ABSTRACT
Purposes
This study investigated the feasibility of adalimumab (ADA) dose reduction and withdrawal strategy in children with stable pediatric non-infectious uveitis (PNIU).
Methods
This open-label prospective pilot trial recruited 18 stable PNIU patients (33 eyes) between two and eighteen years old who were treated with standard doses of ADA (20/40 mg every 2 weeks) plus oral methotrexate. The interval of ADA injection was extended to 4 weeks and followed up for 24 weeks. If the uveitis remained stable, ADA was discontinued and followed up for another 24 weeks. ADA was considered successfully stopped if no relapse occurred during this period. The relapse-free survival rate, best corrected visual acuity (BVCA), anterior chamber cell (ACC), vitritis, macular thickness (MT), and serum ADA levels were evaluated. Approval Number: 2021KYPJ201. ClinicalTrials.gov identifier: NCT05155592.
Results
The relapse-free survival rate was 22.2% (4/18) at 48 weeks. 33.3% (6/18) of patients relapsed when ADA was given every 4 weeks, while 44.5% of patients (8/18) relapsed after ADA was stopped. The four patients successfully withdrawn from ADA were all diagnosed with BD. No statistically significant differences (p > 0.05) were observed in BCVA and MT between baseline and final follow-up. The proportion of ACC and vitritis exhibited an upward trend (p < 0.05) during follow-up. Serum ADA gradually decreased to zero during follow-up in both non-recurrence and recurrence groups.
Conclusions
In PNIU children who reached remission for 6 months, ADA dose reduction and withdrawal were associated with a high risk of inflammation recurrence. Timely adjustment of ADA to the last effective dosage frequency can regain control of the inflammation. Detection of ADA serum levels in patients with recurrence may help find the appropriate interval of ADA use.
Non-infectious uveitis (NIU) is a spectrum of vision-threatening diseases characterized by devastating intraocular inflammation and affects people of all ages.Citation1 Pediatric non-infectious uveitis (PNIU) can be associated with a systemic condition or isolated to the eye, resulting in ocular structure destruction and irreversible visual impairment.Citation2,Citation3 Nowadays, tumor necrosis factor α (TNF-α) inhibitors have shown great effectiveness in the treatment of active NIU.Citation4 Adalimumab (ADA), a fully human TNF-α monoclonal antibody, has proven efficacy in suppressing ocular inflammation and improving visual outcomes in PNIU patients, significantly reducing relapse rate and mean corticosteroid dose.Citation5–7
After achieving sustained remission, tapering or discontinuation of biological agents is often considered, based on a risk-benefit assessment and financial considerations.Citation8 Previous research and the European Alliance of Associations for Rheumatology (EULAR) guidelines have suggested that dose reduction or discontinuation of ADA was feasible in rheumatoid arthritis (RA) patients who have sustained remission for over six months after withdrawal of glucocorticoids.Citation9,Citation10 In addition, studies on stable Behçet’s-disease-associated uveitis (BD-U) patients have shown that extending the dosing interval or reducing the dose of TNF-α monoclonal antibodies is effective, safe, and cost-effective.Citation11,Citation12 To our knowledge, no study has investigated the strategy of ADA dose reduction and withdrawal in stable PNIU. Therefore, determining a robust strategy for ADA reduction and withdrawal in PNIU is urgently needed.
It is worth noting that in patients with RA and psoriatic arthritis (PsA), measurement of ADA trough levels may be helpful during ADA optimization. After three months of standard-dose ADA treatment, serum drug levels may range widely, with 30% to 47% of patients exceeding the optimal treatment window of 5000 to 8000 ng/ml. Measurement of ADA trough levels is helpful in identifying patients suitable for dose optimization. What’s more, monitoring ADA trough level changes during dose reduction is helpful in finding the appropriate dosing interval and achieving the optimal treatment window levels.Citation13–15 However, no research has yet to use ADA trough levels to guide ADA dose reduction and withdrawal in PNIU patients.
Therefore, we designed an open-label prospective pilot study to explore the efficacy of an ADA dose reduction and withdrawal strategy in stable PNIU patients. At the same time, we measured the ADA serum drug level in these patients and observed their changes during ADA dose reduction and withdrawal.
Methods
Study design and patients
We conducted a prospective observational pilot study at Zhongshan Ophthalmic Center, an ophthalmic center in southern China, from February 2021 to February 2022. This study was conducted in compliance with the Helsinki Declaration principles, with approval from the Ethics Committee of Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou, China. All children and their parents consented to participation in the trial and provided written informed consent. ClinicalTrials.gov identifier: NCT05155592.
This study enrolled children between two and eighteen years old diagnosed with non-infectious intermediate uveitis, posterior uveitis, or panuveitis (NIPPU). Patients with stable NIPPU after being treated with standard doses of ADA (20/40 mg every two weeks) plus methotrexate (MTX) were included. Remission was defined as any sign of intraocular inflammation that vanished for at least three months. Stable NIPPU was defined as maintenance of remission for at least 6 months after discontinuation of oral glucocorticoids. Patients with uncontrolled systemic autoimmune disease, ocular microbial infection, potential need for surgery recently, low vision or blindness, and silicone oil or gas tamponade after vitrectomy were excluded.
Sample size estimates
The primary objective of this pilot study was to assess the feasibility of an ADA dose reduction and withdrawal strategy rather than drug efficacy. Moreover, no clinical data exist on the success rate of ADA reduction and discontinuation in PNIU patients: prior data are not available to derive efficacy-based sample size estimates. Therefore, pilot trial sample size estimation (n = 15) was based on medium standardized effect sizes (0.5) for a main trial designed with 90% power and two-sided 5% significance.Citation16
Tapering strategy and study visits
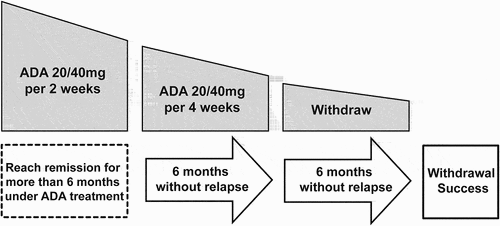
For all included children, based on a shared decision between the patient, their guardian, and the physician, ADA dosing intervals were extended to four weeks, and dosage was maintained as before (20 or 40 mg for patients less than or over 30 kg respectively) (). The original dose and frequency of MTX were maintained. Visits were organized every month and patients were encouraged to contact the outpatient clinic if they experienced any symptoms. If the inflammation stabilized without relapse for six months after drug reduction, the drug would be stopped completely. Then all patients were observed for another six months after drug withdrawal. If there was no relapse during this period, it was considered that the ADA was withdrawn successfully. If inflammation relapsed, it was considered as dose reduction and withdrawal failure and ADA was returned to the initial 20 or 40 mg every two weeks. If the inflammation could not be controlled after increasing the dose to the initial 20 or 40 mg every two weeks, other immunosuppressive drugs such as oral corticosteroids were added. Topical corticosteroids were used based on the severity of anterior chamber inflammation.
Figure 1. An adalimumab dose reduction and withdrawal protocol by extending the dosing interval. (Adalimumab 20 mg for the patient less than 30 Kg and 40 mg for those over 30 Kg).
Demographic characteristics were collected during the initial visit. Individual data including therapeutic management, ophthalmic assessments, systemic disorders, and adverse events (AEs) were evaluated for each patient at every visit throughout the trial. The ophthalmic assessments consisted of best corrected visual acuity (BCVA), anterior chamber cell (ACC) grade, vitritis grade, macular thickness (MT), and ocular complications. BCVA was evaluated using a LogMAR chart for statistical analysis. ACC and vitritis grades were evaluated according to the SUN standard and Nussenblatt scale.Citation17,Citation18 Serum ADA levels were measured via ELISA on venous blood samples collected at baseline and every subsequent four weeks.
Outcome
The primary outcome was the relapse-free rate during follow-up. A relapse was defined as a new flare of uveitis in a patient who was in remission, such as anterior or posterior chamber inflammation, retinal vasculitis, papillitis, or macular edema. The relapse-free rate was defined as the percentage of patients without relapse during follow-up.
Secondary outcomes are the change in BCVA, ACC grade, vitritis grade, and MT as well as changes in ADA serum level throughout the trial. Ocular complications, systemic disorders, and drug-related AEs were also monitored during follow-up.
Statistical analysis
Statistical analysis was conducted using Statistical Package for the Social Sciences (SPSS, Chicago, IL, USA) version 25.0 and GraphPad (GraphPad Software, San Diego, USA) version 9.0. Results were expressed as mean ± standard deviation (SD) for normal data or as median and interquartile range [IQR] [25th, 75th] for non-normally distributed data. The Kolmogorov–Smirnov test or Shapiro–Wilk test was used to assess data normality. Continuous variables were compared using two-tailed Student’s t-test or the Mann-Whitney U-test. The Chi-squared, Fisher’s exact, or McNemar’s tests were applied to compare dichotomous variables, and Wilcoxon's signed-rank test was employed to compare non-normal continuous variables before and after ADA therapy. Statistical significance was considered at a p-value <0.05 in all calculations.
Results
Demographic and clinical features at baseline
This study included a total of 18 Chinese children (33 eyes) aged 8.1 ± 3.0 years old with stable PNIU. Eleven (61.1%) were female and seven (38.9%) were male. Bilateral uveitis presented in 83.3% (15/18) of the patients. Pan-uveitis and intermediate uveitis were diagnosed in 88.9% (16/18) and 11.1% (2/18) of patients, respectively. The etiologies of uveitis included idiopathic uveitis (n = 7, 38.9%), BD-U (n = 7, 38.9%), and juvenile-idiopathic-arthritis-associated uveitis (JIA-U, n = 4, 22.2%). All patients were diagnosed with stable PNIU and treated with ADA plus MTX. At the onset of uveitis in these patients before using ADA plus MTX, the mean decimal BCVA and MT were 0.5 ± 0.2 and 252.2 ± 57.7 μm respectively, while ACC and vitritis existed in 87.9% (29/33) and 90.9% (30/33) of eyes respectively. All were administrated with ADA plus MTX as initial treatment without any other immunosuppressants. The duration of ADA induction period before attaining remission was 5.2 ± 1.5 months. The mean remission duration before dose reduction was 6.5 ± 2.6 months and the follow-up duration of this study was 13.7 ± 2.5 months. Main demographic characteristics and etiologies of uveitis are shown in .
Table 1. Demographic characteristics of children with stable non-infectious uveitis.
Relapse-free rate and treatment characteristics during follow-up
In this cohort, four patients diagnosed with BD remained in remission at the last follow-up (12 months) and were considered to have withdrawn from ADA successfully. The relapse-free rate in our study is 22.2% (4/18) (). 33.3% (6/18) patients had relapsed when ADA was given once a month, while 44.5% patients (8/18) relapsed after ADA was stopped. Among the patients who failed to withdraw from ADA, 50% (7/14) were diagnosed with idiopathic uveitis, 28.6% (4/14) with JIA, and 21.4% (3/14) with BD. At 12 months, the interval of ADA injections was restored to every 2 weeks in six patients (6/18, 33.3%), while ADA injection was maintained every 3 weeks in two patients (2/18, 11.1%) and every 4 weeks in four patients (4/18, 22.2%). ADA was discontinued in two patients (2/18, 11.1%) due to treatment failure. The ADA doses for each patient still using ADA were not changed. As for systemic IMTs, all patients were treated with MTX at baseline with a mean dose of 14.0 ± 2.3 mg/week. At 12 months, three patients (3/18, 16.7%) stopped using oral MTX due to gastrointestinal discomfort and were changed to oral mycophenolate mofetil (MMF) treatment with an average dose of 916.7 ± 144.3 mg/day. The remaining 15 patients (15/18, 83.3%) were still receiving oral MTX (13.2 ± 3.3 mg/week) at 12 months. One patient (1/18, 5.6%) in MTX treatment received additional Cyclosporine A (CsA) 100 mg/day because of poorly controlled ocular inflammation. Three patients (3/18, 16.7%) were still taking low doses of oral glucocorticoids (0.6 ± 0.1 mg/day) in order to control ocular inflammation at 12 months. The treatment characteristics of ADA and systemic treatment are depicted in .
Figure 2. Kaplan-Meier survival curves showing relapse-free survival of all stable NIU patients after ADA dose reduction and withdrawal during the follow-up time. n = patients.
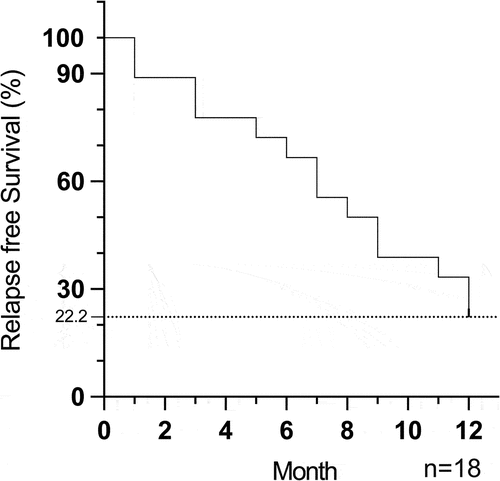
Table 2. Clinical and treatment features of non-infectious uveitis children before and after adalimumab-dose reduction and withdrawal.
Ocular parameter changes after ADA dose reduction
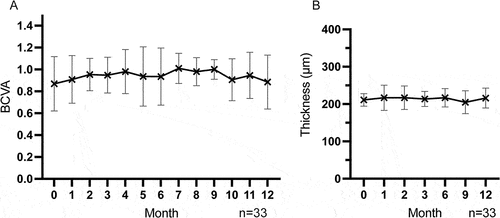
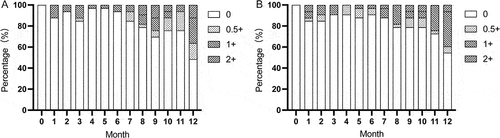
During follow-up, BCVA was maintained at an optimal level without statistical differences (p > 0.05) (). The mean decimal BCVA was 0.9 ± 0.2 at both baseline and last follow-up. During follow-up, no cystoid macular edema (CME) occurred and retinal structure was well maintained. The mean MT at baseline and last follow-up were 211.0 ± 16.8 μm and 216.1 ± 26.5 μm respectively (p > 0.05) (). Inflammatory activities including the presence of ACC and vitritis at any degree were absent at baseline. At 12 months, ACC and vitritis were seen in 51.5% (17/33) and 45.5% (15/33), respectively (). No new ocular complications occurred during follow-up. Changes in ocular parameters and complications after ADA dose reduction are shown in .
ADA serum drug level
As shown in , the mean ADA serum trough level of all patients was 13,854 ± 10,146 ng/ml before ADA tapering, decreasing to 4810 ± 3232 ng/ml at six months (p < 0.05), and then gradually to zero at 12 months (p < 0.05).
Figure 5. (A) All patients’ ADA serum trough level gradually decreased during follow-up, and decreased to 0 at the last visit. (B,C) The ADA serum trough level decreased gradually to 0 over time in both the non-relapse group (B) and relapse group (C) n = patients.
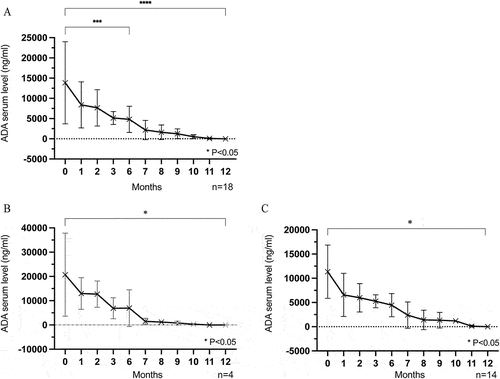
By relapse status, the ADA serum level in the non-relapse and relapse groups gradually decreased from 20,728 ± 17137 ng/ml and 11,355 ± 5499 ng/ml, respectively, at baseline to zero over the follow-up time (, both p < 0.05). This suggests that ADA serum trough levels may not be a decisive factor in ocular inflammation recurrence.
Systemic disorders monitoring and adverse drug events
During the study, no systemic disorders were found. Only three patients experienced an AE of gastrointestinal discomfort due to oral MTX and were promptly switched to MMF. No other AEs occurred during follow-up ().
Discussion
PNIU is one of the leading causes of ocular morbidity and blindness in children.Citation2,Citation3 The treatment of PNIU has become a major challenge for both physicians and parents. Fortunately, several studies have confirmed the efficacy of biological therapy in PNIU treatment. ADA, a fully human TNF-α monoclonal antibody, has demonstrated efficacy in inducing and maintaining PNIU remission, allowing for superior visual outcomes and less corticosteroid usage.Citation5–7 Nevertheless, chronic ADA use may lead to AEs like infections and malignancies.Citation19 What’s more, such treatment is often costly and becomes a serious financial burden on families. Thus, ADA therapy optimization in patients who achieve remission is important in reducing costs and minimizing side effects. According to the EULAR guidelines for RA management, for patients in remission longer than six months, biological agents can be preferentially reduced by dose reduction or dosing interval extension to obtain the maximum economic effect.Citation9 Several studies have shown that the optimization of biological therapy by extending the interval or reducing the dosing in different autoimmune diseases, like RA, Crohn’s disease, psoriasis, or BD-U, is feasible and cost-effective.Citation10–12,Citation20–22 However, detailed optimization strategies for PNIU patients undergoing ADA therapy are lacking. Thus, this study investigated the feasibility of a protocol for ADA dose reduction and withdrawal in patients with PNIU. At the same time, we measured the ADA serum trough level to further explore its possibility in guiding ADA reduction and withdrawal.
In a case series including four adult patients with BD-U in remission, half of them experienced a relapse when anti-TNF treatment was suddenly withdrawn.Citation23 Therefore, another study recommended a dosage regimen by slow but progressive prolongation of the ADA dosing interval up to complete discontinuation so that ADA could be effectively tapered in patients with stable BD-U.Citation12 Thus, the method of gradually lengthening the ADA injection interval in patients with stable PNIU was also adopted in this study, with the ADA injection interval adjusted to four weeks and maintained for 24 weeks. ADA discontinuation was only considered in remission patients during this period. However, in this study, 33.3% of patients relapsed when ADA was extended to once every four weeks, while 44.5% relapsed after ADA discontinuation. Only 22.2% of patients successfully discontinued ADA at 12 months. This may be explained by the difference in immune status between children and adults, which may take a longer time for the use of biological agents to maintain a stable immune status and achieve immune tolerance in children.Citation24 On the other hand, most patients experienced relapse after ADA withdrawal, while almost half maintained a three to four weeks interval of ADA injection at the 12 months. This suggests that a longer period of prolonged-dose interval and inflammation stabilization is needed in PNIU before ADA can be considered for complete discontinuation.
Regarding ophthalmic parameters, no significant differences were observed in BCVA and MT during the follow-up period. Even in patients who relapsed, no significant vision loss or macular edema occurred, with only an increase in the proportion of inflammatory parameters such as ACC and vitritis. After returning to the last effective ADA interval, ocular inflammation was effectively relieved. Fortunately, no new ocular complications occurred during the study. Only three patients had an AE of gastrointestinal discomfort due to oral MTX. This indicates that, under intensive outpatient strategies involving tight control and frequent monitoring of disease activity, the optimization of ADA is relatively safe in PNIU patients who reached remission for a long enough time. Even if uveitis recurs, timely treatment can effectively control the inflammation again without obvious damage to visual function or retinal structure.
In previous studies, the effective therapeutic window of ADA was 5000–8000 ng/ml, with ADA drug levels inversely related to disease activity in different autoimmune diseases.Citation13,Citation25–30 Studies have shown that in the optimization of ADA treatment in RA and PsA patients, detecting ADA serum trough level is helpful in finding patients suitable for dose optimization and attaining appropriate dosing intervals to achieve the optimal window concentration of ADA. However, to the best of our knowledge, no relevant studies have used ADA serum levels to guide ADA dose reduction and withdrawal in children with PNIU. Thus, in this study, ADA trough serum concentrations were measured during follow-up. We found that the ADA trough levels of all patients gradually decreased during follow-up, gradually decreasing to zero at 12 months. During this period, few patients maintained in remission without recurrence even though the serum drug concentration decreased significantly. This may be related to the establishment of immune tolerance in these patients, resulting in the maintenance of uveitis remission after ADA withdrawal. Nevertheless, the majority of patients experienced ocular inflammation relapse, indicating that these patients had not yet achieved immune tolerance and were still dependent on an adequate concentration of serum ADA to control ocular inflammation. Further exploration of alternative biomarkers is required to assess immune tolerance development. However, monitoring ADA serum levels can aid in identifying the appropriate intervals of ADA administration and thereby optimizing the ADA treatment protocol to sustain the best ADA concentration window. Due to a limited follow-up time, it remains to be seen whether patients in the non-relapse group will relapse as a result of prolonged low ADA serum concentration during a longer follow-up period.
This study has some limitations. One is no comparison group to other withdrawal protocols. Another limitation is the small sample size and limited follow-up time. Therefore, larger-scale clinical trials with extended follow-up times are needed to find a safe and feasible ADA dose reduction and withdrawal strategy in PNIU patients.
In conclusion, in stable PNIU patients maintaining remission for six months, reducing or discontinuing ADA treatment is associated with a higher risk of inflammation recurrence. Short-term uveitis relapse has no significant impact on visual function or retinal structure, and timely treatment can effectively control inflammation again. Patients who have not yet achieved immune tolerance rely on adequate serum ADA levels to control ocular inflammation. Comparing the ADA concentrations between relapse and non-inflammation states may provide a basis for individualized determination of ADA dosage intervals.
Authorship
All authors attest that they meet the current ICMJE criteria for authorship.
Ethical approval
This study was conducted in compliance with the principles of the Helsinki declaration, with approval obtained from the Ethics Committee of Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou, China. Approval Number: 2021KYPJ201. All patients and their guardians agreed to the treatment and were given written informed consent. ClinicalTrials.gov identifier: NCT05155592.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Rosenbaum JT, Dick AD. The eyes have it: a rheumatologist’s view of uveitis. Arthritis Rheumatol. 2018;70(10):1533–1543. doi:10.1002/art.40568.
- Edelsten C, Reddy MA, Stanford MR, Graham EM. Visual loss associated with pediatric uveitis in English primary and referral centers. Am J Ophthalmol. 2003;135(5):676–680. doi:10.1016/S0002-9394(02)02148-7.
- Holland GN, Stiehm ER. Special considerations in the evaluation and management of uveitis in children. Am J Ophthalmol. 2003;135(6):867–878. doi:10.1016/S0002-9394(03)00314-3.
- Jaffe GJ, Dick AD, Brézin AP, Nguyen QD, Thorne JE, Kestelyn P, et al. Adalimumab in patients with active noninfectious uveitis. N Engl J Med. 2016;375(10):932–943. doi:10.1056/NEJMoa1509852.
- Díaz-Llopis M, Salom D, Garcia-de-Vicuña C, Cordero-Coma M, Ortega G, Ortego N, et al. Treatment of refractory uveitis with adalimumab: a prospective multicenter study of 131 patients. Ophthalmology. 2012;119(8):1575–1581. doi:10.1016/j.ophtha.2012.02.018.
- Horton S, Jones AP, Guly CM, Hardwick B, Beresford MW, Lee RW, et al. Adalimumab in juvenile idiopathic arthritis–associated uveitis: 5-year follow-up of the bristol participants of the SYCAMORE trial. Am J Ophthalmol. 2019;207:170–174. doi:10.1016/j.ajo.2019.06.007.
- Muñoz-Gallego A, Barral E, Enríquez E, Tejada P, Barceló A, de Inocencio J. Adalimumab for the treatment of refractory noninfectious paediatric uveitis. Int Ophthalmol. 2017;37(3):719–725. doi:10.1007/s10792-016-0293-5.
- Tanaka Y. Stopping tumour necrosis factor-targeted biological DMARDs in rheumatoid arthritis. Rheumatology (Oxford). 2016;55(suppl 2):ii15–ii22. doi:10.1093/rheumatology/kew352.
- Smolen JS, Landewé RBM, Bergstra SA, Kerschbaumer A, Sepriano A, Aletaha D, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82(1):3–18. doi:10.1136/ard-2022-223356.
- van Herwaarden N, van der Maas A, Minten MJ, van den Hoogen FHJ, Kievit W, van Vollenhoven RF, et al. Disease activity guided dose reduction and withdrawal of adalimumab or etanercept compared with usual care in rheumatoid arthritis: open label, randomised controlled, non-inferiority trial. BMJ. 2015;350(apr09 23):h1389. doi:10.1136/bmj.h1389.
- Martín-Varillas JL, Atienza-Mateo B, Calvo-Rio V, Beltrán E, Sánchez-Bursón J, Adán A, et al. Long-term follow-up and optimization of infliximab in refractory uveitis due to Behçet disease: national study of 103 white patients. J Rheumatol. 2021;48(5):741–750. doi:10.3899/jrheum.200300.
- Martín-Varillas JL, Calvo-Río V, Beltrán E, Sánchez-Bursón J, Mesquida M, Adán A, et al. Successful optimization of adalimumab therapy in refractory uveitis due to Behçet’s disease. Ophthalmology. 2018;125(9):1444–1451. doi:10.1016/j.ophtha.2018.02.020.
- Pouw MF, Krieckaert CL, Nurmohamed MT, van der Kleij D, Aarden L, Rispens T, et al. Key findings towards optimising adalimumab treatment: the concentration–effect curve. Ann Rheum Dis. 2015;74(3):513–518. doi:10.1136/annrheumdis-2013-204172.
- van Kuijk AW, de Groot M, Stapel SO, Dijkmans BA, Wolbink GJ, Tak PP. Relationship between the clinical response to adalimumab treatment and serum levels of adalimumab and anti-adalimumab antibodies in patients with psoriatic arthritis. Ann Rheum Dis. 2010;69(3):624–625. doi:10.1136/ard.2009.108787.
- Vogelzang E, Kneepkens E, Nurmohamed M, van Kuijk A, Rispens T, Wolbink G, et al. OP0074 OP0074 a concentration-effect curve of adalimumab in patients with psoriatic arthritis. Ann Rheum Dis. 2014;73(Suppl 2):88–89. doi:10.1136/annrheumdis-2014-eular.1863.
- Whitehead AL, Julious SA, Cooper CL, Campbell MJ. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res. 2016;25(3):1057–1073. doi:10.1177/0962280215588241.
- Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–516.
- Nussenblatt RB, Palestine AG, Chan C-C, Roberge F. Standardizatlon of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92(4):467–471. doi:10.1016/S0161-6420(85)34001-0.
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275–2285. doi:10.1001/jama.295.19.2275.
- Etchevers MJ, Ordás I, Ricart E. Optimizing the use of tumour necrosis factor inhibitors in Crohn’s disease: a practical approach. Drugs. 2010;70(2):109–120. doi:10.2165/11533700-000000000-00000.
- Asahina A, Ohtsuki M, Etoh T, Gu Y, Okun MM, Teixeira HD, et al. Adalimumab treatment optimization for psoriasis: results of a long-term phase 2/3 Japanese study. J Dermatol. 2015;42(11):1042–1052. doi:10.1111/1346-8138.13001.
- Tanaka Y, Smolen JS, Jones H, Szumski A, Marshall L, Emery P. The effect of deep or sustained remission on maintenance of remission after dose reduction or withdrawal of etanercept in patients with rheumatoid arthritis. Arthritis Res Ther. 2019;21(1):164. doi:10.1186/s13075-019-1937-4.
- Adán A, Hernandez V, Ortiz S, Molina JJ, Pelegrin L, Espinosa G, et al. Effects of infliximab in the treatment of refractory posterior uveitis of Behçet’s disease after withdrawal of infusions. Int Ophthalmol. 2010;30(5):577–581. doi:10.1007/s10792-010-9372-1.
- Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282(1821):20143085. doi:10.1098/rspb.2014.3085.
- Jani M, Chinoy H, Warren RB, Griffiths CEM, Plant D, Fu B, et al. Clinical utility of random anti–tumor necrosis factor drug–level testing and measurement of antidrug antibodies on the long-term treatment response in rheumatoid arthritis. Arthritis Rheumatol. 2015;67(8):2011–2019. doi:10.1002/art.39169.
- van Schouwenburg PA, Rispens T, Wolbink GJ. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nat Rev Rheumatol. 2013;9(3):164–172. doi:10.1038/nrrheum.2013.4.
- Mazor Y, Almog R, Kopylov U, Ben Hur D, Blatt A, Dahan A, et al. Adalimumab drug and antibody levels as predictors of clinical and laboratory response in patients with Crohn’s disease. Aliment Pharmacol Ther. 2014;40(6):620–628. doi:10.1111/apt.12869.
- Jani M, Chinoy H, Warren RB, Griffiths CEM, Plant D, Morgan AW, et al. Clinical utility of random anti-tumour necrosis factor drug testing and measurement of anti-drug antibodies on long-term treatment response in rheumatoid arthritis. Lancet. 2015;385(Suppl 1):S48. doi:10.1016/S0140-6736(15)60363-4.
- Cordero-Coma M, Calleja-Antolín S, Garzo-García I, Nuñez-Garnés AM, Álvarez-Castro C, Franco-Benito M, et al. Adalimumab for treatment of noninfectious uveitis: immunogenicity and clinical relevance of measuring serum drug levels and antidrug antibodies. Ophthalmology. 2016;123(12):2618–2625. doi:10.1016/j.ophtha.2016.08.025.
- Leinonen ST, Aalto K, Kotaniemi KM, Kivelä TT. Anti-adalimumab antibodies in juvenile idiopathic arthritis-related uveitis. Clin Exp Rheumatol. 2017;35:1043–1046.