Abstract
Women with polycystic ovary syndrome (PCOS) have unfavorable metabolic profiles. Their offspring may be affected by such risks. The objective of the current study was to disclose associations between preconception health of these women and health of their offspring. 74 women diagnosed with PCOS according to the Rotterdam criteria were screened systematically before conception. Cardiovascular health of their offspring was assessed at 2.5–4 (n = 42) or at 6–8 years of age (n = 32). Multivariate linear regression analysis was performed with adjustments for potential confounders. In the primary analyses the association between preconception Body Mass index (BMI) and offspring BMI was evaluated. Secondly associations between preconception blood pressure, androgens, insulin-resistance (HOMA-IR), and LDL-cholesterol in women with PCOS and BMI and blood pressure of offspring were assessed. Results show that preconception BMI of women with PCOS was positively associated with sex- and age-adjusted BMI of their offspring at 6–8 years of age (β = 0.55 (95% CI: 0.12 to 0.97), p = .012). No other significant associations were found. In conclusion, our data suggest that preconception BMI in PCOS is significantly associated with offspring BMI at 6–8 year of age. If this suggestion could be confirmed this may provide an opportunity for improving the future health of these children.
摘要
多囊卵巢综合征(PCOS)的代谢状况不佳。他们的后代可能会受到这种风险的影响。本研究的目的是揭示这些女性孕前的健康水平和其后代健康之间的关系。74名根据鹿特丹标准诊断为PCOS的女性在孕前进行了系统筛查。在他们的后代2.5-4岁(n = 42)或6-8岁(n = 32)岁时评估其心血管健康。多元线性回归分析用于校正潜在的混杂因素。初步分析中评估了孕前体重指数(BMI)与后代BMI间的关系。其次评估了PCOS患者孕前血压、雄激素、胰岛素抵抗(HOMA-IR)、LDL胆固醇与后代BMI和血压之间的关系。结果显示, PCOS女性孕前BMI与其6-8岁的后代性别和年龄校正的BMI呈正相关(β = 0.55 (95% CI: 0.12 to 0.97), p=.012)。未发现其他的显著相关性。综上, 我们的数据表明PCOS患者的孕前BMI与其6-8岁后代的BMI显著相关。如果该结果得到确认, 可能会为改善这些儿童未来的健康提供可能。
The Chinese abstracts are translated by Prof. Dr. Xiangyan Ruan and her team: Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing 100026, China.
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in female reproductive age with a reported incidence between 6 and 15% [Citation1,Citation2]. The exact etiology of PCOS remains unknown, but the interplay between genetic, intrauterine, and environmental factors is suggested to play a key role in the development of PCOS [Citation3]. According to the 2003 Rotterdam consensus criteria, at least two out of the following three features must be present in PCOS: oligo- or anovulation, clinical or biochemical hyperandrogenism and polycystic ovaries on ultrasound. Metabolic features associated with PCOS are insulin resistance, obesity, dyslipidemia, and later in life onset of type 2 diabetes mellitus [Citation2,Citation4].
Pregnancies in women with PCOS exhibit an increased risk for maternal and neonatal complications (e.g. gestational diabetes and compromised perinatal outcomes) [Citation5,Citation6]. A large Australian study in which PCOS patients were included based on International Classification of Diseases (ICD) codes shows – besides an increased risk for pregnancy complications – also an increased risk for hospitalizations of offspring of women with PCOS (n = 3,626 PCOS offspring, n = 35,340 offspring from controls) until the age of 15 years. Reasons for hospitalizations differed from pulmonic, circulatory, endocrine or neurological pathology [Citation7].
In the general population, strong and consistent evidence supports the notion that the intrauterine environment during pregnancy impacts on health of offspring later in life [Citation8–11]. Data on the general population demonstrate that preconception maternal features influence health of offspring, mainly blood pressure and Body Mass index (BMI). It must be noticed that these preconception parameters were retrieved from questionnaires or interviews with patients during pregnancy or even postpartum up to years after the birth of their offspring. Various cohort studies using this retrospectively retrieved data conclude that prepregnancy obesity and BMI is associated with high blood pressure in offspring [Citation12–14]. Prepregnancy BMI is also reported to be associated with offspring adiposity [Citation15]. In fact maternal obesity is linked with higher childhood adiposity and a higher cardiovascular disease risk [Citation16,Citation17]. Two cohort studies show that prepregnancy BMI and obesity are associated with a high systolic blood pressure in childhood [Citation18,Citation19]. This also accounts for higher insulin and triglyceride levels and lower HDL-cholesterol levels in offspring from obese mothers [Citation13]. In the general population, blood pressure in children is strongly associated with their blood pressure in adult life [Citation20]. A similar tracking phenomenon is seen in BMI: the trajectories of childhood BMI remain quite similar in adulthood [Citation21,Citation22].
At present, sufficient robust data are lacking regarding a possible association between the condition of women with PCOS before conception and health of their offspring. Therefore, we aim to evaluate the association between maternal BMI in women diagnosed with PCOS assessed before conception and offspring BMI. In secondary analyses, the association between prepregnancy or preconception blood pressure, androgens, HOMA-IR, and LDL-cholesterol of women with PCOS on offspring BMI and blood pressure, will be evaluated.
Materials and methods
Design and patient selection
Women with oligomenorrhea or amenorrhea were screened following a standardized procedure in our outpatient clinic. This procedure contains a questionnaire, physical examination, pelvic ultrasound, and a laboratory assessment. The protocol of this evaluation is extensively explained elsewhere [Citation23,Citation24,Citation25]. PCOS was diagnosed using the Rotterdam criteria [Citation4]. Only women with PCOS who gave birth to a life born baby between January 2005 and August 2008 or between September 2008 and December 2010 were eligible to participate in this study with their child. Children were divided in two groups of 2.5–4 years of age and 6–8 years of age. Exclusion criteria for the PCOS offspring were history of diabetes mellitus type 1 or a respiratory infection less than 2 weeks before a visit [Citation26].
The institutional review board of the UMC Utrecht approved the standardized screening of women with PCOS (trial registry number: NCT02309047) and the cross-sectional follow-up study of PCOS offspring (trial registry number: NCT01767051).
Written informed consent was obtained from all participating women at the time of the standardized extensive screening and from the parents at the time of the follow-up study.
Clinical assessments
The offspring follow-up was conducted in the two earlier mentioned age categories. Similar in both groups was a parental questionnaire in which parental and children’s habits and health were evaluated: family history regarding cardiovascular events and diabetes mellitus, anthropometrics, intoxications (smoking, alcohol, and drug use), physical exercise, and chronic diseases. Information on birth and pregnancy complications (Appendix 1) was also asked for: birth weight, gestational diabetes (75-g oral glucose tolerance test: fasting glucose ≥ 5.1 mmol/L, 1 h glucose ≥ 10.0 mmol/L, 2 h glucose ≥ 8.5 mmol/L) [Citation27], pregnancy-induced hypertension (systolic blood pressure was ≥ 140 mm/Hg and/or the diastolic blood pressure was ≥ 90 mm/Hg), preeclampsia (pregnancy-induced hypertension with proteinuria: ≥300 mg/24 h [Citation28], or hemolysis elevated liver enzymes low platelets syndrome.
A detailed description of the study protocol for the PCOS offspring follow-up study was published elsewhere [Citation26]. In brief, during the physical examination of the offspring the following measurements were performed: height, weight and twice in supine position the blood pressure.
We calculated age- and sex-dependent SDS (standard deviation scores or Z-scores) of offspring BMI using Dutch reference data [Citation29]. Sex- and height-dependent blood pressure percentiles were calculated following the 2016 European Society of Hypertension guidelines of high blood pressure in children and adolescents. Blood pressure below the 90th percentile were defined as normal, blood pressure ≥ 90 and < 95th percentile was defined as high-normal and blood pressure ≥ 95th percentile was defined as hypertension [Citation30]. In the multivariate analyses, only systolic blood pressure was used as this was a stronger predictor of high blood pressure and unfavorable cardiometabolic profile in adulthood [Citation31].
Statistical analysis
All data were analyzed using SPSS Statistics (IBM SPSS Inc., Chicago, IL, version 21.0). Means and standard deviations were calculated for all continuous variables after assessing whether they were normally distributed. Dichotomous variables were presented as numbers and percentages. To assess the association between maternal factors and offspring BMI and blood pressure we used linear regression and corrected for maternal age, parity and ethnicity. We used standardized preconception characteristics which resulted in standardized beta coefficients with standardized 95% confidence intervals. To correct for multiple testing we divided p values of .05 into two, since we are testing two independent age groups for each maternal parameter. Therefore p value <.025 was considered statistically significant.
The concept of this study has been visualized in ; the health of offspring is directly and indirectly influenced by their mother’s health during and before a pregnancy. Via the indirect pathway factors such as pregnancy complications and birth weight play a significant role. There is a difficulty in correcting for birth weight and pregnancy complications in our analyses, namely that these factors could also causally influence systolic blood pressure and BMI of the offspring [Citation32]. To avoid any false associations we choose to refrain from correcting for birthweight and pregnancy complications [Citation33]. We did choose however to correct for maternal age, ethnicity, and parity, known confounders in mothers–offspring associations [Citation32]. Two twin pairs were present in the dataset, for whom we adjusted for in our analyses. In the analyses of the younger age group we did not correct for race and twins for it was an all-Caucasian non-twin age group. We choose to analyze our data primarily in two different age groups taking potential effects of catch up growth into account [Citation34]. We fitted models for the entire offspring group (Appendix 2) and compared models with and without an interaction term for age group and thereafter we fitted models for each age group separately.
Figure 1. Schematic representation of the current analysis: Offspring health might be directly and indirectly associated with preconception and pregnancy characteristics in women with PCOS. Abbreviation: BMI: body mass index; BP: blood pressure; HOMA-IR: insulin resistance; in blue: the investigated associations in this paper; in gray: the potential role of pregnancy in mother-offspring associations; LDL: low-density lipoprotein-cholesterol; PCOS: polycystic ovary syndrome.
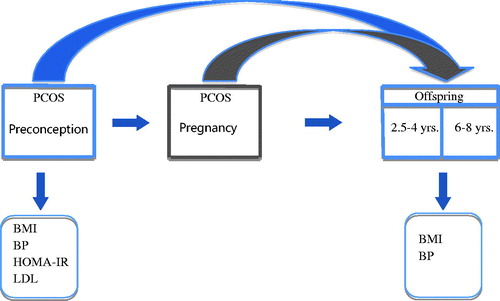
Table 1. Offspring characteristics in the two age groups.
Results
A total of 74 women-child pairs were included: 42 children in the young offspring group (2.5–4 years) and 32 children in the older group (6–8 years). The offspring was female in 49% (n = 36) of all cases (). During preconception the mean age of all 74 women was 31.8 ± 3.7 with a BMI of 25.7 ± 5.9 kg/m2 (). Three children (4.1%) had a high-normal systolic blood pressure, and one child (1.4%) had hypertension [Citation30]. Three (4.1%) children had a BMI > +2 standard deviations and four (5.4%) had a BMI < -2 standard deviations.
Table 2. Preconception characteristics of mothers with PCOS from the two offspring age groups.
Preconception factors and BMI of their offspring
BMI of women and sex- and age-adjusted BMI SDS of their offspring was significantly associated in the older offspring age group (6–8 years old) (β = 0.55 (95% CI: 0.12–0.97), p = .012) (). Such an association could not be observed in the younger children. Furthermore, systolic blood pressure, androgens, HOMA-IR, and LDL-cholesterol of the mother were not significantly associated with BMI SDS for either offspring age-group. No significant p values were observed when adding an interaction term to the models ().
Preconception factors and blood pressure of their offspring
Preconception BMI, systolic blood pressure, androgens, HOMA-IR, and LDL-cholesterol of the mother were not significantly associated with blood pressure SDS for both offspring age-groups ().
Discussion
Main findings
We observed a significant association between preconception BMI of women with PCOS and sex- and age-adjusted BMI of their offspring at 6–8 years, but not at 2.5–4 years of age. No significant associations were identified between preconception systolic blood pressure, androgens, insulin resistance, and lipids, with offspring parameters. To our knowledge, the current preliminary analysis represents the first attempt to study a possible association between preconception health parameters in women with PCOS and health of offspring.
The observed association between preconception BMI of women diagnosed with PCOS and BMI of their offspring is consistent with previous studies in the general population regarding preconception features in women and offspring health [Citation12,Citation14,Citation19,Citation32]. However, we were unable to observe a link between lipid concentrations in women with PCOS and offspring health as described in the general population [Citation35]. Animal studies show that high levels of androgens could be related to an unfavorable cardiovascular profile in offspring [Citation36,Citation37]. We did not find this in our study, potentially due to the relatively small sample size. Another factor possibly influencing our study results could be that the average preconception BMI and blood pressure are not excessively high and quite mild in comparison with international PCOS literature [Citation38]. It has been confirmed that the Netherlands have relatively low rates of overweight in comparison with Western Developed countries [Citation39]. In the younger children we were unable to find an association between preconception BMI and offspring BMI. A reason for this lack of association could be that the effect of maternal BMI could not yet be measured at this young age and impact is more pronounced in older children. Catch up growth might still play a subtle role in the younger age group, considering the fact that 10% of the children in the younger offspring group are small for gestational age () [Citation40]. Unfortunately we could not confirm a difference in age group by adding an interaction term to the models. In our population we could not confirm any association between preconception systolic blood pressure and offspring health.
Table 3. Associations between preconception parameters and BMI and blood pressure of offspring per age group.
Strengths
The strengths of the current study may be summarized as follows; all women underwent an extensive standardized screening before conception, as published before [Citation23,Citation24,Citation41]. Therefore, we are certain that our population consists of women with PCOS following the Rotterdam criteria only. Unlike other studies, we did not use questionnaires or interviews in which we retrospectively asked for preconception BMI, but we used prospectively collected preconception data [Citation7]. Blood pressure in women and offspring was clinically measured twice after a period of rest. To our knowledge this is the first analysis in which factors measured before conception in women with PCOS are linked with data from a standardized screening of offspring.
Limitations
However, the major weakness of the current study is the limited sample size. It should be taken into account when interpreting the results of this preliminary study that we only were able to detect distinct differences. In a recent publication, maternal AMH is thought to have an influence on their intra-uterine offspring [Citation42]. Unfortunately data on anti-müllerian hormone (AMH) is lacking, we did not measure AMH in a standardized manner when screening these women during preconception. Thus, information on the potential link in our population between preconception AMH level and cardiometabolic profile of the offspring remains unknown.
Conclusions
In conclusion, the current study demonstrates that preconception BMI of future mothers with PCOS influences the BMI of their offspring, as already known from studies in the general population. We also know that BMI in childhood age is a tracking phenomenon throughout adulthood [Citation43–45]. According to this phenomenon; obese children are likely to stay obese during early adulthood. The prevalence of overweight and obesity in children is increasing and cardiometabolic profiles are less favorable among overweight children [Citation21,Citation46,Citation47]. The window of opportunity to achieve a favorable BMI in children is during the preconception phase of a future mother with PCOS in need of infertility care. Optimizing maternal health may eventually optimize the health of the generations still to be born.
Acknowledgements
We thank Dr Veltman-Verhulst for her efforts regarding the follow-up study in PCOS offspring.
Disclosure statement
All authors declare complete independence from funders. B.B.R., M.N.B., M.A.W. and M.J.C.E. have nothing to disclose.
Additional information
Funding
References
- Norman RJ, Dewailly D, Legro RS, et al. Polycystic ovary syndrome. Lancet. 2007;370:685–697.
- Fauser BC, Tarlatzis BC, Rebar RW, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38.
- de Melo AS, Dias SV, Rde CC, et al. Pathogenesis of polycystic ovary syndrome: multifactorial assessment from the foetal stage to menopause. Reproduction. 2015;150:11–24.
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41–47.
- Boomsma CM, Eijkemans MJ, Hughes EG, et al. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:673–683.
- Palomba S, de Wilde MA, Falbo A, et al. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update. 2015;21:575–592.
- Doherty DA, Newnham JP, Bower C, et al. Implications of Polycystic Ovary Syndrome for Pregnancy and for the Health of Offspring. Obstet Gynecol. 2015;125:1397–1406.
- Barker DJ, Osmond C, Golding J, et al. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567.
- Barker DJ. The intrauterine environment and adult cardiovascular disease. Ciba Found Symp. 1991;156:3–10.
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736.
- Kermack AJ, Van Rijn BB, Houghton FD, et al. The 'Developmental Origins' hypothesis: relevance to the obstetrician and gynecologist. J Dev Orig Health Dis. 2015;6:415–424.
- Fraser A, Tilling K, Macdonald-Wallis C, et al. Associations of gestational weight gain with maternal body mass index, waist circumference, and blood pressure measured 16 y after pregnancy: the Avon Longitudinal Study of Parents and Children (ALSPAC). Am J Clin Nutr. 2011;93:1285–1292.
- Tsadok MA, Friedlander Y, Paltiel O, et al. Obesity and blood pressure in 17-year-old offspring of mothers with gestational diabetes: insights from the Jerusalem Perinatal Study. Exp Diabet Res. 2011;2011:906154.
- Oostvogels AJ, Stronks K, Roseboom TJ, et al. Maternal prepregnancy BMI, offspring's early postnatal growth, and metabolic profile at age 5-6 years: the ABCD Study. J Clin Endocr Metab. 2014;99:3845–3854.
- Fraser A, Tilling K, Macdonald-Wallis C, et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation. 2010;121:2557–2564.
- Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction. 2010;140:387–398.
- Reynolds RM, Allan KM, Raja EA, et al. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ. 2013;347:4539.
- Wen X, Triche EW, Hogan JW, et al. Prenatal factors for childhood blood pressure mediated by intrauterine and/or childhood growth? Pediatrics. 2011;127:713–721.
- Gaillard R, Steegers EA, Hofman A, et al. Associations of maternal obesity with blood pressure and the risks of gestational hypertensive disorders. The Generation R Study. J. Hypert. 2011;29:937–944.
- Toschke AM, Kohl L, Mansmann U, et al. Meta-analysis of blood pressure tracking from childhood to adulthood and implications for the design of intervention trials. Acta Paediatr. 2010;99:24–29.
- Singh AS, Mulder C, Twisk JW, et al. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008;9:474–488.
- Herman KM, Craig CL, Gauvin L, et al. Tracking of obesity and physical activity from childhood to adulthood: the Physical Activity Longitudinal Study. Int J Pediatr Obes. 2009;4:281–288.
- Broekmans FJ, Fauser BC. Diagnostic criteria for polycystic ovarian syndrome. Endocrine. 2006;30:3–11.
- Broekmans FJ, Knauff EA, Valkenburg O, et al. PCOS according to the Rotterdam consensus criteria: change in prevalence among WHO-II anovulation and association with metabolic factors. BJOG. 2006;113:1210–1217.
- Daan NM, Jaspers L, Koster MP, et al. Androgen levels in women with various forms of ovariandysfunction: associations with cardiometabolic features. Hum Reprod. 2015;30:2376–2386.
- de Wilde MA, Eising JB, Gunning MN, et al. Cardiovascular and metabolic health of 74 children from women previously diagnosed with polycystic ovary syndrome in comparison with a population-based reference cohort. Reprod Sci. 2018;1:1933719117749761.
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2012;35:S11–S63.
- Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Obstet Gyn. 2013;122:1122–1131.
- De TNO groeicalculator voor professionals, 2010. https://groeiweb.pgdata.nl/calculator.asp, last visited February 2nd, 2018.
- Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887–1920.
- Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237–246.
- Yu Z, Han S, Zhu J, et al. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One. 2013;16:8.
- Hernández-Díaz S, Schisterman EF, Hernán MA. The birth weight “paradox” uncovered? Am J Epidemiol. 2006;164:1115–11120.
- Kelishadi R, Haghdoost AA, Jamshidi F, et al. Low birthweight or rapid catch-up growth: which is more associated with cardiovascular disease and its risk factors in later life? A systematic review and cryptanalysis. Paediatr Int Child Health. 2015;35:110–123.
- Gademan MGJ, Vermeulen M, Oostvogels AJJM, et al. Maternal prepregancy BMI and lipid profile during early pregnancy are independently associated with offspring's body composition at age 5–6 years: the ABCD study. PLoS One. 2014;169:e94594.
- Bruns CM, Baum ST, Colman RJ, et al. Insulin resistance and impaired insulin secretion in prenatally androgenized male rhesus monkeys. J Clin Endocrinol Metab. 2004;89:6218–6223.
- Manikkam M, Crespi EJ, Doop DD, et al. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790–798.
- Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–169.
- Global Health Observatory (GHO) data. Overweight and Obesity. World Health Organization. 2016. http://www.who.int/gho/ncd/risk_factors/overweight/en/, last visited April 2nd, 2018.
- Karlberg J, Albertsson-Wikland K, Kwan CW, et al. Early spontaneous catch-up growth. J Pediatr Endocrinol Metab. 2002;15:1243–1255.
- Daan NM, Louwers YV, Koster MP, et al. Cardiovascular and metabolic profiles amongst different polycystic ovary syndrome phenotypes: who is really at risk?. Fertil Steril. 2014;102:1444–1451.
- Tata B, Mimouni NEH, Barbotin AL, et al. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med. 2018;24:834–846.
- Deshmukh-Taskar P, Nicklas TA, Morales M, et al. Tracking of overweight status from childhood to young adulthood: the Bogalusa Heart Study. Eur J Clin Nutr. 2006;60:48–57.
- Johannsson E, Arngrimsson SA, Thorsdottir I, et al. Tracking of overweight from early childhood to adolescence in cohorts born 1988 and 1994: overweight in a high birth weight population. Int J Obes (Lond). 2006;30:1265–1271.
- Bayer O, Krüger H, Von Kries R, et al. Factors associated with tracking of BMI: a meta-regression analysis on BMI tracking. Obesity (Silver Spring). 2011;19:1069–1076.
- Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006;1:11–25.
- Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357:2329–2337.
Appendix 1. Pregnancy and labor complications
Appendix 2. Adjusted linear regression in both offspring groups together.

