Abstract
It is essential that fertility treatment is individualized based on a thorough diagnostic work-up, with treatment tailored to the patients’ requirements. This individualization should be kept in mind during the main decision points that occur before and during treatment. Treatment customization must include consideration of both the woman and her partner involved in the process together, including their collective treatment goals. Once treatment goals have been agreed and diagnostic evaluations performed, personalization based on patient characteristics, together with an understanding of treatment goals and patient preferences, enables the selection of appropriate treatments, protocols, products and their dosing. Following treatment initiation, monitoring and adaptation of product and dose can then ensure optimal outcomes. Currently, it is not possible to base treatment decisions on every characteristic of the patient and personalization is based on biomarkers that have been identified as the most relevant. However, in the future, the use of artificial intelligence coupled with continuous monitoring should enable greater individualization and improve outcomes. This review considers the current state-of-the-art related to decision points during individualized treatment of female infertility, before looking at future developments that might further assist in making individualized treatment decisions, including the use of computer-assisted decision making.
摘要
生育治疗必须是在彻底诊断的基础上, 根据患者的需求进行的个性化治疗。在治疗之前和治疗期间的主要决策要点中应牢记这种个性化。定制治疗方案必须同时考虑参与该过程的妇女及其伴侣, 包括其集体治疗目标。一旦达成了治疗目标并进行了诊断评估, 基于患者特征的个性化设置以及对治疗目标和患者喜好的理解, 就可以选择合适的治疗方法、方案、产品及其剂量。在治疗开始后, 监测和调整产品和剂量可以确保获得最佳结果。目前, 不可能根据患者的每个特征来做出治疗决定, 而个性化是基于已被确定为最相关的生物标志物。然而, 在未来, 人工智能与持续监测的使用应该能够实现更大的个性化并改善结果。这篇综述阐述了目前在女性不孕症个性化治疗过程中与决策点相关的最新技术, 然后探讨了可能有助于进一步制定个性化治疗决策的未来发展, 包括计算机辅助决策的使用。
The Chinese abstracts are translated by Prof. Dr. Xiangyan Ruan and her team: Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing 100026, China.
Introduction
Infertility, despite being defined as a single disease, does not have a single cause, instead there are a number of factors that may contribute to it. As knowledge of the pathogenesis and causes of infertility has developed, it has enabled a greater range of patients to be treated safely and effectively with protocols tailored to patient characteristics. This review considers the current state-of-the-art relating to treatment of female infertility, including individualizing treatment and the main decision points for this individualization (), before looking at developments that might further assist in making treatment decisions.
Figure 1. Decision points during infertility treatment. GnRH: gonadotropin-releasing hormone; hCG: human chorionic gonadotropin; LH: luteinizing hormone.

The decision points and decisions included in this review are general and certain specific consultations/treatments may require other decisions to be made. Specific decisions should be based on discussion of treatment goals and medical history, and a full diagnostic work-up. Over time the trends in fertility treatment have changed, with increased use of single embryo transfer and frozen embryo transfer. The decision of which treatment approach to take will partly be based upon country guidelines and the suggestions made in this review should be used in conjunction with any national or regional guidance. Owing to the number of treatment decisions available, this review will only cover decisions up to, but not including, embryo transfer within the context of assisted reproductive technologies (ART).
Pretreatment
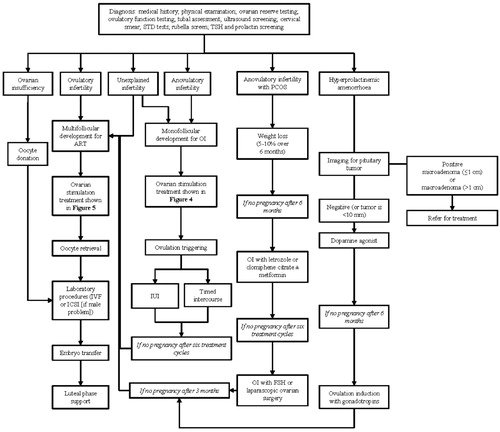
Patients meeting the ICMART criteria for infertility (a disease characterized by the failure to establish a clinical pregnancy after 12 months of regular, unprotected sexual intercourse or due to an impairment of a person's capacity to reproduce either as an individual or with his/her partner) should be referred for specialist treatment if they have not conceived after 12 months of regular, unprotected sexual intercourse [Citation1]. However, some patients should be referred more rapidly, including older women (e.g. >35 years), those with a history of amenorrhea, galactorrhea, menstrual disorders, endometriosis or previous surgery on the ovaries [Citation1,Citation2]. Following referral, initial evaluations and treatment discussions should involve the couple, before separate evaluations are considered. It might be that only a male cause of infertility or a female cause of infertility is present or there may be both male and female causes. Patients can be further categorized (segmented) according to the cause of infertility, and this should guide decisions about whether and how to treat the infertility. An overview of the treatment of female infertility is shown in .
Figure 2. An overview of the treatment of female infertility including the main decision points and treatment suggestions. As infertile women often present with multiple diagnoses and this diagram attempts to segment patient diagnosis to one contributing factor, the suggested treatment may not be most appropriate based on a full diagnosis (e.g. for anovulatory infertility, monofollicular development may not be appropriate if the patient also has tubal factor). ART: assisted reproductive technologies; IUI: intrauterine insemination; OI: ovulation induction; PCOS: polycystic ovary syndrome.
Personalization for fertility treatment
To ensure optimal outcomes it is essential that treatment is individualized according to the characteristics of the patient. At present, it is impossible to base treatment decisions on all known characteristics. Instead, patients are segmented based on biomarkers that have been identified to be of particular relevance. These biomarkers will include those used in diagnosis, as well as those that govern hormonal treatment response as these can help to select the most appropriate hormonal treatment protocol. The biomarkers identified for the treatment of female infertility include female age, antral follicle count (AFC), anti-Müllerian hormone (AMH) concentration, baseline follicle-stimulating hormone (FSH), previous ovarian response to stimulation and actual follicular development during ongoing ovarian stimulation [Citation3–6]. In cases where both female and male infertility are present, both partners should be evaluated and treated.
Diagnosis of female infertility
The aim of treatment depends on the diagnosis, and the following diagnostic tests should be considered:
Complete medical history, including demographics and reproductive, occupational, family, surgical, gynecological, obstetric and sexual histories and lifestyle risk factors. It is important that any history related to thyroid, secondary sexual characteristics, the breasts, galactorrhea, the skin (including acanthosis nigricans, abdominal striae, and hirsutism), assessment of clitoromegaly and structural abnormalities of the cervix, uterus, and pelvis are investigated further. Every specialist center should evaluate this at the first visit.
Physical examination, including body mass index.
Tests for ovarian reserve such as AMH, AFC, basal FSH, and estradiol.
Testing for ovulatory function with a serum progesterone or urinary pregnanediol assessment, approximately 7 days (5–7 days) after estimated ovulation.
Tubal assessment with the type of diagnostic test determined by relevant history of co-morbidities.
Ultrasound screening for uterine/ovarian/morbidities/cervical pathology and for endometriosis (endometriotic ovarian cysts/deep endometriotic nodules), followed by laparoscopic exploration and operative intervention as needed.
Cervical smear, type and screen, testing for sexually transmitted infections and immunity to rubella.
Screening for thyroid-stimulating hormone and prolactin if indicated by history.
These tests should enable a diagnosis to be made, with the main diagnoses being: anovulatory infertility (with or without polycystic ovary syndrome [PCOS]); infertility in ovulatory women (including women with endometriosis, uterine factor infertility, tubal infertility, and unexplained infertility); ovarian insufficiency; and hyperprolactinemic amenorrhea (). Treatable co-morbidities should be addressed before fertility treatment is commenced.
Treatment of female infertility
Treatment of female infertility needs to be considered in the context of the male infertility investigation results, and may include expectant management, reproductive surgery, lifestyle adjustment, and/or medically assisted reproduction, with the aim of:
Monofollicular development – applicable in the context of natural fertilization in vivo, including ovulation induction (OI; pharmacological treatment with the intention of inducing normal ovulatory cycles), and intrauterine insemination (IUI; laboratory processed sperm placed in the uterus to attempt a pregnancy) with or without ovarian stimulation (pharmacological treatment with the intention of stimulating one dominant follicle, or at most 2 dominant follicles that are expected to ovulate).
Multifollicular development – applicable in the context of treatment with ART (interventions that include the in vitro handling of both human oocytes and sperm or of embryos for the purpose of reproduction).
Women diagnosed with ovarian insufficiency will require oocyte donation.
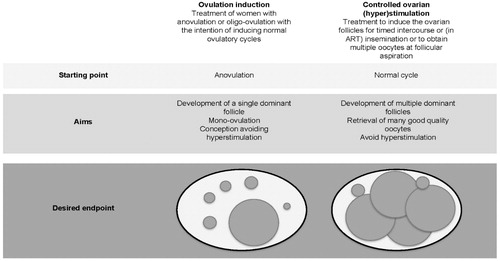
In anovulatory women, the initial aim of treatment should be monofollicular development using OI as described in the section ‘Anovulatory women’ (WHO Type II). In anovulatory women failing treatment with OI, and in women with ovulatory infertility and couples with male infertility, controlled ovarian (hyper)stimulation (COS) aiming at multifollicular development, oocyte retrieval and ART treatment can be indicated ().
Figure 3. Ovulation induction vs. controlled ovarian (hyper)stimulation. ART: assisted reproductive technologies.
If COS or OI with a gonadotropin is needed, it is important to select a protocol, a gonadotropin, and a starting dose before treatment is initiated. Once COS or OI begins, the patient should be monitored using ultrasound and estradiol levels, to ensure that treatment is proceeding as expected. Dose adaptation on day 4–6 and also at later time points is also usually considered based on a patient’s response to treatment [Citation5,Citation7,Citation8]. In the context of COS for ART treatment, dose adaptation is common practice with an analysis of data from a US database that included 23,582 patients undergoing 33,962 IVF cycles finding that 40.7% of cycles included dose adaptation (either an increase, a decrease, or both increases or decreases) [Citation7]. Any specified cutoffs for treatment decisions during a treatment cycle should be based on an audit of data for a specific treatment center and the decisions that could be made are:
Maintain the gonadotropin dose;
Reduce the gonadotropin dose;
Increase the gonadotropin dose;
Add an additional product (e.g. luteinizing hormone [LH]);
Change the gonadotropin product used;
Change the planned agent for ovulation triggering;
Plan a luteal stimulation (dual stimulation protocol);
Change to a freeze-all cycle;
Cancel the cycle.
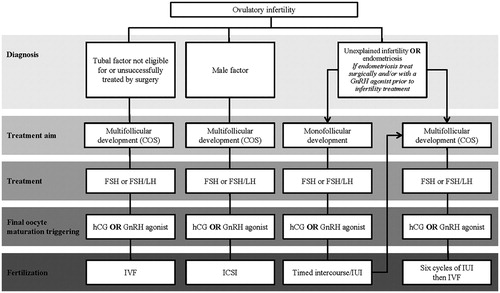
Ovulatory women (including women with unexplained infertility, endometriosis, uterine factor infertility, or tubal infertility)
Ovulatory women with infertility can be treated with COS and ART, and an overview of their treatment is shown in [Citation9]. Conventional COS results in a greater number of oocytes retrieved compared with mild stimulation or natural cycles [Citation10,Citation11]. As the number of oocytes retrieved has been observed to be positively correlated with pregnancy and live birth outcomes, COS should be the preferred treatment option in this population [Citation12–15]. Meta-analyses have demonstrated that gonadotropin-releasing hormone (GnRH) agonists and antagonists have comparable efficacy; however, antagonist protocols are recommended because they offer improved safety owing to a lower risk for ovarian hyperstimulation syndrome (OHSS) [Citation16–22]. The risk for OHSS is also reduced by the use of a GnRH agonist rather than human chorionic gonadotropin (hCG) to trigger ovulation and this can be considered for high responders [Citation17].
Figure 4. Proposed treatment algorithm for ovulatory infertility. As infertile women often present with multiple diagnoses and this diagram attempts to segment patient diagnosis to one contributing factor, the suggested treatment may not be most appropriate based on a full diagnosis. COS: controlled ovarian stimulation; FSH: follicle-stimulating hormone; GnRH: gonadotropin-releasing hormone; hCG: human chorionic gonadotropin; ICSI: intracytoplasmic sperm injection; IUI: intrauterine insemination; IVF: in vitro fertilization; WHO: World Health Organization.
A standard treatment flow chart for ART is shown in , and this should be adapted according to the individual patient’s characteristics. The main diagnoses for ovulatory women are tubal factor infertility, male factor infertility, endometriosis, and unexplained infertility. Ovulatory women can be subdivided according to their expected response to COS for ART; high, normal, and low (poor and suboptimal) responders [Citation23], and treated accordingly ().
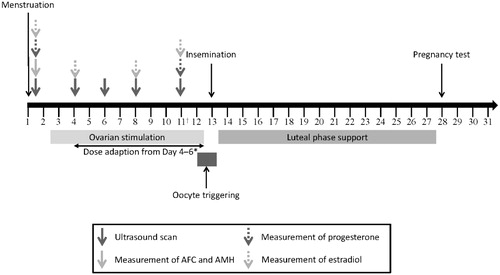
Figure 5. Standard treatment flow for ART treatment. *GnRH antagonist can be used in a flexible or fixed protocol. †If the estradiol level is low in relation to follicles LH supplementation may be considered. If both the estradiol level and follicle count are low, a gonadotropin dose increase should be considered. If the estradiol level is very low, a gonadotropin dose increase should be considered. ‡Additional testing is recommended on day 11 to assess hyperstimulation or premature luteinization. GnRH: gonadotropin-releasing hormone.
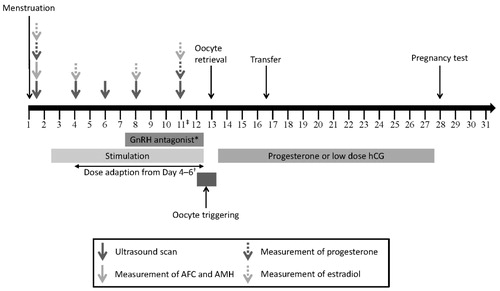
Figure 6. Proposed treatment algorithm for controlled ovarian stimulation according to (predicted) ovarian response. Doses indicated are suggestions, and the dose should be individualized according to all patient characteristics. AFC: antral follicle count; AMH: anti-Müllerian hormone; COS: controlled ovarian stimulation; FSH: follicle-stimulating hormone; GnRH: gonadotropin-releasing hormone; hCG: human chorionic gonadotropin; LH: luteinizing hormone.
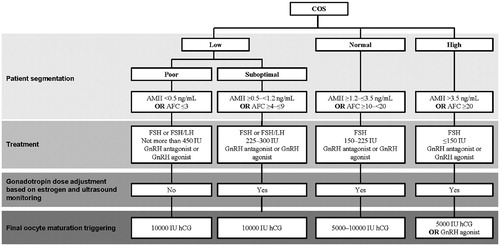
High responders – These patients are characterized by an excessive response to gonadotropin therapy and an increased risk for OHSS [Citation24]. There is no agreed definition for high responders or expected high responder patients [Citation24]. We propose that high responders are patients from whom you would expect to retrieve ≥20 oocytes after COS [Citation25]. A large pool of pre-antral follicles has been identified as a risk factor for high response and hyperstimulation, and both high AFC and AMH have been shown to be good predictors of high response [Citation24,Citation26–31]. Proposed biomarkers for identifying high responders are, therefore, a previous high response (≥20 oocytes were retrieved after COS), AMH >3.5 ng/mL or AFC ≥20 [Citation23,Citation24]. Expected high responders should be initiated with a low dose of FSH (≤150 IU). Above the threshold of 20 oocytes, in addition to an increased risk for OHSS, a decrease in the live birth rate has been reported in a fresh cycle in some studies [Citation14,Citation32] but not in others [Citation33]. Furthermore, increased risk for preterm birth and low birth weight [Citation34] have been observed following transfer in a fresh IVF cycle. To reduce the risk for OHSS, a GnRH antagonist protocol should be used, with a GnRH agonist to trigger ovulation. Depending on the response, treatment should be adjusted (most likely the gonadotropin dose reduced) to ensure that risk of OHSS is minimized. If there is concern about OHSS, the treatment strategy can be amended to a freeze-all approach [Citation35–37].
Normal responders – These are patients who exhibit the expected response (≥10 to <20 oocytes) to stimulation with gonadotropins and are the population for whom most treatment guidelines are intended. Proposed biomarkers for identifying normal responders are a previous normal response (≥10 to <20 oocytes retrieved after COS), AMH ≥1.2 to ≤3.5 ng/mL or AFC ≥10 to <20 [Citation21–23]. As is the recommendation for high responders, follicular development should be monitored by way of estrogen concentration and ultrasound, and the gonadotropin dose should be adjusted as appropriate.
Low (poor and suboptimal) responders – Low responders are patients from whom we would expect to retrieve <10 oocytes after COS. Several tools for personalization have been proposed for the identification of low responders, including the European Society of Human Reproduction and Embryology (ESHRE) Bologna criteria for poor responders, and the Patient-Oriented Strategies Encompassing Individualized Oocyte Number (POSEIDON) criteria [Citation21,Citation22,Citation38].
Poor responders can be identified by the ESHRE Bologna criteria, which were intended to identify a population of poor ovarian responders for clinical trials [Citation38]. However, because the ESHRE Bologna criteria include a broad, heterogeneous group of poor ovarian responders, they are not considered as clinically relevant for making informed treatment decisions [Citation39–42]. Furthermore, the ESHRE Bologna criteria do not aim to differentiate between oocyte quality and oocyte quantity; instead aiming to identify a homogenous population with reduced ovarian reserve, rather than a population with similar prognoses for pregnancy [Citation41]. Despite these shortcomings, we propose that poor responders are defined according to the Bologna criteria, as analyses suggest they are clinically relevant [Citation43]. Therefore, poor responders are patients from whom we would expect to retrieve ≤3 oocytes after conventional COS. Proposed biomarkers for identifying poor responders are a previous poor response (≤3 oocytes retrieved after COS), AMH <0.5 ng/mL or AFC ≤3.
Suboptimal responders are not widely recognized as a patient subgroup and, prior to the development of the ESHRE Bologna criteria, were often combined with either normal responders or poor responders [Citation21–23,Citation44]. In response to this gap, the POSEIDON Group developed a set of criteria by consensus that identify different groups of low prognosis patients (i.e. suboptimal and poor responders) [Citation21,Citation22]. These were developed specifically with the aim of guiding personalized treatment protocols for second and subsequent cycles, following unexpected suboptimal or poor response. To date, the POSEIDON criteria offer the best approach to personalization for poor/suboptimal ovarian responders.
Suboptimal responders are patients from whom we would expect to retrieve ≥4 to ≤9 oocytes after COS. Proposed biomarkers for identifying suboptimal responders are a previous suboptimal response (≥4 to ≤9 oocytes retrieved after COS), AMH ≥0.5 to <1.2 ng/mL or AFC ≥4 to ≤9. A large proportion of patients receiving ART treatment (43.3% in one study) would fulfill the criteria for suboptimal response or expected suboptimal response [Citation23]. Based on recent meta-analyses, there is evidence that suboptimal responders and potentially other patient populations are likely to benefit from r-hLH supplementation [Citation45–49] with respect to implantation, pregnancy, and live birth rates.
Anovulatory women
An overview of the treatment of anovulatory women is shown in . A standard treatment flow chart for ovulation induction and IUI/timed intercourse is shown in , and this should be adapted according to the patient’s characteristics.
Figure 7. Proposed treatment algorithm for anovulatory infertility. ART: assisted reproductive technologies; CC: clomiphene citrate; COS: controlled ovarian stimulation; GnRH: gonadotropin-releasing hormone; hCG: human chorionic gonadotropin; IUI: intrauterine insemination; LOS: laparoscopic ovarian surgery; OI: ovarian induction; WHO: World Health Organization.
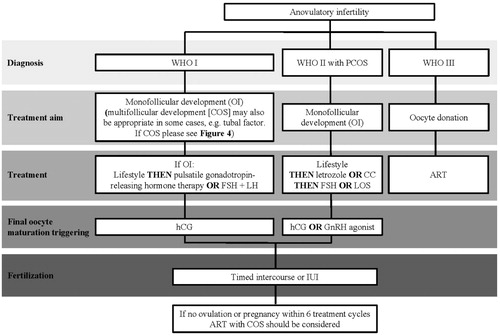
Figure 8. Standard treatment flow for ovulation induction plus IUI/timed intercourse. *If the estradiol level is low in relation to follicles, LH supplementation may be considered. If both the estradiol level and follicle count are low, a gonadotropin dose increase should be considered. If the estradiol level is very low, a gonadotropin dose increase should be considered. †Additional testing is recommended on day 11 to assess hyperstimulation or premature luteinization.
We propose that anovulatory women should be segmented according to response in the same way that ovulatory women are, and suggestions for treatment that are based on response are shown in .
Figure 9. Proposed treatment algorithm for ovulation induction according to (predicted) ovarian response. *The starting dose for subsequent cycles should be selected, taking into account the ovarian response during the previous cycle. AFC: antral follicle count; AMH: anti-Müllerian hormone; hCG: human chorionic gonadotropin; OI: ovulation induction.
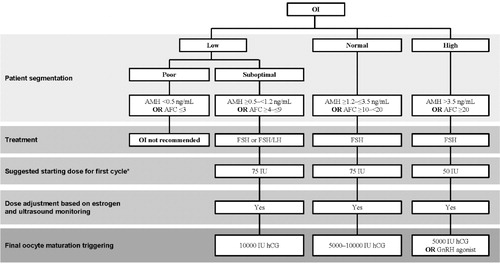
WHO Group I – WHO Group I disorders may be caused by hypothalamic–pituitary failure or dysfunction [Citation50], and typically estradiol levels are low and gonadotropin levels may be low-to-normal. The three conditions included in WHO Group I are hypothalamic amenorrhea, hypogonadotropic hypogonadism, and hypopituitarism, however, it must be noted that WHO Group I does not include all patients with severe FSH and LH deficiency [Citation51]. Treatment should be individualized taking into account the entire clinical manifestation which could include tubal disease, and treatment options include pulsatile GnRH therapy, exogenous gonadotropin therapy with both FSH and LH activity and lifestyle interventions [Citation50].
WHO Group II – WHO Group II disorders are caused by hypothalamic-pituitary-ovarian dysfunction [Citation50], characterized by normal estradiol and FSH concentrations but without the ovulatory LH peak. The most common cause of WHO Group II anovulation is PCOS. Obese women with PCOS should initially be treated using lifestyle interventions, with the aim for weight loss of 5–10% over 6 months, as this has been shown to result in significant clinical improvements [Citation25]. A multicomponent approach is recommended, including diet, exercise, and behavioral strategies. If insulin resistance is suspected, metformin can also be prescribed and anti-androgens and inositol may be beneficial [Citation25]. If these interventions, in combination with regular intercourse, do not result in pregnancy in women with anovulatory infertility and no other infertility within 6–12 months of diagnosis, letrozole or clomiphene citrate should be considered as the first-line treatment for ovulation induction, with or without metformin [Citation25]. However, if anovulatory women do not ovulate with clomiphene citrate/letrozole or do not conceive within six cycles of OI with clomiphene citrate/letrozole and timed intercourse/IUI, they should be offered OI with recombinant-human FSH (hFSH) or a combination of FSH + LH if LH deficient. In these cases, gonadotropins should be used at starting doses of ≤75 IU to avoid multiple pregnancy and OHSS. The initial dose for patients with PCOS should be 50 IU and which can be increased to 75 IU if they do not respond. If the patient does not ovulate with r-hFSH treatment or conceive within three cycles of OI with gonadotrophins and timed intercourse/IUI, they should be treated with COS and ART [Citation9]. The selected treatment can be individualized based on patient characteristics including age, BMI, ovarian reserve markers (AFC, AMH, baseline FSH and estradiol) and diagnosis of infertility [Citation3,Citation5,Citation6]. In case of failed COS, laparoscopic ovarian surgery can also be offered as an alternative to ART [Citation25].
WHO Groups I and II anovulatory women who do not conceive after six attempts at treatment with ovulation induction should be treated with COS and ART [Citation9].
WHO Group III – WHO Group III disorders are caused by ovarian failure [Citation50] including women with ovarian insufficiency and should be treated by oocyte donation.
Hyperprolactinemic amenorrhea – Prolactin concentration should be measured if indicated by clinical history (e.g. a history of amenorrhea) and, if elevated, the first step should be investigation for a pituitary tumor. If a pituitary tumor is diagnosed appropriate referral should be made to facilitate multi-disciplinary input and management. If a tumor is <10 mm or not present, treatment should be initiated with a dopamine agonist [Citation52]. If a pregnancy is not achieved within 6 months, treatment with exogenous FSH could be attempted.
Unexplained female infertility
Unexplained infertility was defined in the latest ICMART Glossary as "Infertility in couples with apparently normal ovarian function, fallopian tubes, uterus, cervix and pelvis and with adequate coital frequency; and apparently normal testicular function, genito-urinary anatomy and a normal ejaculate. The potential for this diagnosis is dependent on the methodologies used and/or those methodologies available" [Citation1]. Women below the age of 35 with unexplained infertility should be advised to try to conceive for a total of 2 years (including up to 1 year before fertility investigations begin) [Citation9], and ART treatment offered to women who do not conceive within 2 years of regular unprotected sexual intercourse [Citation9,Citation53–55], and IUI with COS would be appropriate when the male partner has a total motile sperm count of >10 million. In these cases, gonadotropins should be used at starting doses of ≤75 IU to avoid multiple pregnancy. Clomiphene citrate or tamoxifen is an acceptable alternative to low-dose gonadotropins as they have a similar low risk for multiple pregnancy and birth as low-dose gonadotropins. The addition of a GnRH agonist is not recommended, owing to the lack of an effect on pregnancy rates [Citation53].
Future perspectives
In the future, standardization and tools that enable the objective interpretation of results should ensure consistent, high-quality infertility treatment at all ART centers. This will be complemented by comprehensive diagnostics (genetic, physical and urine/blood) of patients before and during IVF, and objective interpretation supported by artificial intelligence (AI)-decision supporting algorithms. The eventual goal of this would be individualized ovarian stimulation, ovulation triggering, and luteal phase support based on the results from comprehensive diagnostics before treatment, and real-time monitoring of the patient at home with intelligent devices that are connected to the clinic. This real-time monitoring needs to include monitoring of hormone levels and injections, together with ultrasound to measure follicular growth [Citation56]. The concept of ‘continuous’ monitoring of the patient not only during the stimulation, but also during the pregnancy phase, is based on the idea that currently only fragmented information during treatment is compiled, and decisions are taken based on this incomplete picture of the patient. The use of continuous information will enable physicians to better understand the actual course of the treatment and take targeted actions at an earlier point. In addition, home monitoring will bring clinics closer to patients during the stimulation phase and enable physicians to identify incorrect or sub-optimal injection behaviors or adherence issues, thereby improving compliance. These advances would allow precision medicine to be established in infertility treatment. Furthermore, the increase in the available data will allow the development of improved algorithms to individualize treatment protocols and adjust them in real-time. As well as improving outcomes, these protocols should increase patient comfort and provide reassurance and emotional stability during the treatment.
Considering the amount of information that will be collected, AI will be essential to evaluate the results and provide objective recommendations for clinics. The use of AI to evaluate large amounts of data and assist in diagnosis and treatment selection is already being evaluated in several other fields of medicine [Citation57], including diabetes [Citation58,Citation59] and diagnostic imaging [Citation60,Citation61]. The idea of using AI to aid decision-making for infertility treatment has been developed over many years, with an early proposal by Schild et al. [Citation62]. Despite increasing interest, AI is not routinely used by fertility clinics for making treatment decisions, but is starting to be used in infertility laboratories, with a number of tools for embryo monitoring and selection [Citation63–69] currently available for objective judgment and choice of gametes and embryos using AI-picture analytics and decision-supporting algorithms [Citation70]. The aim of these tools is to provide an objective recommendation for embryologists, enabling the standardization of treatment across different centers by removing operator variability/subjectivity. This is an important goal, as center effect and operator variability are major contributors to differences in treatment outcomes for fertility treatment [Citation43,Citation71]. These tools are being designed to work together with the automation of key steps in the embryo lab (e.g. intracytoplasmic sperm injection [ICSI] [Citation72], the culturing of embryos and the freezing of embryos and oocytes [Citation73]). In addition, there are tools in development relating to the partial automation of pick-up instruments, to improve standardization of oocyte retrieval, and partial automation of transfer instruments, to improve standardization of embryo transfer.
Conclusion
The treatment of infertility requires individualization based on patient characteristics and treatment response, and in this review we have highlighted the main decision points in the pretreatment and treatment phases of female infertility. These decisions, however, are currently made using incomplete data, and continuous home monitoring coupled with AI-decision making tools will further improve treatment outcomes in the future.
Disclosure statement
BL has no conflict of interest to declare concerning this paper. WB is an employee of Merck Serono GmbH, Darmstadt, Germany. SL, JK and TDH are employees of Merck KGaA, Darmstadt, Germany. SKS has received grants and non-financial support from Merck and Ferring.
Additional information
Funding
References
- Zegers-Hochschild F, Adamson GD, Dyer S, et al. The International Glossary on Infertility and Fertility Care, 2017. Hum Reprod. 2017;32:1786–1801.
- Cheong Y, Broadley R. Fertility testing and treatment decisions. In: Jha S, Ferriman E, editors. Medicolegal issues in obstetrics and gynaecology. Cham: Springer International Publishing AG; 2018. p. 297–299.
- Grisendi V, La Marca A. Individualization of controlled ovarian stimulation in vitro fertilization using ovarian reserve markers. Minerva Ginecol. 2017;69:250–258.
- Popovic-Todorovic B, Racca A, Blockeel C. Added value today of hormonal measurements in ovarian stimulation in gonadotropin-releasing hormone antagonist treatment cycle. Curr Opin Obstet Gynecol. 2018;30:145–150.
- Mol BW, Bossuyt PM, Sunkara SK, et al. Personalized ovarian stimulation for assisted reproductive technology: study design considerations to move from hype to added value for patients. Fertil Steril. 2018;109:968–979.
- Özcan P, Fiçicioğlu C, Ateş S, et al. The cutoff values of serum AMH levels and starting recFSH doses for the individualization of IVF treatment strategies. Gynecol Endocrinol. 2017;33:467–471.
- Mahony M, Hayward B, Richter K, et al. Occurrence and characteristics of recombinant human follicle-stimulating hormone (r-hFSH) dose adjustments during ovarian stimulation in a real-world US database study of 33,962 IVF patient cycles. Proceedings of the 34th Annual Meeting of the European Society of Human Reproduction and Embryology; 2018 Jul 1–4; Barcelona, Spain; 2018.
- The Practice Committee of the American Society for Reproductive Medicine. Use of exogenous gonadotropins in anovulatory women: a technical bulletin. Fertil Steril. 2009;90:S7–S12.
- National Institute for Health and Care Excellence. CG156: fertility problems: assessment and treatment; 2017; [cited 2018 Jul 5]. London: NICE. Available from: https://www.nice.org.uk/guidance/cg156/chapter/recommendations
- Youssef MA, van Wely M, Al-Inany H, et al. A mild ovarian stimulation strategy in women with poor ovarian reserve undergoing IVF: a multicenter randomized non-inferiority trial. Hum Reprod. 2017;32:112–118.
- Bou Nemer L, Weitzman VN, Arheart KL, et al. In vitro fertilization versus mild stimulation intrauterine insemination in women aged 40 and older. Reprod Sci. 2017;24:609–612.
- Wu CX, Zhang T, Shu L, et al. Cumulative live birth rates per oocytes retrieved cycle: evaluation of clinical outcomes of IVF/ICSI. Zhonghua Fu Chan Ke Za Zhi. 2018;53:160–166.
- Steward RG, Lan L, Shah AA, et al. Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: an analysis of 256,381 in vitro fertilization cycles. Fertil Steril. 2014;101:967–973.
- Magnusson A, Kallen K, Thurin-Kjellberg A, et al. The number of oocytes retrieved during IVF: a balance between efficacy and safety. Hum Reprod. 2018;33:58–64.
- Zhou J, Wang B, Hu Y, et al. Association between the number of oocytes retrieved and cumulative live birth rate in women aged 35–40 years undergoing long GnRH agonist IVF/ICSI cycles. Arch Gynecol Obstet. 2017;296:1005–1012.
- Al-Inany HG, Youssef MA, Ayeleke RO, et al. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev. 2016;4:CD001750.
- Lambalk CB, Banga FR, Huirne JA, et al. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum Reprod Update. 2017;23:560–579.
- Xiao J, Chang S, Chen S. The effectiveness of gonadotropin-releasing hormone antagonist in poor ovarian responders undergoing in vitro fertilization: a systematic review and meta-analysis. Fertil Steril. 2013;100:1594–1601e1–9.
- Pundir J, Sunkara SK, El-Toukhy T, et al. Meta-analysis of GnRH antagonist protocols: do they reduce the risk of OHSS in PCOS? Reprod Biomed Online. 2012;24:6–22.
- Youssef MA, Van der Veen F, Al-Inany HG, et al. Gonadotropin-releasing hormone agonist versus HCG for oocyte triggering in antagonist assisted reproductive technology cycles. Cochrane Database Syst Rev. 2011;1:CD008046.
- Alviggi C, Andersen CY, Buehler K, et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril. 2016;105:1452–1453.
- Humaidan P, Alviggi C, Fischer R, et al. The novel POSEIDON stratification of 'Low prognosis patients in Assisted Reproductive Technology' and its proposed marker of successful outcome. F1000Res. 2016;5:2911.
- Polyzos NP, Sunkara SK. Sub-optimal responders following controlled ovarian stimulation: an overlooked group? Hum Reprod. 2015;30:2005–2008.
- Gat I, Shlush E, Quach K, et al. The continuum of high ovarian response: a rational approach to the management of high responder patient subgroups. Syst Biol Reprod Med. 2015;61:336–344.
- Teede H, Misso M, Costello M, et al. International evidence-based guideline for the assessment and management of polycystic ovary syndrome 2018. Melbourne: Monash University; 2018.
- Broekmans FJ, Verweij PJ, Eijkemans MJ, et al. Prognostic models for high and low ovarian responses in controlled ovarian stimulation using a GnRH antagonist protocol. Hum Reprod. 2014;29:1688–1697.
- Broer SL, Mol BW, Hendriks D, et al. The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril. 2009;91:705–714.
- Nastri CO, Teixeira DM, Moroni RM, et al. Ovarian hyperstimulation syndrome: pathophysiology, staging, prediction and prevention. Ultrasound Obstet Gynecol. 2015;45:377–393.
- Borth R, Lunenfeld B, De Watteville H. Day-to-day variation in urinary gonadotrophin and steroid levels during the normal menstrual cycle. Fertil Steril. 1957;8:233–254.
- Borth R, Lunenfeld B, Stamm O, et al. Gonadotrophin and steroid excretion in a case of twin pregnancy. Acta Obstet Gynecol Scand. 1959;38:417–423.
- Blankstein J, Shalev J, Saadon T, et al. Ovarian hyperstimulation syndrome: prediction by number and size of preovulatory ovarian follicles. Fertil Steril. 1987;47:597–602.
- Sunkara SK, Rittenberg V, Raine-Fenning N, et al. Nomogram for predicting live birth from egg number: an analysis of 400,135 IVF cycles. Hum Reprod. 2011;1:i34.
- Arce J-C, Klein BM, La Marca A. The rate of high ovarian response in women identified at risk by a high serum AMH level is influenced by the type of gonadotropin. Gynecol Endocrinol. 2014;30:444–450.
- Sunkara SK, La Marca A, Seed PT, et al. Increased risk of preterm birth and low birthweight with very high number of oocytes following IVF: an analysis of 65 868 singleton live birth outcomes. Hum Reprod. 2015;30:1473–1480.
- Griesinger G, Schultz L, Bauer T, et al. Ovarian hyperstimulation syndrome prevention by gonadotropin-releasing hormone agonist triggering of final oocyte maturation in a gonadotropin-releasing hormone antagonist protocol in combination with a “freeze-all” strategy: a prospective multicentric study. Fertil Steril. 2011;95:2029–2033, 2033e1.
- Herrero L, Pareja S, Losada C, et al. Avoiding the use of human chorionic gonadotropin combined with oocyte vitrification and GnRH agonist triggering versus coasting: a new strategy to avoid ovarian hyperstimulation syndrome. Fertil Steril. 2011;95:1137–1140.
- Garcia-Velasco JA. Agonist trigger: what is the best approach? Agonist trigger with vitrification of oocytes or embryos. Fertil Steril. 2012;97:527–528.
- Ferraretti AP, La Marca A, Fauser BC, et al. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26:1616–1624.
- Younis JS, Ben-Ami M, Ben-Shlomo I. The Bologna criteria for poor ovarian response: a contemporary critical appraisal. J Ovarian Res. 2015;8:76.
- Venetis CA. The Bologna criteria for poor ovarian response: the good, the bad and the way forward. Hum Reprod. 2014;29:1839–1841.
- Ferraretti AP, Gianaroli L. The Bologna criteria for the definition of poor ovarian responders: is there a need for revision? Hum Reprod. 2014;29:1842–1845.
- Papathanasiou A. Implementing the ESHRE 'poor responder' criteria in research studies: methodological implications. Hum Reprod. 2014;29:1835–1838.
- Lehert P, Chin W, Schertz J, et al. Predicting live birth for poor ovarian responders: the PROsPeR concept. Reprod Biomed Online. 2018;37:43–52.
- Polyzos NP, Sunkara SK. Reply: is it necessary to recognize the sub-optimal responder. Hum Reprod. 2015;30:2959.
- Alviggi C, Conforti A, Esteves SC, et al. Recombinant luteinizing hormone supplementation in assisted reproductive technology: a systematic review. Fertil Steril. 2018;109:644–664.
- Conforti A, Esteves SC, Di Rella F, et al. The role of recombinant LH in women with hypo-response to controlled ovarian stimulation: a systematic review and meta-analysis. Reprod Biol Endocrinol. 2019;17:18.
- Lehert P, Kolibianakis EM, Venetis CA, et al. Recombinant human follicle-stimulating hormone (r-hFSH) plus recombinant luteinizing hormone versus r-hFSH alone for ovarian stimulation during assisted reproductive technology: systematic review and meta-analysis. Reprod Biol Endocrinol. 2014;12:17.
- Mochtar MH, Danhof NA, Ayeleke RO, et al. Recombinant luteinizing hormone (rLH) and recombinant follicle stimulating hormone (rFSH) for ovarian stimulation in IVF/ICSI cycles. Cochrane Database Syst Rev. 2017;5:CD005070.
- Santi D, Casarini L, Alviggi C, et al. Efficacy of follicle-stimulating hormone (FSH) alone, FSH + luteinizing hormone, human menopausal gonadotropin or FSH + human chorionic gonadotropin on assisted reproductive technology outcomes in the “personalized” medicine era: a meta-analysis. Front Endocrinol (Lausanne). 2017;8:114.
- National Collaborating Centre for Women’s and Children’s Health. Fertility: assessment and treatment for people with fertility problems – NICE Clinical Guideline; 2013; [cited 2019 Mar 12]. London: NICE. Available from: https://www.nice.org.uk/guidance/cg156/evidence/full-guideline-pdf-188539453
- Yasmin E, Davies M, Conway G, et al. British Fertility Society. 'Ovulation induction in WHO Type 1 anovulation: guidelines for practice'. Produced on behalf of the BFS Policy and Practice Committee. Hum Fertil (Camb). 2013;16:228–234.
- Liu X, Tang C, Wen G, et al. The mechanism and pathways of dopamine and dopamine agonists in prolactinomas. Front Endocrinol (Lausanne). 2018;9:768.
- Cohlen B, Bijkerk A, Van der Poel S, et al. IUI: review and systematic assessment of the evidence that supports global recommendations. Hum Reprod Update. 2018;24:300–319.
- The Practice Committee of the American Society for Reproductive Medicine. Effectiveness and treatment for unexplained infertility. Fertil Steril. 2006;86:S111–S114.
- Collège National des Gynécologues et Obstétriciens Français (CNGOF). Extrait des mises à jour en gynécologie et obstétrique. J Gynecol Obstet Biol Reprod. 2010;39:S1–S342.
- Gerris J, Delvigne A, Dhont N, et al. Self-operated endovaginal telemonitoring versus traditional monitoring of ovarian stimulation in assisted reproduction: an RCT. Hum Reprod. 2014;29:1941–1948.
- Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2:230–243.
- Rigla M, Garcia-Saez G, Pons B, et al. Artificial intelligence methodologies and their application to diabetes. J Diabetes Sci Technol. 2018;12:303–310.
- Contreras I, Vehi J. Artificial intelligence for diabetes management and decision support: literature review. J Med Internet Res. 2018;20:e10775.
- Fazal MI, Patel ME, Tye J, et al. The past, present and future role of artificial intelligence in imaging. Eur J Radiol. 2018;105:246–250.
- Mayo RC, Leung J. Artificial intelligence and deep learning – radiology's next frontier? Clin Imaging. 2018;49:87–88.
- Schild W, Lunenfeld B, Gavish G. Computer-aided diagnosis with an application to endocrinology. IBM J Res Dev. 1978;22:518–532.
- Schoolcraft W, Meseguer M, Alliance GF. Paving the way for a gold standard of care for infertility treatment: improving outcomes through standardization of laboratory procedures. Reprod Biomed Online. 2017;35:391–399.
- VerMilyea MD, Tan L, Anthony JT, et al. Computer-automated time-lapse analysis results correlate with embryo implantation and clinical pregnancy: a blinded, multi-centre study. Reprod Biomed Online. 2014;29:729–736.
- Conaghan J, Chen AA, Willman SP, et al. Improving embryo selection using a computer-automated time-lapse image analysis test plus day 3 morphology: results from a prospective multicenter trial. Fertil Steril. 2013;100:412–419e5.
- Diamond MP, Suraj V, Behnke EJ, et al. Using the Eeva test adjunctively to traditional day 3 morphology is informative for consistent embryo assessment within a panel of embryologists with diverse experience. J Assist Reprod Genet. 2015;32:61–68.
- Adamson GD, Abusief ME, Palao L, et al. Improved implantation rates of day 3 embryo transfers with the use of an automated time-lapse-enabled test to aid in embryo selection. Fertil Steril. 2016;105:369–375e6.
- Aparicio-Ruiz B, Basile N, Perez Albala S, et al. Automatic time-lapse instrument is superior to single-point morphology observation for selecting viable embryos: retrospective study in oocyte donation. Fertil Steril. 2016;106:1379–1385e10.
- Chen AA, Tan L, Suraj V, et al. Biomarkers identified with time-lapse imaging: discovery, validation, and practical application. Fertil Steril. 2013;99:1035–1043.
- Simopoulou M, Sfakianoudis K, Maziotis E, et al. Are computational applications the “crystal ball” in the IVF laboratory? The evolution from mathematics to artificial intelligence. J Assist Reprod Genet. 2018;35:1545.
- Arvis P, Lehert P, Guivarc'h-Leveque A. Simple adaptations to the Templeton model for IVF outcome prediction make it current and clinically useful. Hum Reprod. 2012;27:2971–2978.
- Zhang Z, Dai C, Huang JY, et al. Robotic immobilization of motile sperm for clinical intracytoplasmic sperm injection. IEEE Trans Biomed Eng. 2018;66:444–452.
- Roy TK, Brandi S, Peura TT, Chapter 20 Gavi-automated vitrification instrument. Methods Mol Biol. 2017;1568:261–277.

