toxicol. 2007;20:99–107.10.1021/tx060199e
- Gueraud F, Atalay M, Bresgen N, et al. Chemistry and biochemistry of lipid peroxidation products. Free Radic. Res. 2010;44:1098–1124.10.3109/10715762.2010.498477
- Winczura A, Zdzalik D, Tudek B. Damage of DNA and proteins by major lipid peroxidation products in genome stability. Free Radic. Res. 2012;46:442–459.10.3109/10715762.2012.658516
- Catala A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids. 2009;157:1–11.10.1016/j.chemphyslip.2008.09.004
- Guichardant M, Bacot S, Moliere P, et al. Hydroxy-alkenals from the peroxidation of n-3 and n-6 fatty acids and urinary metabolites. Prostaglandins, Leukot. Essent. Fatty Acids. 2006;75:179–182.10.1016/j.plefa.2006.05.006
- Chung FL, Chen HJ, Nath RG. Lipid peroxidation as a potential endogenous source for the formation of exocyclic DNA adducts. Carcinogenesis. 1996;17:2105–2111.10.1093/carcin/17.10.2105
- Petrova kV, Jalluri RS, Kozekov ID, et al. Mechanism of 1, N2-etheno-2′-deoxyguanosine formation from epoxyaldehydes. Chem. Res. Toxicol. 2007;20:1685–1692.10.1021/tx7001433
- Burcham PC. Genotoxic lipid peroxidation products: their DNA damaging properties and role in formation of endogenous DNA adducts. Mutagenesis. 1998;13:287–305.10.1093/mutage/13.3.287
- Voulgaridou GP, Anestopoulos I, Franco R, et al. DNA damage induced by endogenous aldehydes: current state of knowledge. Mutat. Res. 2011;711:13–27.10.1016/j.mrfmmm.2011.03.006
- Nair U, Bartsch H, Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: a review of published adduct types and levels in humans. Free Radic. Biol. Med. 2007;43:1109–1120.10.1016/j.freeradbiomed.2007.07.012
- Chou PH, Kageyama S, Matsuda S, et al. Detection of lipid peroxidation-induced DNA adducts caused by 4-oxo-2(E)-nonenal and 4-oxo-2(E)-hexenal in human autopsy tissues. Chem. Res. Toxicol. 2010;23:1442–1448.10.1021/tx100047d
- Matsuda T, Tao H, Goto M, et al. Lipid peroxidation-induced DNA adducts in human gastric mucosa. Carcinogenesis. 2013;34:121–127.10.1093/carcin/bgs327
- Kawai Y, Uchida K, Osawa T. 2′-deoxycytidine in free nucleosides and double-stranded dna as the major target of lipid peroxidation products. Free Radic. Biol. Med. 2004;36:529–541.10.1016/j.freeradbiomed.2003.12.006
- Hecht SS, Young-Sciame R, Chung FL. Reaction of alpha-acetoxy-N-nitrosopiperidine with deoxyguanosine: oxygen-dependent formation of 4-oxo-2-pentenal and a 1, N2-ethenodeoxyguanosine adduct. Chem. Res. Toxicol. 1992;5:706–712.10.1021/tx00029a018
- Rindgen D, Nakajima M, Wehrli S, et al. Covalent modifications to 2′-deoxyguanosine by 4-oxo-2-nonenal, a novel product of lipid peroxidation. Chem. Res. Toxicol. 1999;12:1195–1204.10.1021/tx990034o
- Berdyshev EV, Goya J, Gorshkova I, et al. Characterization of sphingosine-1-phosphate lyase activity by electrospray ionization-liquid chromatography/tandem mass spectrometry quantitation of (2E)-hexadecenal. Anal. Biochem. 2011;408:12–18.10.1016/j.ab.2010.08.026
- Kasai H, Kawai K. 4-oxo-2-hexenal, a mutagen formed by omega-3 fat peroxidation: occurrence, detection and adduct formation. Mutat. Res. 2008;659:56–59.10.1016/j.mrrev.2008.02.003
- Chen HJ, Chung FL. Epoxidation of trans -4-Hydroxy-2-nonenal by fatty acid hydroperoxides and hydrogen peroxide. Chem. Res. Toxicol. 1996;9:306–312.10.1021/tx9501389
- Lee SH, Blair IA. Characterization of 4-Oxo-2-nonenal as a novel product of lipid peroxidation. Chem. Res. Toxicol. 2000;13:698–702.10.1021/tx000101a
- Blair IA. Lipid hydroperoxide-mediated DNA damage. Exp. Gerontol. 2001;36:1473–1481.10.1016/S0531-5565(01)00133-4
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128.10.1016/0891-5849(91)90192-6
- Wu T, Sempos CT, Freudenheim JL, et al. Serum iron, copper and zinc concentrations and risk of cancer mortality in US adults. Ann. Epidemiol. 2004;14:195–201.10.1016/S1047-2797(03)00119-4
- Yamaguchi K, Mandai M, Toyokuni S, et al. Contents of endometriotic cysts, especially the high concentration of free iron, are a possible cause of carcinogenesis in the cysts through the iron-induced persistent oxidative stress. Clin. Cancer Res. 2008;14:32–40.10.1158/1078-0432.CCR-07-1614
- Kato J, Kobune M, Nakamura T, et al. Normalization of elevated hepatic 8-hydroxy-2’-deoxyguanosine levels in chronic hepatitis C patients by phlebotomy and low iron diet. Cancer Res. 2001;61:8697–8702.
- Torti SV, Torti FM. Iron and cancer: more ore to be mined. Nat. Rev. Cancer. 2013;13:342–355.10.1038/nrc3495
- Nair J, Sone H, Nagao M, et al. Copper-dependent formation of miscoding etheno-DNA adducts in the liver of Long Evans cinnamon (LEC) rats developing hereditary hepatitis and hepatocellular carcinoma. Cancer Res. 1996;56:1267–1271.
- Toyokuni S, Sagripanti JL. Iron-mediated DNA damage: sensitive detection of DNA strand breakage catalyzed by iron. J. Inorg. Biochem. 1992;47:241–248.10.1016/0162-0134(92)84069-Y