ABSTRACT
Purpose
Post-operative vision impairment is common among patients who have undergone cataract surgery in low-resource settings, impacting quality of clinical outcomes and patient experience. This prospective, multisite, single-armed, pragmatic validation study aimed to assess whether receiving tailored recommendations via the free Better Operative Outcomes Software Tool (BOOST) app improved surgical outcomes, as quantified by post-operative unaided distance visual acuity (UVA) measured 1–3 days after surgery.
Methods
During the baseline data collection round, surgeons in low and middle-income countries recorded clinical characteristics of 60 consecutive cataract cases in BOOST. Additional data on the causes of poor outcomes from 20 consecutive cases with post-operative UVA of <6/60 (4–12 weeks post-surgery) were entered to automatically generate tailored recommendations for improvement, before 60 additional consecutive cases were recorded during the follow-up study round. Average UVA was compared between cases recorded in the baseline study round and those recorded during follow-up.
Results
Among 4,233 cataract surgeries performed by 41 surgeons in 18 countries, only 2,002 (47.3%) had post-operative UVA 6/12 or better. Among the 14 surgeons (34.1%) who completed both rounds of the study (1,680 cases total), there was no clinically significant improvement in post-operative average UVA (logMAR units ±SD) between baseline (0.50 ± 0.37) and follow-up (0.47 ± 0.36) rounds (mean improvement 0.03, p = 0.486).
Conclusions
Receiving BOOST-generated recommendations did not result in improved UVA beyond what could be expected from prospective monitoring of surgical outcomes alone. Additional research is required to assess whether targeted support to implement changes could potentiate the uptake of app-generated recommendations and improve outcomes.
Introduction
Despite the substantial improvement in quality of life that can be achieved through cataract surgery, a large proportion of people in low-resource settings continue to have some level of vision impairment post-operatively.Citation1–9 Cataract is the leading cause of blindness and the second leading cause of moderate and severe vision impairment globally.Citation10,Citation11 It affects over 100 million people, 17 million of whom are blind, and 90% of people with vision impairment due to cataract live in low and middle income countries (LMICs).Citation8,Citation10 Cataract surgery is a relatively simple and cost-effective intervention, which can restore sight, improve quality of life and wellbeing, and reduce poverty.Citation8,Citation12–17 Tools that facilitate improvement in clinical outcomes of cataract surgery have been shown to maximise the benefits of cataract surgery for individuals and communities in LMICs and improve the overall quality of surgical services in these settings.Citation8,Citation18,Citation19
Approximately 25 million people are estimated to undergo cataract surgery globally each year and outcomes often differ due to a range of social, economic, and health system factors.Citation8,Citation20,Citation21 LMICs generally have poorer clinical and patient-reported outcomes and more research is required to investigate interventions that improve these outcomes.Citation8,Citation9,Citation22 Effective cataract surgical coverage, a measure of both population coverage and quality, has been approved as a progress indicator of Universal Health Coverage.Citation4,Citation12,Citation23 Thus, easy-to-use tools that support real-time collection of surgical outcome data and monitor quality improvement of cataract surgeries in low-resource settings are urgently needed.Citation8,Citation24
The Better Operative Outcomes Software Tool (BOOST) is a freely-downloadable app which has been validated for anonymous clinical auditing and benchmarking of visual outcomes of cataract surgery.Citation3,Citation24 A second component of the app captures the characteristics of cases with poor visual outcomes and provides tailored recommendations to encourage surgeons to adjust practice and optimise future outcomes.Citation25
Although the advantages of clinical audits and benchmarking of surgical results are well-established,Citation18,Citation26–31 the benefit of tailored recommendations for improved surgical performance through BOOST is yet to be investigated, as are evidence-based quality improvement interventions in LMICs more broadly.Citation32,Citation33 Therefore, the primary aim of this study was to evaluate BOOST as a quality improvement tool for cataract surgery in LMICs by comparing post-operative unaided distance visual acuity before and after receiving the tailored recommendations. In addition, we aimed to investigate associations between visual outcomes and patient and surgeon characteristics.
Materials and methods
The current study followed a prospective, multisite, single-armed, pragmatic before-and-after design. Ethical approval was granted through the Joint Research Ethics Committee of the School of Medicine, Dentistry and Biomedical Sciences at Queen’s University, Belfast (ref: 18.48/v2), and was conducted in accordance with the tenets of the Declaration of Helsinki. Participating surgeons and service managers provided written consent after the nature of the study was explained.
Recruitment and eligibility
Ophthalmologists, general physicians, and non-physician cataract surgeons were recruited between November 2018 and May 2020 from cataract surgery centres having existing collaborations with The Fred Hollows Foundation, Orbis International, SightSavers, Aravind Eye Hospitals, and Seva Foundation.Citation34 Additional centres were recruited through online promotion via the Community Eye Health Journal and 2018 World Ophthalmology Congress, and direct referrals from co-authors. Currently practicing cataract surgeons in LMICs who performed more than six cataract surgeries per week and had been operating for at least one year were eligible for inclusion in the study. Surgeons required the support of their surgical centre managers to take part in the study, but surgeon participation was entirely voluntary within each centre.
At commencement, surgeons completed a questionnaire providing information on location, qualifications, experience, surgical volume and case mix, and the use of current cataract outcome auditing tools. Surgeons who reported being unlikely to consistently operate during the study period or to meet the target number of 140 surgeries were not enrolled into the study.
BOOST procedures
BOOST is a free app available for use on desktop personal computers and Android phones and tablets in English, French, Spanish, Russian, Simplified Chinese, Vietnamese, and Bahasa Indonesian.Citation24 No party benefited financially from BOOST app downloads. Users can remain anonymous and de-identified data can be captured while on- or offline and uploaded to the study server on connection to the internet. All accounts are password protected and access across multiple devices is possible.
Participants were trained to enter the data via instructional manuals and online resources available on the BOOST website.Citation35 Technical support was provided via the project manager (EM), with email reminders to encourage progression through each study round. The app has two phases (Phase I and II) as described below. This study included two rounds of Phase I data collection (baseline and follow-up).
Phase I – baseline
In the baseline round of Phase I data collection, surgeons were asked to use BOOST to record patient age and gender, operated eye, pre-operative best corrected visual acuity, surgical technique (extracapsular cataract extraction [ECCE], intracapsular cataract extraction [ICCE], phacoemulsification, small incision cataract surgery [SICS]), implantation of an intra-ocular lens (IOL), and unaided post-operative distance visual acuity (UVA) 1–3 days post-surgery (an indicator of final UVA in settings where follow-up is poor)Citation3,Citation31,Citation36 for 60 consecutive surgical patients aged 30 years and above with no known ocular co-morbidities (such as glaucoma or diabetic retinopathy). Prospective data collection started on 1 November 2018 but retrospective cases performed on or after 1 January 2018 were eligible for inclusion. UVA was measured under usual clinical conditions with no restrictions on chart type or assessor qualifications and was entered into the BOOST app using each surgeon’s preferred metric (feet, meters, logMAR, or decimal).
After entering 60 consecutive Phase I cases, a summary of patient and surgical characteristics was generated (Supplemental Figures S1 and S2) and participants were prompted to move to Phase II data collection.
Phase II
The characteristics of 20 cases with UVA of <6/60 at four to 12 weeks post-surgery were recorded in the Phase II tab of the app. These cases could be entered into the app prior to or after completing the baseline round of Phase I. Retrospective cases, cases already entered in the baseline round of Phase I, and cases with ocular co-morbidities discovered after surgery were eligible. Surgeons were asked to select one of three reasons for the poor outcome (Case selection [i.e., ocular co-morbidity], Surgical complication, or need for Optical correction [i.e., residual refractive error]). After completion of the baseline round of Phase I and Phase II, tailored recommendations for improving future outcomes were provided according to the most common cause of poor UVA for each surgeon (example recommendations in Supplemental Table S1).
Phase I – follow-up
Upon completing Phase II, surgeons were asked to collect data on an additional 60 consecutive cases in the Phase I tab of the app.
Sample size and statistical methods
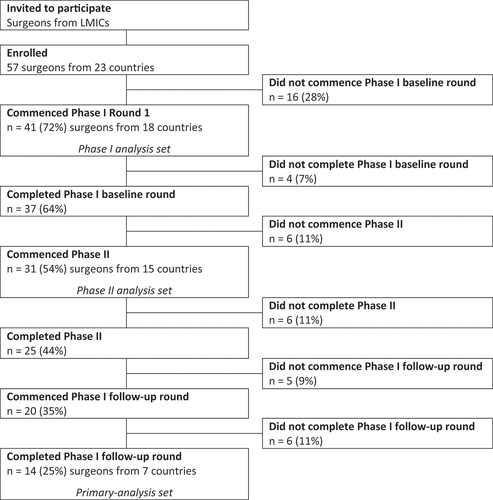
A target sample size of 75 surgeons was initially selected to achieve 80% power to detect a 20% difference in UVA between Phase I baseline and follow-up rounds with a 5% type I error rate. Recruitment ended prior to the target being reached due to COVID-19 related delays and limited resources. The primary analysis set included surgeons who completed all study rounds (i.e., a per-protocol set). All other analysis of Phase I data included data from all surgeons who commenced Phase I.
UVA was converted to logMAR for analyses (Light perception = 2.30, No perception of light = 3.00). Associations between surgeon and patient characteristics and post-operative UVA in Phase I were assessed using univariable linear regression. The proportion of Phase I patients with each level of vision impairment (mild, moderate, severe, and blindness as classified in the International Classification of Diseases, 11th Revision)Citation37 was estimated with logit-transformed 95% CIs.
To assess the impact of receiving BOOST-generated recommendations, mean post-operative UVA was compared between Phase I baseline and follow-up rounds among the primary-analysis set using linear regression.Citation38 This was repeated as a sensitivity analysis among all surgeons/Phase I cases with non-missing data by adjusting for pre-operative best corrected visual acuity, patient age, region, national World Bank income status (low, lower-middle, or upper-middle), surgeon qualification and experience, surgical technique, and IOL implantation. Each covariate was chosen a priori as a potential predictor of the outcome.
Each 95% CI for estimates of proportions and mean differences was calculated using robust standard errors to account for intra-surgeon correlation.
Results
Surgeons
All 57 surgeons from 23 LMICs who responded to the invitation met enrolment requirements. Among these, 16 (28.1%) did not commence the baseline round of Phase I, leaving 41 (71.9%) surgeons from 18 countries for inclusion (Supplemental Table S2). Most surgeons who commenced data collection were operating in Asia and Oceania (n = 25, 61.0%), were male (n = 29, 70.7%), and had trained as ophthalmologists (n = 28, 68.3%, ). The majority were already recording surgical outcomes at the time of enrolment (n = 25, 61.0%); two via BOOST, ten via spreadsheets, and the remainder using paper, electronic medical records, or other platforms. Fourteen surgeons (34.1%) from seven countries completed all data collection rounds ().
Table 1. Baseline characteristics of surgeons who commenced Phase I of the Better Operative Outcomes Software Tool study (n = 41).
Phase I surgical cases
Phase I surgical cases were recorded between January 2018 and May 2020 by 41 surgeons (n = 4,233 cases total, between 1 and 202 cases per surgeon). Patients had a mean (±SD) age of 64 ± 11.1 years (range 30–107) and over half were female (n = 2,309, 54.5%, ). Almost half (n = 2,013, 47.6%) were considered to have severe vision impairment or blindness (corrected visual acuity < 6/60) prior to surgery. The majority of patients underwent SICS (n = 2,562, 60.5%) and over one third underwent phacoemulsification (n = 1,491, 35.2%). Most patients had IOL implantation (n = 4,200, 99.2%) regardless of surgeon qualifications (98.2% non-physicians, 100% general physicians, 99.4% ophthalmologists).
Table 2. Characteristics of Phase 1 (baseline and follow-up rounds) surgical cases performed by surgeons who commenced phase I of the better operative outcomes software tool study. (n = 41 surgeons).
The mean logMAR VA among all Phase I cases improved from 1.24 ± 0.70 preoperatively (≈6/105 corrected) to 0.48 ± 0.36 post-operatively (6/18 unaided, mean improvement 0.76 ± 0.69). Post-operative UVA was worse among older patients (p < 0.001) and for patients in Africa (p = 0.015, ). Compared to patients undergoing SICS, mean UVA was better for those who underwent phacoemulsification (p = 0.010) but worse for those with ECCE (p = 0.005).
Table 3. Unaided post-operative distance visual acuity in phase I of the better operative outcomes software tool study according to surgeon and patient characteristics (n = 41 surgeons, 4233 cases).
More than half (n = 2,231, 52.7%) of patients had some level of vision impairment post-operatively (<6/12, ). Roughly 1 in 17 patients (n = 248, 5.9%) had severe vision impairment or blindness (<6/60) after surgery. Only 18 surgeons (43.9%) met the previous WHO target of ≥ 80% of cases with UVA ≥ 6/18, while only 25 surgeons (61.0%) met the target of ≤ 5% of cases with UVA < 6/60.Citation39
Phase II surgical cases
A total of 574 Phase II cases were recorded by 31 surgeons between December 2018 and March 2020. Mean patient age was 66.8 ± 11.2 years and 51.7% of patients were female (n = 297, Supplemental Table S3). The cause of poor vision was accredited to case selection (i.e., ocular co-morbidity) among 39.7% (n = 228), surgical complications in 30.3% (n = 174), and need for optical correction (i.e., residual refractive error) among 30.0% (n = 172) of patients.
Primary analysis
Among the 14 surgeons who completed both Phase I rounds, the post-operative UVA in the follow-up round ranged from an average of 0.25 better to 0.36 logMAR units worse than the baseline round (Supplemental Figure S3). The average post-operative UVA for all surgeons in the primary analysis was 0.50 ± .037 (≈6/18-) in the baseline round and 0.47 ± 0.36 logMAR units (≈6/18+, SD 0.36) in the follow-up round, with a mean difference between rounds of −0.03 (95% CI −0.11 to 0.06, p = 0.486). Similar results were observed in the sensitivity analysis which included all surgeons who commenced Phase I (adjusted mean difference −0.02 logMAR units, 95% CI −0.08 to 0.04, p = 0545, Supplemental Table S4).
There was a slightly higher proportion of cases without post-operative vision impairment (UVA ≥6/12) in the follow-up round (n = 435/840, 51.8%) compared to baseline (n = 395/840, 47.0%, ) among all surgeons in the primary analysis set. This ranged from 16.2% fewer to 10.8% more cases without post-operative vision impairment in the follow-up round for individual surgeons ().
Figure 2. Proportion of cases in each Phase I round with (a) no vision impairment (unaided visual acuity 6/12 or better) and (b) severe vision impairment/blindness (unaided visual acuity worse than 6/60) at 1–3 days after surgery. Circles represent baseline round data from each surgeon who commenced Phase I of the study (n = 41). Squares represent the follow-up round for surgeons who entered follow-up round data (n = 20). Solid shapes represent surgeons who were included in the primary analysis (n = 14). Surgeons ordered separately in each plot according to percentage of cases for each vision impairment category in the baseline round.
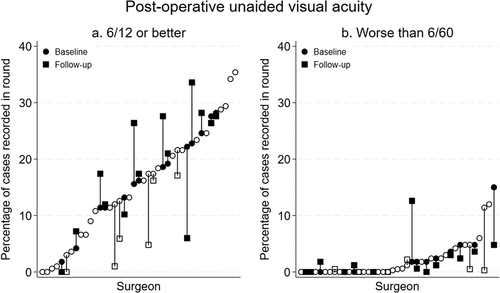
Table 4. Proportion of Phase I cases with post-operative vision impairment in the Better Operative Outcomes Software Tool study.
The percentage of cases with severe post-operative vision impairment or blindness (UVA <6/60) decreased slightly in the follow-up round (n = 52, 6.2%) from the baseline round (n = 60, 7.1%). This ranged from 10.2% fewer to 10.8% more in the follow-up round compared to the baseline round for individual surgeons ().
Similar proportions were observed among surgeons excluded from the primary analysis ().
Discussion
In this large, international quality improvement study, we found post-operative vision impairment in a high proportion of individuals following cataract surgery in LMICs. There was no clinically meaningful improvement in post-operative visual acuity after receiving tailored recommendations for improved surgical performance from the BOOST app. Additional support, financial or otherwise, may be needed to promote concrete action on these suggestions. Similar proportions of poor outcomes were attributable to pre-existing ocular comorbidity, need for optical correction, and surgical complications.
Implications of findings
BOOST may benefit surgeons or surgical centres with no existing cataract surgical outcome monitoring systems.Citation25,Citation32 In contrast to programs designed for cataract surgeons in high-income nations who have an interest in refining already-acceptable outcomes, Phase II of the BOOST app aims to reduce severe vision impairment through provision of tailored recommendations to improve practice.Citation40
The presence of pre-existing conditions and unrecognised ocular co-morbidities, such as retinal disease or glaucoma, was a common cause of poor outcomes in this study. This is consistent with existing calls for comprehensive screening to identify and ensure patients with existing co-morbidities are aware of potential risks and benefits of cataract surgery,Citation41,Citation42 and reinforces the need for comprehensive, routine pre-operative examination of both eyes and detailed history taking.Citation43,Citation44
Surgeons identified residual refractive error as the cause of almost a third of poor outcomes. Hence, access to resources such as pre-operative ocular biometry and stockpiles of IOLs in a range of powers may reduce the number of patients with severe post-operative vision impairment. Another third of poor outcomes was attributable to surgical complications. Additional support is required to address underlying causes of inconsistent surgical protocols, failure to recognise and manage complications, lack of training, inadequate equipment, and lack of regular review mechanisms.Citation45
Insufficient time, resources, incentives, and motivation may have limited participants’ ability to act on the tailored recommendations, thereby limiting potential to improve surgical outcomes within the study timeframe.
Strengths and limitations
Strengths include the pragmatic nature of the study, which reflects real-world conditions and real-time monitoring by surgeons. The study included a large number of surgical cases across a variety of facilities, geographic areas, skill levels, and types of surgery, thus representing a wide range of potential users within LMICs.
However, both baseline and follow-up involved prospective monitoring of outcomes in BOOST. It is possible that differences observed between baseline and follow-up may have occurred naturally over time in the absence of the Phase II intervention. Data were not collected from surgeons who do not engage in auditing or from those who use another platform to monitor outcomes. Several surgeons were regularly recording their surgical outcomes prior to the study. Thus, we are unable to comment on the efficacy of clinical auditing and benchmarking alone.
Participation was based on a sample of convenience and may not be representative of the wider population. Surgeons with a low volume of cases were not enrolled in the study so we are unable to comment on whether using BOOST would benefit this group. In addition, surgeons were required to record 20 cases with severe post-operative vision impairment in Phase II, possibly precluding those with lower numbers of very poor outcomes. This lack of representativeness was amplified due to significant attrition; in the primary analysis no low-income countries and only one participant from Africa were included, and a higher percentage of cases were performed via phacoemulsification.
A high proportion of enrolled surgeons did not complete the study due to inability to meet target number of surgeries and impacts of the COVID-19 pandemic on routine cataract surgical procedures, potentially inducing selection bias and lowering statistical power.
Details of ocular biometry parameters, IOL power, target and observed refraction were not available as BOOST was specifically designed for data collection to be quickly and easily implemented. Thus, in-depth analyses of refractive outcomes and the proportion of patients who obtained spectacle correction were not possible. Finally, data was not collected on whether BOOST-generated recommendations were acted upon or perceived barriers to their implementation.
Interpretation within the context of the wider literature
Since 1998, WHO has defined a “good” surgical outcome for cataract surgery as UVA of 6/18 or better using available correction, and currently recommends that at least 80% of patients should achieve this level of vision.Citation39,Citation46 In 2020, the threshold for a good surgical outcome was increased to UVA ≥ 6/12.Citation8,Citation12 While reporting of cataract surgical outcomes varies widely across surgical centres internationally, many countries where data are available do not achieve this target.Citation4,Citation32 Published estimates of the level of post-operative vision impairment as currently defined in low-resource settings are scarce. The proportion of cases in the current study with UVA of 6/18 or better at 1–3 days was higher than that in several studies conducted in low-resource settings reported over the last decade.Citation3,Citation4,Citation24 This may be due to higher degrees of surgeon training and experience in the current cohort, coupled with the increasing number of surgeons practicing phacoemulsification and the declining number of ECCEs performed.Citation3
Future research
Exploration of BOOST user experience is underway to investigate how the platform meets the needs of surgeons and clinic staff. Further research is required into surgeon adherence with BOOST recommendations and the barriers that prevent recommendations being implemented. Research into the impact of providing resources to support addressing the main causes of poor outcomes identified in Phase II of the BOOST app is ongoing in a new study in Ethiopia and Zambia. Given almost one third of cases with poor outcomes recorded in Phase II of the app were attributed to the need for refractive correction, an understanding of which interventions minimise residual refractive error could lead to meaningful improvements in visual outcomes.
Conclusions
Although there was insufficient evidence that receiving BOOST-generated recommendations improved visual outcomes in this study, further investigation into its usefulness is warranted given the high level of attrition and lack of resourcing to implement the recommendations. The global monitoring paradigm for cataract quality has shifted, with effective cataract surgical coverage now endorsed by WHO as a global progress indicator for Universal Health Coverage.Citation12 Simple tools are needed to ensure surgeons can adequately monitor quality of surgical outcomes in low resource settings and identify shortcomings where resources can be invested to improve performance.
Acknowledgments
The BOOST consortia (The Fred Hollows Foundation, Sightsavers International, Aravind Eye Care Systems, Orbis International, International Agency for the Prevention of Blindness, International Council of Ophthalmology) provided in-kind support and had a role in the design and implementation of the study. Feedback on the study design and ethics approval was provided by Queen’s University Belfast.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Polack S. Restoring sight: how cataract surgery improves the lives of older adults. Commu Eye Health. 2008;21:24–25.
- Kuper H, Polack S, Mathenge W, et al. Does cataract surgery alleviate poverty? Evidence from a multi-centre intervention study conducted in Kenya, the Philippines and Bangladesh. PLOS ONE. 2010;5(11):e15431. doi:10.1371/journal.pone.0015431.
- Congdon N, Yan X, Lansingh V, et al. Assessment of cataract surgical outcomes in settings where follow-up is poor: PRECOG, a multicentre observational study. Lancet Glob Health. 2013;1(1):e37–45. doi:10.1016/S2214-109X(13)70003-2.
- Ramke J, Gilbert CE, Lee AC, et al. Effective cataract surgical coverage: an indicator for measuring quality-of-care in the context of universal health coverage. PLOS ONE. 2017;12(3):e0172342. doi:10.1371/journal.pone.0172342.
- Baltussen RMPM, Sylla M, Mariotti SP. Cost-effectiveness analysis of cataract surgery: a global and regional analysis. Bull World Health Organ. 2004;82:338–345.
- Wadud Z, Kuper H, Polack S, et al. Rapid assessment of avoidable blindness and needs assessment of cataract surgical services in Satkhira district, Bangladesh. Br J Ophthalmol. 2006;90(10):1225–1229. doi:10.1136/bjo.2006.101287.
- Essue BM, Li Q, Hackett ML, et al. A multicenter prospective cohort study of quality of life and economic outcomes after cataract surgery in Vietnam: the VISIONARY study. Ophthalmology. 2014;121(11):2138–2146. doi:10.1016/j.ophtha.2014.05.014.
- Burton MJ, Ramke J, Marques AP, et al. The lancet global health commission on global eye health: vision beyond 2020. Lancet Glob Health. 2021;9(4):e489–e551. doi:10.1016/S2214-109X(20)30488-5.
- Han X, Zhang J, Liu Z, et al. Real-world visual outcomes of cataract surgery based on population-based studies: a systematic review. Br J Ophthalmol. 2023;107(8):1056–1065. doi:10.1136/bjophthalmol-2021-320997.
- Steinmetz JD, Bourne RRA, Briant PS, et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the right to sight: an analysis for the global burden of disease study. Lancet Glob Health. 2021;9(2):e144–e160. doi:10.1016/S2214-109X(20)30489-7.
- Bourne R, Steinmetz JD, Flaxman S, et al. Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the global burden of disease study. Lancet Glob Health. 2021;9(2):e130–e143. doi:10.1016/S2214-109X(20)30425-3.
- World Health Organization. Discussion Paper: Proposed Global Targets for 2030 on Integrated People-Centred Eye Care. Geneva: World Health Organization; 2020.
- International Agency for the Prevention of Blindness. Vision Atlas Evidence Series: Universal Eye Health: A Global Action Plan 2014–2019 Indicators. London: The International Agency for the Prevention of Blindness; 2020.
- Gupta VB, Rajagopala M, Ravishankar B. Etiopathogenesis of cataract: an appraisal. Indian J Ophthalmol. 2014;62(2):103–110. doi:10.4103/0301-4738.121141.
- Lamoureux EL, Fenwick E, Pesudovs K, Tan D. The impact of cataract surgery on quality of life. Curr Opin Ophthalmol. 2011;22(1):19–27. doi:10.1097/ICU.0b013e3283414284.
- Frampton G, Harris P, Cooper K, Lotery A, Shepherd J. The clinical effectiveness and cost-effectiveness of second-eye cataract surgery: a systematic review and economic evaluation. Health Technol Assess. 2014;18(68):1–205, v–vi. doi:10.3310/hta18680.
- Yong G-Y, Mohamed-Noor J, Salowi MA, Adnan TH, Zahari M, Grzybowski A. Risk factors affecting cataract surgery outcome: the Malaysian cataract surgery registry. PLOS ONE. 2022;17(9):e0274939. doi:10.1371/journal.pone.0274939.
- Polack S, Kuper H. Measuring the impact of cataract services in the community. Commun Eye Health. 2014;27:15–15.
- Congdon N, Dodson S, Chan VF, Mathenge W, Moo E. Improving the practice of cataract surgical outcome measurement. Commun Eye Health. 2019;31:91–92.
- Wang W, Yan W, Fotis K, et al. Cataract surgical rate and socioeconomics: a global study. Invest Ophthalmol Vis Sci. 2017;57(14):5872–5881. doi:10.1167/iovs.16-19894.
- Yan W, Wang W, van Wijngaarden P, Mueller A, He M. Longitudinal changes in global cataract surgery rate inequality and associations with socioeconomic indices. Clin Exp Ophthalmol. 2019;47(4):453–460. doi:10.1111/ceo.13430.
- McCormick I, Butcher R, Evans JR, et al. Effective cataract surgical coverage in adults aged 50 years and older: estimates from population-based surveys in 55 countries. Lancet Glob Health. 2022;10(12):e1744–e1753. doi:10.1016/S2214-109X(22)00419-3.
- World Health Organization. Integrated people-centred eye care, including preventable vision impairment and blindness. 2021. Seventy-Fourth World Health Assembly Geneva.
- Congdon N, Suburaman G-B, Ravilla T, et al. Transforming research results into useful tools for global health: BOOST. Lancet Glob Health. 2016;4(2):e96. doi:10.1016/S2214-109X(15)00267-3.
- Lecumberri M, Moser CL, Loscos-Arenas J. Evaluation of the better operative outcome software tool to predict cataract surgical outcome in the early postoperative follow-up. BMC Ophthalmol. 2023;23(1):317. doi:10.1186/s12886-023-03058-1.
- Limburg H. Monitoring cataract surgical outcomes: methods and tools. Commu Eye Health. 2002;15:51–53.
- Kruk ME, Gage AD, Arsenault C, et al. High-quality health systems in the sustainable development goals era: time for a revolution. Lancet Glob Health. 2018;6(11):e1196–e1252. doi:10.1016/S2214-109X(18)30386-3.
- Lindfield R. The value of clinical audit to improve cataract quality. Commu Eye Health. 2014;27:55–55.
- Salowi MA, Choong YF, Goh PP, Ismail M, Lim TO. CUSUM: a dynamic tool for monitoring competency in cataract surgery performance. Br J Ophthalmol. 2010;94(4):445–449. doi:10.1136/bjo.2009.163063.
- Yorston D, Gichuhi S, Wood M, Foster A. Does prospective monitoring improve cataract surgery outcomes in Africa? Br J Ophthalmol. 2002;86(5):543–547. doi:10.1136/bjo.86.5.543.
- Limburg H, Foster A, Gilbert C, Johnson GJ, Kyndt M. Routine monitoring of visual outcome of cataract surgery. Part 1: development of an instrument. Br J Ophthalmol. 2005;89(1):45–49. doi:10.1136/bjo.2004.045351.
- Ramke J, Zwi AB, Silva JC, et al. Evidence for national universal eye health plans. Bull World Health Organ. 2018;96(10):695–704. doi:10.2471/BLT.18.213686.
- Yoshizaki M, Ramke J, Zhang JH, et al. How can we improve the quality of cataract services for all? A global scoping review. Clin Exp Ophthalmol. 2021;49(7):672–685. doi:10.1111/ceo.13976.
- World Health Organization. Core competencies for the eye health workforce in the WHO African region. Vol Geneva. 2019; 9290234180.
- BOOST Consortia. BOOST better operative outcomes software tool. https://boostcataract.org/.
- Bani A, Wang D, Congdon N. Early assessment of visual acuity after cataract surgery in rural Indonesia. Clin Exp Ophthalmol. 2012;40(2):155–161. doi:10.1111/j.1442-9071.2011.02667.x.
- World Health Organization. International Classification of Diseases, Eleventh Revision (ICD-11). World Health Organization; 2018. https://icd.who.int/browse11/l-m/en.
- Hanley JA, Negassa A, Edwardes M, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157(4):364–375. doi:10.1093/aje/kwf215.
- World Health Organization. Informal Consultation on Analysis of Blindness Prevention Outcomes. Geneva: World Health Organization; 1998.
- Sheard R. Optimising biometry for best outcomes in cataract surgery. Eye (Lond). 2014;28(2):118–125. doi:10.1038/eye.2013.248.
- Khanna RC, Rathi VM, Guizie E, et al. Factors associated with visual outcomes after cataract surgery: a cross-sectional or retrospective study in Liberia. PLOS ONE. 2020;15(5):e0233118. doi:10.1371/journal.pone.0233118.
- Cheng KKW, Anderson MJ, Velissaris S, et al. Cataract risk stratification and prioritisation protocol in the COVID-19 era. BMC Health Serv Res. 2021;21(1):153.
- Keay L, Lindsley K, Tielsch J, Katz J, Schein O. Routine preoperative medical testing for cataract surgery. Cochrane Database Syst Rev. 2019;1(1):CD007293. doi:10.1002/14651858.CD007293.pub4.
- Lindfield R, Kuper H, Polack S, et al. Outcome of cataract surgery at one year in Kenya, the Philippines and Bangladesh. Br J Ophthalmol. 2009;93(7):875–880. doi:10.1136/bjo.2008.152744.
- Gupta S, Haripriya A, Ravindran RD, Ravilla T. Differences between male and female residents in case volumes and learning in cataract surgery. J Surg Educ. 2021;78(4):1366–1375. doi:10.1016/j.jsurg.2020.12.017.
- World Health Organization. World Report on Vision. 2019.