ABSTRACT
Most breast cancer survivors receiving chemotherapy have severe cognitive impairment, often referred to as “chemobrain.” Polydatin (PLD) is known to have many biological activities. Thus, this study aimed to determine whether symptoms of chemobrain can be prevented or relieved by PLD. The chemobrain models were established by intraperitoneal injection of doxorubicin (DOX, 2 mg/kg) in rats once a week for 4 weeks (DOX group and DOX+PLD group). In the PLD group and DOX+PLD group, PLD (50 mg/kg) was administered orally to rats every day. We found that PLD treatment significantly protected against DOX-induced learning and memory impairment, restored hippocampal histopathological architecture. Furthermore, PLD suppressed DOX-induced oxidative stress through up-regulating Nrf2, inhibited inflammatory response by activating the NF-κB pathway, and reduced hippocampal apoptosis. Therefore, the present study indicated that PLD offered neuroprotection against DOX-induced chemobrain. PLD may assist in preventing chemobrain after chemotherapy in patients with cancers.
GRAPHICAL ABSTRACT
Polydatin attenuated doxorubicin-induced hippocampal nerve damage to alleviate cognitive impairment by inhibiting oxidative stress, inflammatory response, and apoptosis in rats.
Chemotherapy leaves a growing number of cancer patients alive, but patients receiving chemotherapeutic agents are often plagued with some side effects [Citation1]. Cancer survivors have developed slow processing speed, memory impairment, inability to concentrate, and language difficulty, which is often attributed to the receipt of chemotherapy, and called chemotherapy-induced cognitive impairment (also known as “chemobrain”) [Citation2]. This phenomenon greatly deteriorates the survivors’ quality of life and prevents them from resuming their pre-cancer lives. Breast cancer patients were found to be the most reported patients with impaired cognitive function after chemotherapy [Citation3,Citation4].
Paclitaxel and doxorubicin (DOX) are still the most commonly used chemotherapeutic agents to manage breast cancer [Citation5]. Despite DOX is known not to target the central nervous system or even across the blood–brain barrier (BBB), it could still impair cognitive functions and induces chemobrain in rodents and patients [Citation6,Citation7]. Therefore, prevention or alleviation of chemobrain is important for the treatment and rehabilitation of cancer patients, especially breast cancer patients. Several agents (such as cholinesterase inhibitors, modafinil, and anti-inflammatory agents) have been tried clinically to treat chemobrain, but little effect has been achieved [Citation8,Citation9]. So it is necessary to find new methods to combat chemotherapy-induced cognitive impairment so as to improve the quality of life in cancer survivors.
Polydatin (PLD) is a glycoside of resveratrol (Res) extracted from the root of Polygonum cuspidatum. Although PLD and Res could transform each other in vivo, PLD is the main substance in serum after administration of PLD or Res, suggesting that PLD has better oral absorption and metabolic stability than Res [Citation10,Citation11]. Furthermore, PLD exhibits diverse biological functions, including anti-inflammatory [Citation12], antioxidant [Citation13,Citation14] and immunoregulation [Citation15]. Studies have shown that PLD can alleviate chemotherapy-induced oxidative stress and lipid peroxidation to protect organisms [Citation16]. More importantly, PLD can cross the BBB to prevent Parkinson’s disease [Citation17], reduce brain edema following cerebral infarction [Citation18], protect against cerebral nerve injury caused by ischemia/reperfusion injury [Citation19,Citation20] and alleviate cognitive impairment caused by chronic drinking [Citation21]. Nevertheless, there have been no reports on the effects of PLD on chemotherapy-induced cognitive impairment.
In the present study, we aimed to study the effects of PLD on symptoms of chemobrain and disruptions in the hippocampus in rat models of DOX-induced chemobrain.
Materials and methods
Animal model
Animal studies and treatment were approved by the Hebei Medical University Ethics Committee and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Six-week-old male Sprague–Dawley rats (Beijing HFK Bioscience Co. Ltd., China), weighting 210–230 g, were used for the experiments. Rats were kept in a regulated environment (temperature, 22 ± 1°C; relative humidity, 45–55%; 12-h light/dark cycle).
Rats were randomly divided into four groups: sham-operated group (Sham, n = 6), doxorubicin-treated group (DOX, n = 6), polydatin administered group (PLD, n = 6), and doxorubicin-treated and polydatin administered group (DOX+PLD, n = 6). To generate the rat model of chemotherapy, 2 mg/kg of DOX (Melone Pharmaceutical Co., Ltd., China) was intraperitoneally (IP) injected for the DOX group once a week for 4 weeks. For PLD groups, 50 mg/kg of PLD (Aladdin, China) was taken orally every day for 3 weeks. In the DOX+PLD group, 2 mg/kg of DOX was IP injected once a week for 4 weeks, and the PLD was taken orally at 50 mg/kg per day for 4 weeks. Rats in the sham group were administered an equal volume of solvent. Behavioral analyses of each group were observed after 28 days.
Morris water-maze task
The Morris water-maze task [Citation22,Citation23] was conducted in a circular pool of 160 cm in diameter. The pool was filled to a depth of 24 cm with water. The pool temperature was maintained at 22 ± 2°C. A platform with a diameter of 10 cm placed in the third quadrant, 30 cm from the pool’s edge and submerged 1 cm below water level.
We placed the rat on the platform for 20 s. The rat was then placed into the pool from one of the four quadrant walls. The maximum swim time was set to 60 s. If the rat located the platform before 60 s, it was immediately removed from the pool. If the platform was not located within 60 s, the rat was guided to the platform for 15 s before being removed from the pool. Rats were tested for 5 days, training them twice a day in each quadrant. The average latency of the four quadrants was recorded to evaluate the spatial learning ability of the rats.
To examine spatial memory, a probe test was administered 1 day after the last training. The platform was removed from the pool, and the rat was then placed into the pool from the third quadrant wall. The rat was allowed to swim freely for 60 s, and its swimming tracks were recorded.
Step-down avoidance task
In order to evaluate the short-term memory, the step-down avoidance task was performed. The rat was placed on a 7 × 25 cm platform which was 2.5 cm in height. In the training sessions, the rat was shocked (0.5 mA, 2 s) after they stepped down off the platform and stand on the grid. Two hours after training, the latency time in each group was measured. Grid was not energized. The interval of time that the rat stepped down and stayed on the grid was defined as latency time. A latency time greater than 180 s was counted as 180 s.
Hematoxylin-Eosin (HE) staining
Brain samples from different groups were fixed, dehydrated, and embedded in paraffin. Then, paraffin tissue blocks were sliced at 5 μm thickness. The slices were dried at 60°C in an oven for 4 h, dewaxed twice in xylene (Aladdin, China) for 15 min each, hydrated in graded concentrations of ethanol at 95%, 85%, and 75% for 2 min each, stained with hematoxylin (Solarbio, China) for 5 min, treated by distilled water for 20 min, stained with eosin Y (Sangon, China) for 3 min, dehydrated by graded concentrations of ethanol (75%, 85%, and 95%). Last, the slices were observed using a light microscope.
Toluidine blue staining
Paraffin slices were stained with toluidine blue O (Solarbio, China) for 10 min, washed with distilled water for 2 min, treated by acetone, dehydrated and mounted with neutral balsam. Afterward, the slides were visualized under a light microscope.
Assessments of oxidative stress biomarkers
The tissue samples were added to 9 volumes of saline, homogenized with a tissue homogenizer, and centrifugated at 430 × g for 10 min. The levels of malondialdehyde (MDA) and glutathione (GSH) were measured using maleic dialdehyde assay Kit (Nanjing Jiancheng Bioengineering Institute, China) and Glutathione (GSH) Assay Kit (Nanjing Jiancheng Bioengineering Institute, China).
Assessments of inflammatory markers
The tissue samples were added to 9 volumes of saline, homogenized with a tissue homogenizer, and centrifugated at 430 × g for 10 min. The levels of tumor necrosis factor alpha (TNF-α), prostaglandin E2 (PEG-2) and cyclooxygenase-2 (COX-2) were assessed using tumor necrosis factor alpha (CLOUD-CLONE CORP.WUHAN, China), ELISA Kit for prostaglandin E2 (CLOUD-CLONE CORP.WUHAN, China) and ELISA Kit for cyclooxygenase-2 (CLOUD-CLONE CORP.WUHAN, China).
TUNEL staining
The tissue slices were soaked in phosphate-buffered saline (PBS) for 5 min and permeabilized with 0.1% Triton X-100. The TUNEL Apoptosis Assay Kit (Wanleibio, China) was used to label apoptotic cells. DAPI was added for 5 min to stain the nuclei. The samples were washed with PBS and then observed under the fluorescence microscope.
Western blot analysis
The hippocampus tissues were homogenized in liquid nitrogen and lysed in the lysis buffer (Beyotime, Shanghai, China) containing 1 mM PMSF (Beyotime, China). Protein content was measured using a BCA protein assay kit (Beyotime, China). Proteins were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Beyotime, China) and transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA), which were incubated with cleaved caspase-3 antibody (CST, USA), cleaved caspase-9 (Abcam, Cambridge, UK), Nrf-2 antibody (Proteintech, Wuhan, China), p-IκBα antibody (Bioss, Beijing, China), IκBα antibody (Bioss, China), p-p65 antibody (Bioss, China), p65 antibody (Bioss, China) Histone H3 (ABGENT, USA) and β-actin antibody (Santa Cruz, CA, USA). The membranes were subsequently incubated with goat anti-mouse IgG secondary antibody (Beyotime, China) or goat anti-rabbit IgG secondary antibody (Beyotime, China). The antigen-antibody reaction was visualized by ECL assay (Beyotime, China). Histone H3 was used as the control of Nrf2. β-actin was used as the control of other proteins.
Statistical analysis
All the statistical analyses were performed using the GraphPad Prism 8.0 (GraphPad Inc., San Diego, CA, USA). Student’s t-test was used to compare the differences between the two groups, and multiple group comparisons were analyzed with one-way analysis of variance (ANOVA). Each experiment was repeated at least three times. Data were presented as mean ± SD (standard deviation). A value of P < 0.05 was considered statistically significant.
Results
PLD improved spatial learning and memory
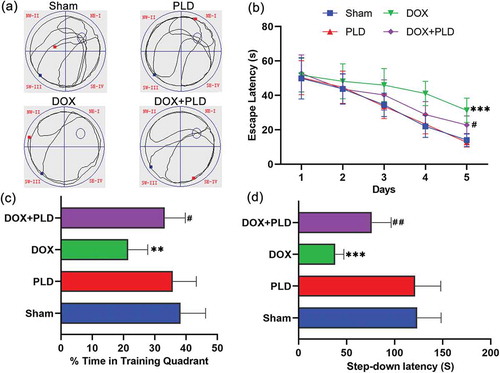
We employed the Morris water-maze task to assess spatial learning and memory in rats (n = 6). Traces of movement of rats are presented in ). The DOX group observed a decrease in spatial memory ability relative to levels exhibited in the sham group, whereas the DOX+PLD group exhibited an increase in spatial memory relative to that observed in the DOX group ()). The escape latency in the DOX group was significantly longer than that in the sham group, and this difference began on the second day. The latency difference between the DOX+PLD group and the sham group began on the third day. Over time, the latency in the DOX+PLD group was significantly shorter than that in the DOX group ()). Besides, rats in the DOX group spent more time in the target quadrant than rats in the DOX+PLD group ()). Short-term memory was assessed by the step-down avoidance task. The latency time of the step-down in the DOX group was 38.1 ± 9.0 s, which was significantly less than the sham group (123.5 ± 24.8 s). Co-administration of PLD significantly increased the step-through latency time (76.2 ± 20.2 s) in the DOX+PLD group ()). Moreover, there was no difference between the sham group and the PLD group ()). These results demonstrate that PLD did not show neurotoxicity. DOX leads to decreased spatial learning/memory and short-term memory, while PLD improves rat’s learning and memory abilities in the DOX-induced chemobrain models.
Figure 1. Spatial learning and memory ability were assessed by Morris water maze and step-down avoidance task. (a) The swim tracks of the rats during Morris water-maze testing. (b) Comparison of the latency to find the platform among the four groups in Morris water-maze testing. The 1, 2, 3, 4, and 5 represent the latency day. (c) Percentage of time in the training quadrant. (d) Step-down latency time. Each group consisted of 6 rats. Values represent mean ± SD, ** p < 0.01, *** p < 0.001, vs. Sham group. #p < 0.05, ##p < 0.01, vs. DOX group.
PLD ameliorated DOX-induced hippocampal neurodegenerative changes
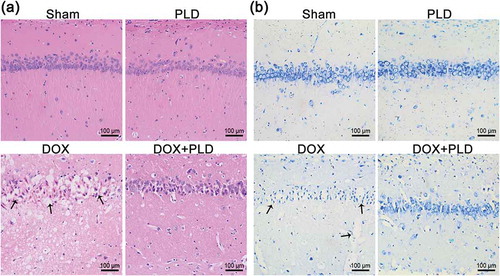
As shown in ), the sham group and PLD group had normal histological structures of the hippocampus. DOX resulted in nuclear pyknosis and degeneration of neuronal cells in the hippocampus. Co-administration of PLD and DOX partially restored the histological features of the hippocampus ()). The extent of neurodegeneration in the hippocampus was further assessed using toluidine blue staining ()). DOX resulted in complete loss of histological architecture of hippocampal neurons. Conversely, co-administration of PLD and DOX partially restored neuron features manifested by the rounded neuronal build and defined nuclei. Sham group and PLD group showed no difference ()).
Figure 2. PLD alleviated neurodegeneration induced by DOX. (a) Histological sections of hippocampus were observed by HE staining (magnification, ×200). Black arrows indicated nuclear pyknosis and degeneration in the DOX group. (b) Histological sections of the hippocampus were observed by toluidine blue staining (magnification, ×200). Black arrows indicated the degenerated neurons possessing pyknotic nuclei in the DOX group.
PLD affected DOX-induced oxidative stress and pro-inflammatory response
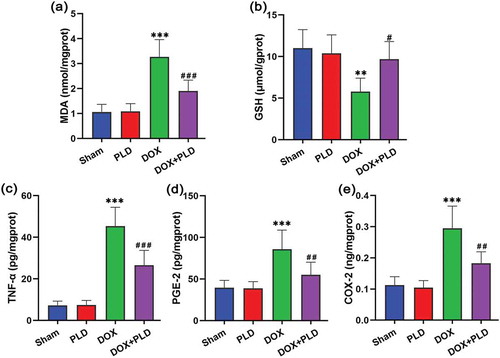
In order to assess the effect on hippocampal oxidative stress, we detected the levels of GSH and MDA () and ). DOX caused a significant increase in the content of MDA (3.2 ± 0.7 nmol/mgprot), while co-administration of PLD reduced this elevation (1.9 ± 0.4 nmol/mgprot) ()). DOX reduced GSH levels (5.8 ± 1.6 μmol/gprot), while co-administration of PLD prominently restored GSH to normal levels (9.7 ± 2.1 μmol/gprot) ()). We further studied the effect of PLD on DOX-induced pro-inflammatory response (). TNF-α levels were significantly increased by DOX (45.3 ± 9.1 pg/mgprot), while the administration of PLD reduced this elevation (16.5 ± 7.2 pg/mgprot) ()). Besides, PGE-2 levels were increased in the DOX group (85.9 ± 22.9 pg/mgprot), and restored to normal levels in the DOX+PLD group (55.0 ± 15.2 pg/mgprot) ()). DOX caused an increase in COX-2 levels (0.3 ± 0.1 ng/mgprot), while PLD treatment could attenuate DOX-induced elevation (0.1 ± 0.03 ng/mgprot) ()). In addition, there was no statistical difference between the PLD group and the sham group (). These results indicated that DOX triggered oxidative stress and inflammatory response in the hippocampal tissues. PLD treatment significantly attenuated DOX-induced oxidative stress and inflammatory response.
Figure 3. Effects of PLD treatment on DOX-induced oxidative stress and pro-inflammatory response in hippocampal tissues. (a) The content of MDA was expressed as nmol/mgprot. (b) The content of GSH was expressed as μmol/gprot. (c) The content of TNF-α was expressed as pg/mgprot. (d) The content of PGE-2 was expressed as pg/mgprot. (e) The content of COX-2 was expressed as ng/mgprot. Data are presented as mean ± SD (n = 6). **p < 0.01, ***p < 0.001, vs. Sham group. #p < 0.05, ##p < 0.01, ###p < 0.001, vs. DOX group.
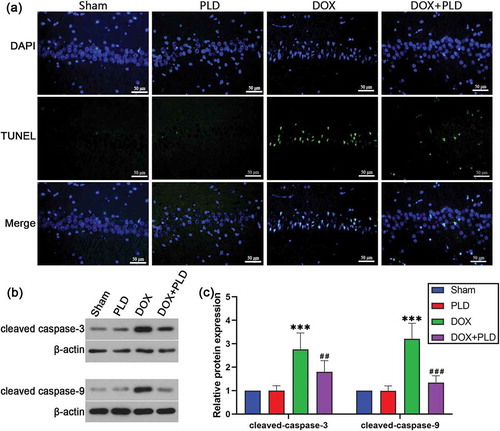
PLD inhibited DOX-induced neuronal apoptosis
The number of TUNEL positive cells in the DOX group was higher than that in the sham group, while co-administration of PLD could reduce the number of TUNEL positive cells ()). The levels of cleaved caspase-3 and cleaved caspase-9 were higher in the DOX group than the sham group. Conversely, co-administration of PLD decreased the expression of cleaved caspase-3 and cleaved caspase-9 (). These results indicated that PLD inhibited neuronal apoptosis in the hippocampus of rats.
Figure 4. Effect of PLD on DOX-induced neuronal apoptosis in the hippocampus. (a) Immunofluorescence staining for TUNEL (green), DAPI (blue), and merged images. (b and c) The expression of cleaved caspase-3 and cleaved caspase-9 were assessed by Western blot. Data are presented as mean ± SD (n = 6). ***p < 0.001, vs. Sham group. ##p < 0.01, ###p < 0.001, vs. DOX group.
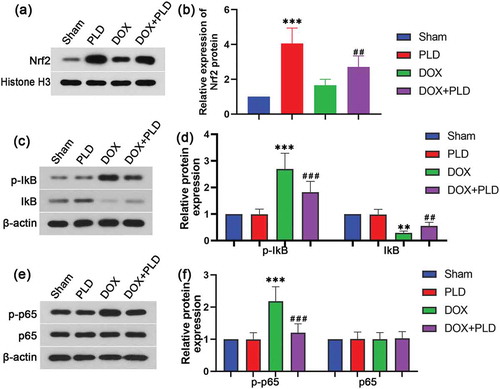
Effect of PLD on the signal pathway in the hippocampus of rats with DOX-induced chemobrain
We further explore the effect of PLD on oxidative stress and proinflammatory response-related signaling pathways. Nuclear factor E2-related factor 2 (Nrf2) is a major transcription factor of the antioxidant stress [Citation24]. We found that PLD could significantly increase the expression of Nrf2. Nrf2 levels in the DOX+PLD group was much higher than that in the DOX group (). Nuclear factor kappa B (NF-κB) is associated with inflammatory responses [Citation25]. So we assayed the expression of NF-κB pathway-associated proteins. DOX treatment enhanced the expression of p-IκB and p-p65, indicating that DOX activated the NF-κB pathway. In contrast, co-administration of PLD could inhibit the activation of IκB and p65 (). These results demonstrated that PLD prevented DOX-induced oxidative stress and pro-inflammatory response through Nrf2 and NF-κB pathway.
Figure 5. Effect of PLD on the signal pathway in the hippocampus of rats with DOX-induced chemobrain. Protein expression of Nrf2 (a and b), p-IκB (c and d), IκB (c and d), p-p65 (e and f) and p65 (e and f) was evaluated by Western blot. Data are presented as mean ± SD (n = 6). **p < 0.01, ***p < 0.001, vs. Sham group. ##p < 0.01, ###p < 0.001, vs. DOX group.
Discussion
Many chemotherapeutic agents could directly or indirectly affect brain function [Citation26,Citation27]. DOX is one of the most widely used chemotherapeutic agents for breast cancer patients [Citation5]. It is well known that DOX inhibits DNA synthesis and topoisomerase II to interrupt chromosome replication, thereby inhibiting tumor growth [Citation28]. DOX could also generate free radicals, such as the lipid peroxides and superoxide radicals, which cause the non-targeted cytotoxicity of DOX [Citation29,Citation30]. Although DOX is cytotoxic, neither it nor its metabolites readily pass the BBB. How could it cause chemobrain? Substantially, DOX stimulates some cytokines (such as inflammatory factors) release [Citation31], which could pass the BBB and lead to brain damage [Citation32]. In addition, DOX impaired learning and memory in rodents [Citation6,Citation33], and patients treated with DOX performed poorly on cognitive and visual-spatial skills tests [Citation7,Citation34]. Hence, we have selected to study DOX-induced cognitive impairment and to test PLD as a possible treatment. We found that treatment with PLD inhibited DOX-induced cognitive deficits at both neurobehavioral and hippocampal histopathological levels.
Oxidative stress is the key to mediating DOX-induced cognitive deficits [Citation35]. The structure of DOX has a quinone moiety that undergoes a redox cycling transformation, which produces large amounts of reactive oxygen species (ROS). The consequence of increased ROS is a disarranged equilibrium between oxidants and antioxidants resulting in oxidative stress [Citation36]. Nrf2 drives an antioxidant defense mechanism to deal with the increased production of ROS [Citation37]. Nrf2 dysfunction exacerbates cellular oxidative stress [Citation38]. Our results indicated that DOX-induced cognitive impairment was associated with increased oxidative stress in hippocampal tissues as depicted by significant elevation of MDA levels and reduction of GSH and Nrf2 levels. Treatment with PLD could reverse levels of these markers of oxidative stress, implying that the neuroprotective effects of PLD in preventing DOX-induced cognitive impairment could be partially attributed to PLD’s antioxidant capacity.
It has been reported that ROS produced from a redox cycling process activates the redox-responsive transcription factor NF-κB, which in turn activates the transcription of multiple genes including inflammatory cytokines [Citation39]. In a previous observational clinical study, researchers observed the elevation of TNF-α after DOX treatment in the context of multiagent chemotherapy regimens [Citation40]. Other researchers revealed that DOX administration increased brain TNF-α level in wild-type mice and rats [Citation41,Citation42]. Inflammation appears to be one of the main causes of DOX-induced cognitive impairment [Citation31]. In addition, researchers have found that PLD treatment could reduce inflammation correlated with inhibition of TLR4/NF-κB signaling [Citation43]. Consistently, this study showed that DOX dramatically increased the levels of TNF-α, PGE-2, and COX-2 in hippocampal tissues, suggesting that DOX heightened neuroinflammatory response in rats. In contrast, PLD treatment suppressed such an inflammation response. Furthermore, PLD could attenuate the activation of IκB and p65. Therefore, the anti-inflammatory effect of PLD may be due to its ability to block ROS-induced activation of the NF-κB signaling pathway through inhibiting the phosphorylation of IκB and p65.
Activation of apoptotic signaling pathways is considered as an important contributor to DOX-induced cognitive impairment [Citation44]. In a rat model of permanent middle cerebral artery occlusion, PLD treatment mitigated the neurobehavioral deficits, sequentially rescued neuronal apoptosis [Citation45]. In our study, we found that PLD restored cleaved caspase-3 and cleaved caspase-9 to normal levels, suggesting that PLD treatment attenuated chemobrain through reducing apoptosis.
Previous studies have confirmed that PLD and Res have similar chemical structures, and they can mutually transform in vivo [Citation11]. Thus, they exerted similar pharmacological actions, such as neuroprotective [Citation46–Citation48] and antioxidant effects [Citation10,Citation49]. A publication showed that rats were orally administered with the same amount of PLD or Res, respectively. At different times, the concentration of PLD was significantly higher than that of Res in vivo [Citation10]. PLD is more stable and shows better bioavailability than Res in vivo [Citation10,Citation50,Citation51]. Therefore, this study prioritized the protective effect of PLD on chemobrain. Our results have demonstrated that the administration of PLD can protect chemobrain. Although whether the protective effects observed in the brain are directly attributable to PLD or transformed Res requires further investigation, we believe that the conclusions of this article have some significance for clinical treatment.
Conclusion
We indicated that PLD presents a promising neuroprotective agent which could protect against DOX-induced cognitive impairment. DOX treatment caused cognitive impairment in SD rats, and PLD could prevent these deficits through its antioxidant, anti-inflammatory, and anti-apoptotic activities. Therefore, PLD may be a promising and potential adjuvant therapeutic agent to alleviate these deficits associated with DOX-induced chemobrain.
Author contribution
Participated in research design: J.Z. Cui, Y.F. Tong
Conducted experiments: Y.F. Tong, K.J. Wang, S.H. Sheng
Performed data analysis: Y.F. Tong, K.J. Wang
Wrote or contributed to the writing of the manuscript: Y.F. Tong, J.Z. Cui
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data described in this article are openly available in the Open Science Framework at DOI:10.17605/OSF.IO/TPA6U.
References
- Ren X, Boriero D, Chaiswing L, et al. Plausible biochemical mechanisms of chemotherapy-induced cognitive impairment (“chemobrain”), a condition that significantly impairs the quality of life of many cancer survivors. Biochimica et Biophysica Acta Mol Basis Dis. 2019 Jun 1;1865(6):1088–1097. PubMed PMID: 30759363; PubMed Central PMCID: PMCPMC6502692. eng.
- Nelson CJ, Nandy N, Roth AJ. Chemotherapy and cognitive deficits: mechanisms, findings, and potential interventions. Palliat Support Care. 2007 Sep;5(3):273–280. PubMed PMID: 17969831; eng.
- Fardell JE, Vardy J, Johnston IN, et al. Chemotherapy and cognitive impairment: treatment options. Clin Pharmacol Ther. 2011;90(3):366–376.
- Runowicz CD, Leach CR, Henry NL, et al. American cancer society/American society of clinical oncology breast cancer survivorship care guideline. CA Cancer J Clin. 2016;66(1):43–73.
- Fraguas-Sanchez AI, Martin-Sabroso C, Fernandez-Carballido A, et al. Current status of nanomedicine in the chemotherapy of breast cancer. Cancer Chemother Pharmacol. 2019 Jul;31(84):689–706. PubMed PMID: 31367789; eng.
- Konat GW, Kraszpulski M, James I, et al. Cognitive dysfunction induced by chronic administration of common cancer chemotherapeutics in rats. Metab Brain Dis. 2008 Sep;23(3):325–333. PubMed PMID: 18690526; eng.
- Jansen CE, Dodd MJ, Miaskowski CA, et al. Preliminary results of a longitudinal study of changes in cognitive function in breast cancer patients undergoing chemotherapy with doxorubicin and cyclophosphamide. Psychooncology. 2008 Dec;17(12):1189–1195. PubMed PMID: 18506671; eng.
- Portela MA, Rubiales AS, Centeno C. The use of psychostimulants in cancer patients. Curr Opin Support Palliat Care. 2011 Jun;5(2):164–168. PubMed PMID: 21532350; eng.
- Davis J, Ahlberg FM, Berk M, et al. Emerging pharmacotherapy for cancer patients with cognitive dysfunction. BMC Neurol. 2013 Oct;24(13):153. PubMed PMID: 24156319; PubMed Central PMCID: PMCPMC4015674. eng.
- Wang HL, Gao JP, Han YL, et al. Comparative studies of polydatin and resveratrol on mutual transformation and antioxidative effect in vivo. Phytomedicine. 2015 May 15;22(5):553–559. PubMed PMID: 25981921; eng.
- Xu LQ, Xie YL, Gui SH, et al. Polydatin attenuates d-galactose-induced liver and brain damage through its anti-oxidative, anti-inflammatory and anti-apoptotic effects in mice. Food Funct. 2016 Nov 9;7(11):4545–4555. PubMed PMID: 27714005; eng.
- Ji H, Zhang X, Du Y, et al. Polydatin modulates inflammation by decreasing NF-kappaB activation and oxidative stress by increasing Gli1, Ptch1, SOD1 expression and ameliorates blood-brain barrier permeability for its neuroprotective effect in pMCAO rat brain. Brain Res Bull. 2012 Jan 4;87(1):50–59. PubMed PMID: 22001340; eng.
- Liu H, Zhao S, Zhang Y, et al. Reactive oxygen species-mediated endoplasmic reticulum stress and mitochondrial dysfunction contribute to polydatin-induced apoptosis in human nasopharyngeal carcinoma CNE cells. J Cell Biochem. 2011 Dec;112(12):3695–3703. PubMed PMID: 21815196; eng.
- Ye J, Piao H, Jiang J, et al. Polydatin inhibits mast cell-mediated allergic inflammation by targeting PI3K/Akt, MAPK, NF-kappaB and Nrf2/HO-1 pathways. Sci Rep. 2017 Sep 19;7(1):11895. 10.1038/s41598-017-12252-3. PubMed PMID: 28928455; PubMed Central PMCID: PMCPMC5605538. eng.
- Du QH, Peng C, Zhang H. Polydatin: a review of pharmacology and pharmacokinetics. Pharm Biol. 2013 Nov;51(11):1347–1354. PubMed PMID: 23862567.
- Ince S, Arslan Acaroz D, Neuwirth O, et al. Protective effect of polydatin, a natural precursor of resveratrol, against cisplatin-induced toxicity in rats. Food Chem Toxicol. 2014;72:147–153.
- Huang B, Liu J, Meng T, et al. Polydatin prevents lipopolysaccharide (LPS)-induced Parkinson’s disease via regulation of the AKT/GSK3β-Nrf2/NF-κB signaling axis. Front Immunol. 2018;9:2527. PubMed PMID: 30455692; PubMed Central PMCID: PMCPMC6230593. eng.
- Chen FY, Fang XY, Zhang H. Effect of polydatin on expression of p53 and Notch1 in brain tissue of ischemic cerebrovascular disease. J Biol Regul Homeost Agents. 2018 Jan–Feb;32(1):133–138. PubMed PMID: 29504377; eng.
- Gao Y, Chen T, Lei X, et al. Neuroprotective effects of polydatin against mitochondrial-dependent apoptosis in the rat cerebral cortex following ischemia/reperfusion injury. Mol Med Rep. 2016 Dec;14(6):5481–5488. PubMed PMID: 27840959; PubMed Central PMCID: PMCPMC5355690. eng.
- Li RP, Wang ZZ, Sun MX, et al. Polydatin protects learning and memory impairments in a rat model of vascular dementia. Phytomedicine. 2012 Jun 15;19(8–9):677–681. PubMed PMID: 22483554; eng.
- Zhang Y, Li S, Wang W, et al. Beneficial effects of polydatin on learning and memory in rats with chronic ethanol exposure. Int J Clin Exp Pathol. 2015;8(9):11116–11123. PubMed PMID: 26617831; PubMed Central PMCID: PMCPMC4637646. eng.
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984 May;11(1):47–60. PubMed PMID: 6471907; eng. .
- Fardell JE, Vardy J, Shah JD, et al. Cognitive impairments caused by oxaliplatin and 5-fluorouracil chemotherapy are ameliorated by physical activity. Psychopharmacology (Berl). 2012 Mar;220(1):183–193. PubMed PMID: 21894483; eng.
- Yu H, Shi L, Zhao S, et al. Triptolide attenuates myocardial ischemia/reperfusion injuries in rats by inducing the activation of Nrf2/HO-1 defense pathway. Cardiovasc Toxicol. 2016 Oct;16(4):325–335. 10.1007/s12012-015-9342-y. PubMed PMID: 26391895; eng.
- Ridder DA, Schwaninger M. NF-kappaB signaling in cerebral ischemia. Neuroscience. 2009 Feb 6;158(3):995–1006. PubMed PMID: 18675321; eng.
- Joshi G, Sultana R, Tangpong J, et al. Free radical mediated oxidative stress and toxic side effects in brain induced by the anti cancer drug adriamycin: insight into chemobrain. Free Radic Res. 2005 Jan 01;39(11):1147–1154.
- Meyers CA. How chemotherapy damages the central nervous system. J Biol. 2008;7(4):11. PubMed PMID: 18439322; PubMed Central PMCID: PMCPMC2397491. eng.
- Cummings J, Anderson L, Willmott N, et al. The molecular pharmacology of doxorubicin in vivo. Eur J Cancer (Oxford, England: 1990). 1991;27(5):532–535. PubMed PMID: 1647181; eng.
- Singal PK, Li T, Kumar D, et al. Adriamycin-induced heart failure: mechanism and modulation. Mol Cell Biochem. 2000 Apr;207(1–2):77–86. PubMed PMID: 10888230; eng.
- De Beer EL, Bottone AE, Voest EE. Doxorubicin and mechanical performance of cardiac trabeculae after acute and chronic treatment: a review. Eur J Pharmacol. 2001 Mar 9;415(1):1–11. PubMed PMID: 11245845; eng.
- Aluise CD, Sultana R, Tangpong J, et al. Chemo brain (chemo fog) as a potential side effect of doxorubicin administration: role of cytokine-induced, oxidative/nitrosative stress in cognitive dysfunction. Adv Exp Med Biol. 2010;678:147–156. PubMed PMID: 20738017; eng.
- Trask PC, Esper P, Riba M, et al. Psychiatric side effects of interferon therapy: prevalence, proposed mechanisms, and future directions. J Clin Oncol. 2000 Jun;18(11):2316–2326. PubMed PMID: 10829053; eng.
- Macleod JE, DeLeo JA, Hickey WF, et al. Cancer chemotherapy impairs contextual but not cue-specific fear memory. Behav Brain Res. 2007 Jul 19;181(1):168–172. PubMed PMID: 17509697; PubMed Central PMCID: PMCPMC4012416. eng.
- Raffa RB, Tallarida RJ. Effects on the visual system might contribute to some of the cognitive deficits of cancer chemotherapy-induced ‘chemo-fog’. J Clin Pharm Ther. 2010 Jun;35(3):249–255. PubMed PMID: 20831527; PubMed Central PMCID: PMCPMC3249620. eng.
- Joshi G, Aluise CD, Cole MP, et al. Alterations in brain antioxidant enzymes and redox proteomic identification of oxidized brain proteins induced by the anti-cancer drug adriamycin: implications for oxidative stress-mediated chemobrain. Neuroscience. 2010 Mar 31;166(3):796–807. PubMed PMID: 20096337; PubMed Central PMCID: PMCPMC2852883. eng.
- Sies H. Oxidative stress: from basic research to clinical application. Am J Med. 1991 Sep 30;91(3c):31s–38s. PubMed PMID: 1928209; eng.
- Yoh K, Hirayama A, Ishizaki K, et al. Hyperglycemia induces oxidative and nitrosative stress and increases renal functional impairment in Nrf2-deficient mice. Genes Cells. 2008 Nov;13(11):1159–1170. PubMed PMID: 19090810; eng.
- Ungvari Z, Bailey-Downs L, Sosnowska D, et al. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011 Aug;301(2):H363–72. PubMed PMID: 21602469; PubMed Central PMCID: PMCPMC3154665. eng.
- Trachootham D, Lu W, Ogasawara MA, et al. Redox regulation of cell survival. Antioxid Redox Signal. 2008 Aug;10(8):1343–1374. PubMed PMID: 18522489; PubMed Central PMCID: PMCPMC2932530. eng.
- Aluise CD, Miriyala S, Noel T, et al. 2-Mercaptoethane sulfonate prevents doxorubicin-induced plasma protein oxidation and TNF-alpha release: implications for the reactive oxygen species-mediated mechanisms of chemobrain. Free Radic Biol Med. 2011 Jun 1;50(11):1630–1638. PubMed PMID: 21421044; eng.
- Tangpong J, Cole MP, Sultana R, et al. Adriamycin-mediated nitration of manganese superoxide dismutase in the central nervous system: insight into the mechanism of chemobrain. J Neurochem. 2007 Jan;100(1):191–201. PubMed PMID: 17227439; eng.
- Butterfield DA. The 2013 SFRBM discovery award: selected discoveries from the butterfield laboratory of oxidative stress and its sequela in brain in cognitive disorders exemplified by Alzheimer disease and chemotherapy induced cognitive impairment. Free Radic Biol Med. 2014 Sep;74:157–174. PubMed PMID: 24996204; PubMed Central PMCID: PMCPMC4146642. eng.
- Li R, Li J, Huang Y, et al. Polydatin attenuates diet-induced nonalcoholic steatohepatitis and fibrosis in mice. Int J Biol Sci. 2018;14(11):1411–1425. PubMed PMID: 30262993; PubMed Central PMCID: PMCPMC6158724. eng.
- Tangpong J, Miriyala S, Noel T, et al. Doxorubicin-induced central nervous system toxicity and protection by xanthone derivative of Garcinia mangostana. Neuroscience. 2011 Feb;23(175):292–299. PubMed PMID: 21074598; PubMed Central PMCID: PMCPMC3136166. eng.
- Shah FA, Kury LA, Li T, et al. Polydatin attenuates neuronal loss via reducing neuroinflammation and oxidative stress in rat MCAO models. Front Pharmacol. 2019;10:663. PubMed PMID: 31293416; PubMed Central PMCID: PMCPMC6606791. eng.
- Corpas R, Grinan-Ferre C, Rodriguez-Farre E, et al. Resveratrol induces brain resilience against Alzheimer neurodegeneration through proteostasis enhancement. Mol Neurobiol. 2019 Feb;56(2):1502–1516. 10.1007/s12035-018-1157-y. PubMed PMID: 29948950; eng.
- Dou Z, Rong X, Zhao E, et al. Neuroprotection of resveratrol against focal cerebral ischemia/reperfusion injury in mice through a mechanism targeting gut-brain axis. Cell Mol Neurobiol. 2019 Aug;39(6):883–898. 10.1007/s10571-019-00687-3. PubMed PMID: 31140018; eng.
- Li L, Tan HP, Liu CY, et al. Polydatin prevents the induction of secondary brain injury after traumatic brain injury by protecting neuronal mitochondria. Neural Regen Res. 2019 Sep;14(9):1573–1582. PubMed PMID: 31089056; PubMed Central PMCID: PMCPMC6557083. eng.
- Huang K, Chen C, Hao J, et al. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating Sirt1 to resist AGEs-induced upregulation of fibronectin and transforming growth factor-β1 in rat glomerular mesangial cells. Mol Cell Endocrinol. 2015;399:178–189.
- Amri A, Chaumeil JC, Sfar S, et al. Administration of resveratrol: what formulation solutions to bioavailability limitations? J Control Release. 2012 Mar 10;158(2):182–193. PubMed PMID: 21978644; eng.
- Liu LT, Guo G, Wu M, et al. The progress of the research on cardio-vascular effects and acting mechanism of polydatin. Chin J Integr Med. 2012 Sep;18(9):714–719. PubMed PMID: 22936326; eng.

