ABSTRACT
This study aims to investigate the correlation between the ability of L. acidophilus to modulate miRNA expression and prevent Th17-dominated β-lactoglobulin (β-Lg) allergy. In vitro immunomodulation was evaluated by measuring splenocyte proliferation, Th17-related immune response and miRNA expression in β-Lg-sensitized splenocytes cultured with live L. acidophilus. Next, the allergic mouse model was used to evaluate anti-allergy capability of lactobacilli. The β-Lg challenge led to induction of up-regulation of miR-146a, miR-155, miR-21 and miR-9 expression in both in vivo and in vitro, along with increased Th17-related cytokine levels and mRNA expression of RORγt and IL-17. However, treatment of live L. acidophilus significantly suppressed hypersensitivity responses and Th17 cell differentiation. Moreover, administration of live L. acidophilus reduced expression of four miRNAs, especially miR-146a and miR-155. In addition, the decreased expression of the miRNAs in the spleen of the L. acidophilus-treated group was closely associated with decrease of IL-17 and RORγt mRNA expression.
Graphical Abstract
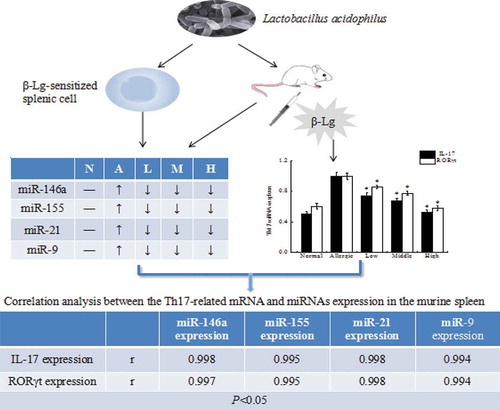
Lactobacillus acidophilus relieve ß-lactoglobulin allergy by regulating miRNAs expressioon, which may provide new insights for the preventative therapy of Th17-induced allergic diseases
Cow’s milk allergy (CMA) is a highly common allergic disease that affects infants and children who absorb and utilize the high-quality milk protein. Upon antigenic stimulation, activation of naive CD4+ T cells causes them to expand and differentiate into Th1, Th2, Th17 and CD4+ CD25+ regulatory T (Treg) cells. In the past, Th2-dominant immunity has been found to exhibit a close relationship with CMA. Recently, increasing clinical and laboratory experiments demonstrated that Th17/Treg immunity imbalances participated in the pathogenesis of allergic inflammation [Citation1,Citation2]. Accordingly, our previous study also observed that bovine β-lactoglobulin (β-Lg)-induced allergy was characterized by inflammatory cell infiltration and Th17 cell differentiation in the colons and lungs of sensitized mice [Citation3]. However, the molecular mechanism of augmented abnormal Th17 responses, resulting in CMA, has not been thoroughly elucidated to date.
There is emerging evidence indicating that microRNAs (miRNAs), as important regulators of host gene expression, control lymphocyte activation and functions and are closely associated with autoimmune and other chronic inflammatory diseases [Citation4–Citation7]. Previous research found that miRNAs were associated with the Th1/Th2 balance, which is a crucial contributor to the development of allergic disease [Citation8]. Subsequently, Takuya Niimoto et al. reported miR-146a/b, miR-150, and miR-155 promoted IL-17 expression in the PBMC of rheumatoid arthritis patients [Citation9]. Yao et al. showed that miR-155 directly targeted cytokine signaling, which functioned as a negative regulator of the Janus kinase (JAK)/STAT signaling pathway and resulted in an increase in the proportion and numbers of Th17 cells in the CD4+ T cell lineage [Citation10]. MiR-21 was reported to significantly enhance the secretion of pro-inflammatory cytokines required by Th17 induction [Citation11]. However, whether the above mentioned miRNAs may be involved in Th17 cell development and tissue-specific inflammation in CMA has not been thoroughly elucidated to date.
Probiotics are defined as live or killed organisms that confer a health benefit on the host when consumed in adequate numbers [Citation12]. Abundant data support the therapeutic role of probiotics in the treatment of allergic diseases [Citation13,Citation14]. Recent studies discussed the effect of probiotics strains on regulating the host immune response at the post-transcriptional level by regulating miRNAs expression. Giahi et al. observed that Lactobacillus rhamnosus GG (LGG) significantly inhibited NF-κB pathways for the production of inflammatory cytokines by down-regulating miR-146a in LGG-treated dendritic cells [Citation15]. Moreover, Liang et al. found that certain miRNAs, which were involved in gut inflammation and the mucosal barrier via host-microbiota interaction, were induced by gut microbiota in the early life of dairy calves [Citation16]. To date, few studies have investigated the relationship between modulation of miRNAs and antiallergic capacities of probiotics in CMA.
Our previous studies have shown that heat-killed L. acidophilus KLDS 1.0738 was able to ameliorate β-Lg allergy in mice by inhibiting Th17 cell activation and inducing Treg cell response [Citation17]. However, additional studies focused on the beneficial immunomodulatory effect of live probiotics. Therefore, this study aims to expand upon our previous work to 1) explore the potential roles of miRNAs in the development and deterioration of the CMA, 2) evaluate the immunomodulatory effects of live L. acidophilus KLDS 1.0738 in the expression of miRNAs in vivo and in vitro, and 3) analyze the correlation between the down-regulation of miRNAs by live L. acidophilus and Th17 cell polarization in the β-Lg-sensitized murine model.
Materials and methods
Animals
Female BALB/c mice aged 5–6 weeks and with an average weight of 23 ± 0.92 g were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd (Beijing, China). Animals were acclimatized to new surroundings for 1 week before experimentation. All mice were freely fed a milk-free diet and water under environmentally controlled hygienic conditions (temperature, 22 ± 2°C; light-dark cycle, 12−12 h). The animal experiment procedures conformed to the rules of the Care and Use of Laboratory Animals of Northeast Agricultural University.
Preparation of bacteria strain
The L. acidophilus KLDS 1.0738 was provided by the Key Laboratory of Dairy Science of Northeast Agriculture University (Harbin, China). The L. acidophilus was cultured in MRS at 37°C for 16 h and harvested by centrifuging at 8000 × g for 5 min at 4°C. The bacteria were later washed three times with sterile saline and adjusted to final concentrations of approximately 107, 108, or 109 colony-forming units (CFU/mL) as the low, medium, and high doses of the live strains and stored at -80°C.
Establishment of animal model
BALB/c mice were randomly sub-divided into 5 groups (n = 6 per group): N: normal group; A: β-Lg allergic group; live L. acidophilus KLDS 1.078 group (low dose (L): 107 CFU/mL; middle dose (M): 108 CFU/mL; high dose (H): 109 CFU/mL). Mice in the β-Lg allergic group and L. acidophilus-treated group mice were both intraperitoneally injected with 0.2 mL 1 mg/mL β-Lg (Sigma, USA) on days 7, 14 and 21, which was dissolved in a mixture of Freund’s adjuvant (Sigma, USA) and sterile saline. Mice in L. acidophilus-treated groups were intragastrically administered 0.2 mL (5 mL/kg wt) bacterial suspension three times a week from days 1 to 28. Normal group mice were treated with sterile saline. All mice were orally challenged with β-Lg (20 mg/mouse) on day 28, except the normal group. After that step, blood was obtained by removing the eyeballs, and the mice were sacrificed, after which the spleen, colon and feces were aseptically removed.
Preparation of splenocytes
Splenocytes isolated from allergic mice were prepared by pressing tissue through a cell strainer and the red blood cells were lysed with lysis reagent (BD Biosciences, USA). After centrifugation and washing, the splenocytes were adjusted to 5 × 106 cells/mL and suspended with RPMI-1640 medium.
Cell proliferation assay
The cell growth inhibitory effects were measured by MTT assay. The collected splenocytes, which were derived from β-Lg-sensitized mice, were seeded in 96-well culture plates (5 × 106 cells/200 μL/well) and later incubated at 37°C for 48 h with ConA (5 μg/mL), β-Lg (1 mg/mL) or β-Lg + various concentrations of L. acidophilus suspension (low dose: 107 CFU/mL, medium dose: 108 CFU/mL or high dose: 109 CFU/mL). Dissolved 3-[4,5-dimethylthiazol-2-yl]- 2,5-diphenyl- tetrazolium bromide (Sigma, USA) was added to each well for 4 h before the end of culture. The supernatants were discarded, and dimethyl sulfoxide (Sigma, USA) was added to each well. The absorbance was measured at 540 nm, and proliferation ratios were calculated according to the following equation: proliferation ratio = (Test OD540-Control OD540)/Control OD540 × 100%.
Cytokines and antibody production from β-Lg-sensitized mouse splenocytes
The spleens obtained from the β-Lg allergic group were further used for vitro cytokines and IgE antibody production assays. Splenocytes (5 × 106 cells/mL) prepared as described above were cultured in 96-well culture plates in RPMI-1640 medium containing 1 mg/mL β-Lg in the absence (allergic group) or presence of different doses of live L. acidophilus (low dose: 107 CFU/mL, medium dose: 108 CFU/mL, high dose: 109 CFU/mL). After incubation for 48 h, the levels of cytokines (IL-21, IL-17, IL-6, TNF-α), total IgE and β-Lg-specific IgE in the culture supernatant were measured using an ELISA quantitation kit (Tiangen, China) according to the manufacturer’s instructions. The contents were measured spectrophotometrically (optical density at 450 nm).
Evaluation of the antiallergic properties of L. acidophilus in β-Lg -sensitized mice
The antiallergic effects of L. acidophilus KLDS 1.0738 were investigated as follows: 1. After 1 h of β-Lg challenge, hypersensitivity symptoms were evaluated as per a described scoring system with minor adjustments [Citation17]. 2. The mice were weighed weekly for 4 weeks. 3. The colon was removed under sterile conditions and stained with hematoxylin and eosin (H&E). Histological assessment of the colon was performed by a pathologist. 4. The feces were gathered and inoculated on selective medium (Hopebio, China) for identification of Lactobacilli, Bifidobacteria, Enterobacter and Enterococcus. 5 The levels of β-Lg-specific IgE and histamine in serum were determined by ELISA (Tiangen, China; Bioss, China). The detection procedures were performed according to the manufacturer’s instructions. Optical density was determined at 450 nm.
RNA isolation and real-time quantitative PCR
The total RNA extracted from murine splenocytes, spleens and colons was measured using a Total RNA kit (Tiangen, China) in accordance with the manufacturer’s instructions. Synthesis of cDNA was done using 6 μL of RNA template with FastQuant cDNA (Tiangen, China). Amplification of cDNA used RealMasterMix (SYBR Green) (Tiangen, China) and was run in an ABI 7500 Fast ReaL-Time PCR thermal cycler. Thermal cycling conditions for all reactions were as follows: 1 cycle at 95°C for 30 s, 40 cycles at: 90°C for 5 s, 60°C for 30 s and 68°C for 30 s. The sequences of Th17-related cytokines and four miRNAs (miR-146a, miR-155, miR-21 and miR-9) are shown in and , respectively. β-actin and U6 were used as the reference genes. The relative expression levels were expressed in CT values and calculated using the 2−ΔΔCT method.
Table 1. Quantitative RT-PCR primers designed for the detection of Th17 cell response.
Table 2. Quantitative RT-PCR primers designed for the detection of miRNAs.
Statistical analysis
All data were expressed as the mean ± standard deviation (SD). Data comparison between groups was performed using the ANOVA-LSD post hoc test. Analysis of data was conducted using SPSS version 19 (SPSS Inc, Chicago). The correlation between the Th17-related mRNA and different miRNAs expression in the murine spleen was tested by Spearman’s correlation analysis. A P-value of less than 0.05 was considered to be statistically significant.
Results
Immunomodulating effects of L. acidophilus KLDS 1.0738 in vitro
First, the ability of live lactobacilli to inhibit allergic inflammation was evaluated by culturing β-Lg-sensitized murine splenocytes in the presence or absence of various concentrations of L. acidophilus in vitro. shows that higher proliferative activities of splenocytes in response to β-Lg antigen stimulation was observed. In contrast, L. acidophilus KLDS 1.0738 had a significant inhibitory effect on β-Lg-sensitized murine splenocyte growth (P < 0.05). Further analyses showed that treatment of L. acidophilus KLDS 1.0738 significantly suppressed total IgE and β-Lg-specific IgE production in comparison with the cells treated with β-Lg antigen (). Additionally, decreased levels of IL-17, IL-21, IL-6 and TNF-α were also observed in the L. acidophilus-treated group (), especially in the high dosage groups (P < 0.05).
Figure 1. The immunomodulatory properties of live L. acidophilus KLDS 1.0738 in β-Lg-sensitized murine splenocytes.
Female BALB/c mice were intraperitoneally injected on days 7, 14 and 21 with 0.2 mL 1 mg/mL β-Lg adsorbed in Freund’s adjuvant. After final orally β-Lg challenge, splenocytes (5 × 106 cells/ml) were prepared and cultured with 1 mg/mL β-Lg in the absence (A: allergic group) or presence of various concentrations of live L. acidophilus (L: low dose, 107 CFU/mL; M: medium dose, 108 CFU/mL; H: high dose, 109 CFU/mL). The cells cultured with RPMI-1640 medium alone was used as normal control (N). The cells and culture supernatants were collected, and the proliferative response (A), total IgE (B), β-Lg-specific IgE (C), cytokines (D) and the expression of miRNAs (E) were measured. Experiments were repeated 3 times, and the values are the average ± SD. *P < 0.05 versus allergic group by the ANOVA-LSD post hoc test.
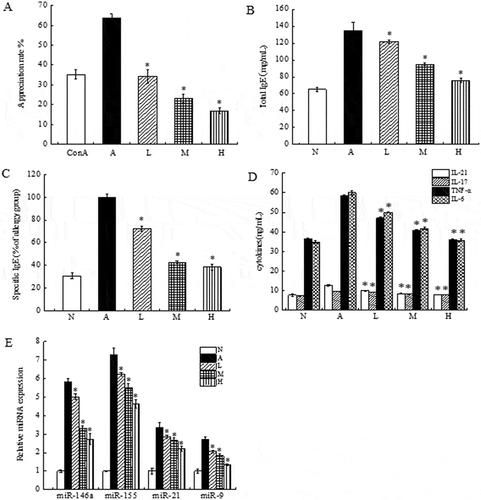
Next, we investigated the effect of L. acidophilus on the expression of inflammation-related miRNAs in β-Lg-sensitized murine splenocytes. As shown in , the splenocytes stimulated with β-Lg led to significantly increased expression of four miRNAs. Specifically, the levels of miR-146a and miR-155 in the allergic group were up-regulated more than 5-fold compared to the normal group. In contrast, treatment of L. acidophilus KLDS 1.0738 significantly reduced the expression of miR-146a, miR-155, miR-21 and miR-9 compared with those in the allergic group (P < 0.05).
Suppressive effect of L. acidophilus KLDS 1.0738 on hypersensitivity
Based on in vitro results, we continued to explore the protective effect of live lactobacilli against β-Lg-induced inflammation in a murine model. Compared to the allergic group, oral administration of live L. acidophilus KLDS 1.0738 tended to attenuate the severity of allergic symptoms, including reducing weight loss and improving hypersensitivity score (). The histological analysis results showed that the increase in β-Lg-induced inflammatory cell infiltration was reduced after treatment with L. acidophilus strains (). Furthermore, live probiotics increased the numbers of Bifidobacteria and Lactobacilli but decreased the numbers of Enterobacter and Enterococcus compared with the allergic group (). In addition, allergic mice treated with L. acidophilus KLDS 1.0738 showed a significant reduction in β-Lg-specific IgE and histamine concentration in serum (,). mRNA expression of IL-17 and RORγt in the colon and spleen were also lower in the live bacteria-treated group than those in the β-Lg-sensitized group () (P < 0.05).
Figure 2. Protective effect of live L. acidophilus KLDS 1.0738 strains against β-Lg- -induced allergy in BALB/c mice.
BALB/c mice were sensitized intraperitoneally on days 7, 14 and 21 with 1 mg/mL of β-Lg plus Freund’s adjuvant at a total volume of 0.2 mL (A: allergic group). L. acidophilus bacteria suspension (L: low dose, 107 CFU/mL; M: medium dose, 108 CFU/mL; H: high dose, 109 CFU/mL) was intragastrically given to the β-Lg-challenged mice three times a week from days 1 to 28. Control mice were treated with sterile saline (N). Body weight (A), anaphylactic score (B), colon H&E staining (C), fecal microflora (D), the production of β-Lg-specific IgE (E) and histamine (F) in serum were measured in the mice. All results are representative for 3 independent experiments. Results of experiments are means ± SD (n = 6 mice per group). *P < 0.05 versus allergic group by the ANOVA-LSD post hoc test.
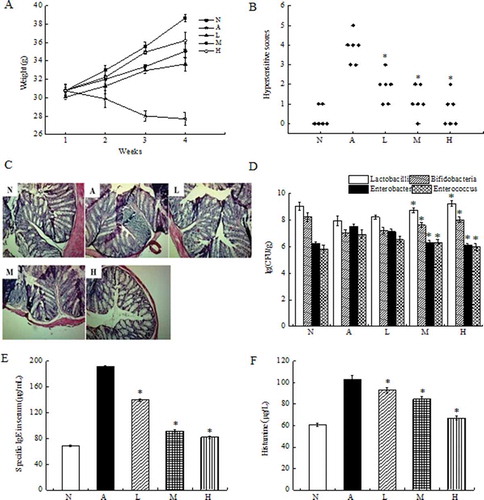
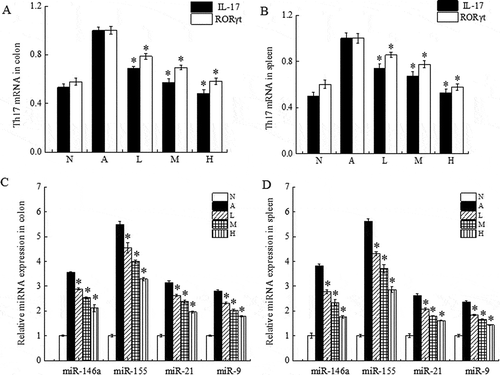
Figure 3. Effect of oral administration of live L. acidophilus KLDS 1.0738 on the expression of miRNAs, IL-17 and RORγt in spleen and colon tissue of β-Lg-sensitized mice.
After antigen challenge, the expression of miRNAs, mRNA expression of IL-17 and RORγt in the colon (A, C) and spleen (B, D) were measured by Real-Time PCR. All results are representative for 3 independent experiments. The results are means ± SD (n = 6 mice per group). *P < 0.05 versus allergic group by the ANOVA-LSD post hoc test. N: Normal group; A: Allergic group; L: Low dose (107 CFU/mL); M: Middle dose (108 CFU/mL); H: High dose L. acidophilus (109 CFU/mL).
L. acidophilus reduces miRNAS expression in β-Lg-sensitized mouse
Altered miRNA expression in the mouse spleen and colon tissue is shown in . Sensitizing mice with β-Lg allergen led to induction of high expression of miRNAs related to inflammation, such as miR-146a, miR-155, miR-21 and miR-9. In particular, miR-146a and miR-155 levels in the spleen and colon of allergic mice were more than 5 times higher than those in the normal group (P < 0.05). However, oral administration of live L. acidophilus KLDS 1.0738 significantly decreased allergen-induced miRNAs levels in both the spleen and colon tissue in a dose-dependent manner (P < 0.05).
Correlation between miRNAS and Th17-related cytokines
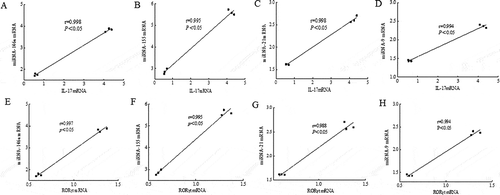
Further experiments were performed to examine the correlation between L. acidophilus strains modulating miRNAs levels and improving Th17 response. As shown in , there was a significant linear correlation between the expression of inflammation-related miRNAs and Th17-related mRNA in the spleens of the high dose L. acidophilus group compared to the allergic group (P < 0.05). The decreased expression of miR-146a, miR-155, miR-21 and miR-9 were positively related to decreased IL-17 mRNA expression, as well as RORγt mRNA expression (P < 0.05).
Figure 4. The correlation between the Th17-related mRNA and different miRNAs expression in the murine spleen.
The correlation of the gene expressions of IL-17 and miR-146a (A), miR-155 (B), miR-21 (C), miR-9 (D), and the correlation of the gene expressions of RORγt and miR-146a (E), miR-155 (F), miR-21 (G) and miR-9 (H) in the murine spleen of high dose of L. acidophilus group (dots on the left side) compared to allergy group (dots on the right side). A value of P < 0.05 was considered significantly correlation.
Discussion
CMA is an inflammatory disease characterized by IgE-mediated hypersensitivity and excessive eosinophil infiltration along with abnormal β-Lg-specific Th17/Treg cell imbalance toward Th17 dominance. Thus, regulators of Th17 cell differentiation and function are considered to be potential factors for preventing allergic inflammation.
Recently, several studies reported that miRNAs serve as important regulators of the immune response, participating in a wide variety of immunological and inflammatory disorders, including allergic diseases. Sonkoly et al. demonstrated that miR-155 was markedly induced by both T-cell activators in PBMCs and allergens in patients with chronic skin inflammation [Citation18]. Feng et al. indicated that miR-146a played important pro-inflammatory roles in murine models of asthma [Citation19]. Similarly, we observed that stimulation with β-Lg enhanced the expression of four miRNAs (miR-146a, miR-155, miR-21 and miR-9) in comparison to the normal group, accompanied by marked lymphocyte inflammatory influx into the colon and a high IgE level in the serum. These findings implied that the aberrant expression of miRNAs might be related to the aggravated β-Lg allergy.
Next, we further investigated the functional role of miRNAs in regulating the Th17 cell. To the best of our knowledge, Th17 cells can be induced by differentiation cytokines (such as IL-21, TNF-α, IL-17, IL-6) and transcription factors (such as STAT3 and RORγt), all of which are involved in the destruction due to allergic inflammation. Our results suggest that the expression of the miRNAs was positively correlated with both Th17-related cytokines production in β-Lg-stimulated splenocytes (data not shown) and mRNA expression of Th17 transcription factors in the β-Lg-sensitized murine model. The data published in recent years indicated the role of specific miRNA in fitting into the known regulatory circuits underlying Th17 cell biology. For example, miR-16 and miR-146a were shown able to target the 3ʹ-UTR of TNF-α and later regulate TNF-α signaling [Citation20,Citation21]. miR-16 was also found to be related to up-regulation of RORγt and down-regulation of FoxP3 in peripheral blood mononuclear cells of rheumatoid arthritis patients, which finally led to inducing Th17 cell proliferation [Citation22]. Additionally, Du et al. demonstrated that miR-326 could promote Th17 differentiation by targeting Ets-1, a negative regulator of Th17 differentiation [Citation23]. Murugaiyan et al. showed that miR-21 could promote Th17 differentiation by targeting and depleting SMAD-7, a negative regulator of TGF-β signaling [Citation24]. In addition, miR-146a attracted special attention because of its NF-κB–dependent induction and targeted attenuation of the NF-κB signaling transducers TRAF6 and IRAK1 [Citation25]. Although we did not detect the direct link between miRNAs and STAT3 or STAT5, Yao et al. confirmed that miR-155 could activate the IL-6/JAK/STAT3 signaling pathway for Th17 differentiation through down-regulation of suppressor of cytokine signaling-1 (SOCS-1) protein translation [Citation10]. In contrast, Yan et al. showed that miR-155 inhibition in experimental autoimmune myocarditis resulted in reducing the severity of disease and inhibiting the Th17 immune response [Citation26]. Consistent with this finding, miR-155−/- mice with chronic and autoimmune inflammation presented with defective Th17 differentiation [Citation27]. Therefore, we hypothesize that inhibition of dysregulated miRNAs might be a promising therapeutic strategy for Th17-induced allergic diseases.
Lactobacilli as probiotic bacteria are well known for their immunomodulatory effects in T-cell-mediated inflammatory diseases [Citation28]. Our previous study found that Lactobacillus acidophilus could alleviate β-Lg allergy by suppressing the aberrant balance of Th1/Th2 responses with decreasing the level of Th2 related cytokines such as IL-4 and increasing the level of Th1 related cytokines such as IFN-γ (data not shown). The identification of Th17 cells revised the traditional concept that Th1 and Th2 cells predominantly account for the inflammatory diseases. Therefore, we further reported the modulatory effects of heat-killed probiotic or probiotic cell wall extracts in the balance between Th17 and Treg responses, including decreased Th17 related cytokines and key transcription factors, reduced Th17 frequencies and Th17/Treg ratio in CD4+ splenocytes, and suppressed IL-6/STAT3 pathway in murine models of β-Lg allergy [Citation3]. In the present study, we discussed the anti-inflammation mechanism of live probiotics. As expected, in addition to alleviating the β-Lg-induced inflammatory reaction, administration of live lactobacilli also exerted inhibitory effects on the Th17 allergic response in in vitro and in vivo experiments. However, the underlying mechanisms for lactobacilli inhibited allergen-activated Th17 and Th2 responses have not been thoroughly elucidated to date.
Research on certain miRNAs advanced our understanding of the impact of probiotic bacteria on intestinal barrier function. It was determined that both miRNAs expression and intestinal microbiota-mediated host gene expression were important aspects in the control of innate immunity [Citation29]. Subsequent reports investigated the interaction between probiotics and miRNAs response. For instance, Archambaud et al. demonstrated that oral treatment with two Lactobacillus strains modulated the expression of miR-192, miR-200b and miR-215 during L. monocytogenes infection [Citation30]. Zhao et al. indicated that LGG supplementation protected the liver from ethanol-induced liver inflammation by decreasing ethanol-elevated miR-122a in a mouse model of chronic ethanol exposure [Citation31]. Kalani et al. found that miR-155, as a pro-inflammatory molecule, was inhibited by L. acidophilus in LPS-treated human umbilical vein endothelial cells [Citation32]. Accordingly, we focused on the effect of probiotics strains in modulating the miRNAs induced by β-Lg allergy. Our initial in vitro studies showed that treatment of live lactobacilli strains down-regulated four miRNAs in β-Lg-sensitized splenocytes, especially miR-146a and miR-155. Consistent with the results in vitro, administration of live L. acidophilus KLDS 1.0738 to β-Lg-sensitized mice also prevented high expression of inflammatory miRNA in spleen and colon tissue in a dose-dependent manner. Further analysis revealed that the expression levels of the four miRNAs were positively correlated with mRNA expression levels of IL-17 and RORγt in the L. acidophilus -treated group compared with those in the allergic group. Collectively, these data suggest that live probiotics as immune modulating agents, have the ability to inhibit miRNAs expression, which might be linked to decreased Th17 differentiation in CMA. In addition, miRNA was also involved in the regulation of allergen-induced Th2 responses in allergic airways disease [Citation33]. This raises the question whether manipulating the miRNA could provide a novel approach to influence Th2 related inflammation in β-Lg allergy. Thus, The relationship between miRNAs and Th2 cell activation and differentiation in β-Lg allergy will be discussed in future works.
Additionally, further research indicated that the immunomodulatory activity of probiotic bacteria was relevant to the activation of the Toll-like receptors (TLRs) signal, which induced the release of downstream inflammatory cytokines [Citation34,Citation35]. In the previous study, we also found that administration of L. acidophilus KLDS 1.0738 to sensitized mice elevated TLR2 transcription levels, as well as the TLR2 protein levels in colon tissue, along with a significant inhibitory response in IL-17, TNF-α and IL-6 production [Citation36]. As the primary role players in innate immunity, several miRNAs have been shown to negatively regulate the expression of key proteins participating in the TLRs/NF-κB signaling pathway [Citation37,Citation38]. Furthermore, regulating TLR-responsive miRNAs, such as miR-155, miR-21 and miR-146a, was able to inhibit or enhance the TLR-triggered inflammatory response [Citation39–Citation41]. Therefore, whether miRNAs mediating TLR ligands initiated by TLR2-recognition of bacterial components were responsible for reducing allergic inflammation warrants further research.
Conclusions
The present in vitro and in vivo studies revealed that the increase in miRNA levels may be involved in the development of Th17 inflammation during CMA. However, live L. acidophilus KLDS 1.0738 supplementation not only decreased the expression of four miRNAs (miR-146a, miR-155, miR-21 and miR-9) but also attenuated β-Lg-induced Th17-dominated allergy in mice. Our present understanding of the role of miRNAs in the regulation of allergic diseases is still notably limited. However, these findings help to elucidate the mechanism of beneficial microorganisms and open new avenues for preventative therapy of food allergies.
Authors contribution
Jun-juan Wang and Ai-li Li designed and performed the experiments, participated in writing the manuscript. Si-han Li, Qi-min Zhang, Wei-wei Ni and Mei-na Li analyzed the data. Xiang-chen Meng, Chun Li, Shi-long Jiang, Jian-cun Pan and Yuan-yuan Li helped to carry out the studies and write the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Glosson-Byers NL, Sehra S, Stritesky GL, et al Th17 cells demonstrate stable cytokine production in a proallergic environment. J Immunol. 2014;193:2631–2640.
- Lifschitz C, Szajewska H. Cow’s milk allergy: evidence-based diagnosis and management for the practitioner. Eur J Pediatr. 2015;174:141–150.
- Zhang Y, Li AL, Sun YQ, et al Lactobacillus acidophilus regulates STAT3 and STAT5 signaling in bovine beta-lg-sensitized mice model. Dairy Sci Technol. 2016;96:501–512.
- Lu TX, Hartner J, Lim EJ, et al MicroRNA-21 limits in vivo immune response-mediated activation of the IL-12/IFN-gamma pathway, Th1 polarization, and the severity of delayed-type hypersensitivity. J Immunol. 2011;187:3362–3373.
- Rebane A, Akdis CA. MicroRNAs: essential players in the regulation of inflammation. J Allergy Clin Immunol. 2013;132:15–26.
- Rebane A, Runnel T, Aab A, et al MicroRNA-146a alleviates chronic skin inflammation in atopic dermatitis through suppression of innate immune responses in keratinocytes. J Allergy Clin Immunol. 2014;134:836–847.e811.
- Solinas C, Corpino M, Maccioni R, et al Cow’s milk protein allergy. J Matern Fetal Neonatal Med. 2010;23(Suppl 3):76–79.
- Mattes J, Collison A, Plank M, et al Antagonism of microRNA-126 suppresses the effector function of TH2 cells and the development of allergic airways disease. Proc Natl Acad Sci U S A. 2009;106(44):18704–18709.
- Niimoto T, Nakasa T, Ishikawa M, et al MicroRNA-146a expresses in interleukin-17 producing T cells in rheumatoid arthritis patients. BMC Musculoskelet Disord. 2010;11:209.
- Yao R, Ma YL, Liang W, et al MicroRNA-155 modulates Treg and Th17 cells differentiation and Th17 cell function by targeting SOCS1. PLoS One. 2012;7:e46082.
- Liu YL, Wu W, Xue Y, et al MicroRNA-21 and -146b are involved in the pathogenesis of murine viral myocarditis by regulating TH-17 differentiation. Arch Virol. 2013;(158):1953–1963. DOI:10.1007/s00705-013-1695-6
- Hashemi A, C R V, Comelli EM. Probiotics in early life: a preventative and treatment approach[J]. Food Funct. 2016;7(4):1752–1768.
- Yang J, Ren F, Zhang H, et al Induction of regulatory dendritic cells by lactobacillus paracasei L9 prevents allergic sensitization to bovine beta-Lactoglobulin in mice. J Microbiol Biotechnol. 2015;(25):1687–1696. DOI:10.4014/jmb.1503.03022
- Zhu J, Zhao LA, Guo HY, et al Immunomodulatory effects of novel bifidobacterium and lactobacillus strains on murine macrophage cells. Afr J Microbiol Res. 2011;5:8–15.
- Giahi L, Aumueller E, Elmadfa I, et al Regulation of TLR4, p38 MAPkinase, IκB and miRNAs by inactivated strains of lactobacilli in human dendritic cells. Benef Microbes. 2012;3:91–98.
- Liang G, Malmuthuge N, Guan Le L, et al Model systems to analyze the role of miRNAs and commensal microflora in bovine mucosal immune system development. Mol Immunol. 2015;66:57–67.
- Li AL, Meng XC, Duan CC, et al Suppressive effects of oral administration of heat-killed Lactobacillus acidophilus on T helper-17 immune responses in a bovine β-lactoglobulin-sensitized mice model. Biol Pharm Bull. 2013;36:202–207.
- Sonkoly E, Janson P, Majuri ML, et al MiR-155 is overexpressed in patients with atopic dermatitis and modulates T-cell proliferative responses by targeting cytotoxic T lymphocyte–associated antigen 4. J Allergy Clin Immunol. 2010;126:1–20.
- Feng MJ, Shi F, Qiu C, et al MicroRNA-181a, -146a and -146b in spleen CD4+ T lymphocytes play proinflammatory roles in a murine model of asthma. Int Immunopharmacol. 2012;13:347–353.
- K D T, M P B, K J C, et al NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci. 2006;103(33):12481–12486.
- A V C, E K O, Knip M. MicroRNAs in rheumatoid arthritis: altered expression and diagnostic potential. Autoimmun Rev. 2015;14(11):1029–1037.
- Wu YH, Liu W, Xue B, et al Upregulated expression of microRNA-16 correlates with Th17/Treg cell imbalance in patients with rheumatoid arthritis. DNA Cell Biol. 2016;35:853–860.
- Du C, Liu C, Kang J, et al Corrigendum: microRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol. 2009;10(12):1252–1259.
- Murugaiyan G, A P D C, A K A, et al MicroRNA-21 promotes Th17 differentiation and mediates experimental autoimmune encephalomyelitis. J Clin Investigation. 2015;125(3):1069–1080.
- Y F X, Shu R, S Y J, et al miRNA-146 negatively regulates the production of pro-inflammatory cytokines via NF-κB signalling in human gingival fibroblasts. J Inflamm. 2014;11(1):38.
- Yan L, Hu F, Yan X, et al Inhibition of microRNA-155 ameliorates experimental autoimmune myocarditis by modulating Th17/Treg immune response. J Mol Med (Berl). 2016;94:1063–1079.
- Zhang A, Wang K, Zhou C, et al Knockout of microRNA-155 ameliorates the Th1/Th17 immune response and tissue injury in chronic rejection. J Heart Lung Transplant. 2016;36(2):175–184.
- Kim SW, Kim HM, Yang KM, et al Bifidobacterium lactis inhibits NF-kappaB in intestinal epithelial cells and prevents acute colitis and colitis-associated colon cancer in mice. Inflamm Bowel Dis. 2010;16:1514–1525.
- Dalmasso G, Nguyen HT, Yan Y, et al Microbiota modulate host gene expression via microRNAs. PLoS One. 2011;6:e19293.
- Archambaud C, Nahori MA, Soubigou G, et al Impact of lactobacilli on orally acquired listeriosis. Proc Natl Acad Sci U S A. 2012;109:16684–16689.
- Zhao H, Zhao C, Dong Y, et al Inhibition of miR122a by Lactobacillus rhamnosus GG culture supernatant increases intestinal occludin expression and protects mice from alcoholic liver disease. Toxicol Lett. 2015;234:194–200.
- Kalani M, Hodjati H, Sajedi Khanian M, et al Lactobacillus acidophilus increases the anti-apoptotic micro RNA-21 and decreases the pro-inflammatory micro RNA-155 in the LPS-treated human endothelial cells. Probiotics Antimicrob Proteins. 2016;8:61–72.
- Mattes J, Collison A, Plank M, et al Antagonism of microRNA-126 suppresses the effector function of TH2 cells and the development of allergic airways disease. Proc Natl Acad Sci U S A. 2009;06(44):18704–18709.
- Gabriela P, Castillo NA, Alejandra LB. Oral administration of a probiotic Lactobacillus modulates cytokine production and TLR expression improving the immune response against Salmonella enterica serovar Typhimurium infection in mice. BMC Microbiol. 2011;11:1–12.
- Yao P, Tan F, Gao H, et al Effects of probiotics on Tolllike receptor expression in ulcerative colitis rats induced by 2,4,6-trinitrobenzene sulfonic acid. Mol Med Rep. 2017;15:1973–1980. DOI:10.3892/mmr.2017.6226
- Li AL, Sun YQ, Du P, et al The Effect of Lactobacillus actobacillus peptidoglycan on bovine β-Lactoglobulin-sensitized mice via TLR2/NF-κB pathway. Iran J Allergy Asthma Immunol. 2017;16:147.
- Case SR, Martin RJ, Jiang D, et al MicroRNA-21 inhibits toll-like receptor 2 agonist–induced lung inflammation in mice. Exp Lung Res. 2011;37:500–508.
- Quinn EM, Wang JH, O’Callaghan G, et al MicroRNA-146a is upregulated by and negatively regulates TLR2 signaling. PLoS One. 2013;8:e62232.
- Sun Y, Cai J, Ma F, et al miR-155 mediates suppressive effect of progesterone on TLR3, TLR4-triggered immune response. Immunol Lett. 2012;146:25–30.
- Bi D, Cui J, Chu Q, et al MicroRNA-21 contributes to suppress cytokines production by targeting TLR28 in teleost fish. Mol Immunol. 2017;83:107–114.
- Jiang SY, Xue D, Xie YF, et al The negative feedback regulation of microRNA-146a in human periodontal ligament cells after porphyromonas gingivalis lipopolysaccharide stimulation. Inflamm Res. 2015;64:441–451.

