Abstract
Background
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder with complex pathophysiological mechanism. It is reported that even a modest weight loss of 5–10% substantially may improve the reproductive and metabolic profile. This study aims to assess the efficacy of the low dose of liraglutide (0.6 mg QD) combined with metformin (0.85 mg BID) in weight loss in Chinese Han women with PCOS.
Methods
We included clinical data of 102 obese/overweight (≥18 years, body mass index ≥28 kg/m2 or ≥24 kg/m2) women who were diagnosed with PCOS from October 2016 to March 2018 in Wuhan Union Hospital initially. They were treated with dinae-35, low dose of liraglutide (0.6 mg QD) and metformin (0.85 mg BID) for 12 weeks. The demographic and clinical data were retrieved retrospectively, and weight loss was the main outcome measure. Student’s paired t-test and Wilcoxon rank sum test were used to compare the differences before and after therapy, p < 0.05 was considered statistically significant.
Results
Participants(n = 102)had lost a mean of 7.20 ± 3.42 kg of body weight (95%CI: 6.55–7.86, p < 0.001), and the mean reduction of BMI was 2.87 ± 1.36 kg/m2 (95%CI: 0.02–0.27, p < 0.001). A total of 88.24% of participants lost more than 5% of their body weight.
Conclusion
The combination of low dose of liraglutide and metformin was associated with significant reduction of body weight in Chinese Han women with PCOS. Additionally, a larger randomized double-blind multicenter controlled clinical trial is needed to confirm that.
Trial registration
The study was registered on http://www.chictr.org.cn as ChiCTR1900024384.
Plain English summary
Polycystic ovary syndrome (PCOS) is a common endocrine disorder, which is characterized by chronic anovulation and hyperandrogenism, among women of reproductive age. Obesity is one of the pathophysiological principles of PCOS, and excess free fatty acids are related with insulin resistance and hyperandrogenemia. And even 5–10% weight loss may improve reproductive and metabolic disorders. Liraglutide, a long acting glucagon-like peptide-1 analogue, is approved by the FDA for weight management, and some researches show there is a synergy between metformin and liraglutide. But for Chinese Han women with PCOS, the dose and effect of combination of metformin and liraglutide in weight loss are worthy of further investigation. In this study, the data of 102 patients in Wuhan Union Hospital who were diagnosed with PCOS were retrospectively analyzed. And even a low dose of liraglutide (0.6 mg QD) combined with metformin (0.85 g BID) resulted in a significant weight loss.
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder with psychological, reproductive and metabolic features [Citation1], which prevalence is about 8%–13% in reproductive-aged women [Citation2,Citation3]. According to Rotterdam criteria, PCOS can be diagnosed with two of three features: clinical or biochemical hyperandrogenism, ovulatory dysfunction, and polycystic ovaries on ultrasound [Citation4]. Women with PCOS usually present different clinical manifestations including metabolic disorders [Citation5], reproductive dysfunctions [Citation6] and psychological features [Citation7,Citation8].
The majority of women with PCOS are overweight or obese. It is reported that obesity affect fertility by many processes, such as mitochondrial dynamics derangements, disrupted meiosis, impairment of ovarian follicles development [Citation5]. Moreover, obesity may reduce the utilization of glucose by peripheral tissues and aggravating insulin resistance [Citation9]. The excess insulin promotes androgen production in ovarian theca cells as a response to luteinizing hormone stimulation, and result in follicular arrest and anovulation [Citation10]. Otherwise, it is closely related to nonalcoholic fatty liver disease, atherosclerosis, cardiovascular metabolic risks, and other diseases. Consequently, losing weight and ameliorating insulin resistance are essential in the treatment of PCOS.
Even a modest weight loss of 5–10% may improve reproductive and metabolic profile [Citation5]. Nowadays, ways to lose weight include lifestyle interventions, medication, and bariatric surgery. Lifestyle interventions is the first line of treatments but is not sustainable in daily life as many women usually regain lost weight because of poor compliance [Citation11,Citation12]. Bariatric surgery is an effective way and usually reserved for patients with a body mass index (BMI) >40 kg/m2 or with BMI >35 kg/m2 and one or more significant comorbid conditions, when nonsurgical methods of weight loss have failed. But bariatric surgery may cause nutritional deficiencies and other postoperative complications [Citation13]. As for pharmacological interventions, metformin is an established treatment for PCOS with good safety and toleration. As an insulin sensitizer, it can not only improve insulin resistance significantly, but also has effects on menstrual disorders, anovulation, metabolic, and cardiovascular abnormalities [Citation14,Citation15]. However, some meta-analysises show its effect on weight loss with lifestyle changes always unsatisfactory [Citation16].
Liraglutide, known as a long-acting glucagon-like peptide-1 (GLP-1) analogue, can improve glucose homeostasis by increasing the endogenous secretion of insulin that is induced by glucose ingestion and inhibiting glucagon secretion [Citation17]. In addition, liraglutide can reduce body weight via delaying gastric emptying and inhibiting appetite [Citation18]. It is proved that liraglutide has significant and sustained effect on weight loss in overweight people with or without diabetes [Citation19]. As an anti-diabetic therapy is approved at doses up to 1.8 mg [Citation20], while higher doses are required for weight loss in many countries [Citation19, Citation21]. However, higher doses usually linked potential higher frequency of gastrointestinal side effects like nausea and dyspepsia [Citation22,Citation23].
It is reported metformin combined with liraglutide could enhance weight-lowering capacity. And we wonder whether the combination of metformin (0.85 mg BID) and a lower dose liraglutide (0.6 mg QD) could get weight loss satisfactorily with fewer side effects in Chinese Han women with PCOS. At the same time, Dinae-35 was used to regulate menstrual cycles for all the participants.
Materials and methods
The study population included 102 overweight or obese women who were initially diagnosed with PCOS between October 2016 and March 2018 in Wuhan Union Hospital ().
According to the guidelines for prevention and control of overweight and obesity in Chinese adults, we define overweight as 24 ≤ BMI < 28 kg/m2 and obesity as BMI ≥ 28 kg/m2. The diagnosis of PCOS was made based on the 2003 Rotterdam criteria with the presence of at least two of the following features: ovulatory dysfunction, clinical and/or biochemical hyperandrogenism and polycystic ovaries on ultrasound (ultrasound shows that the number of follicles with diameters of 2–9 mm on one side or both sides is equal or greater than 12). We excluded women who were diagnosed with thyroid disease, hyperprolactinemia, congenital adrenal hyperplasia, Cushing’s syndrome and androgenic tumor. Furthermore, we excluded pregnant or lactating woman and the patients who had angiocardiopathy, severe liver disease and kidney dysfunction. And people who used medicines that affect glucose and lipid metabolism recently were not included.
Participants started with liraglutide 0.6 mg once daily and metformin 0.85 g twice a day. And dinae-35 was prescribed that taking one pill per day for 21 days, and then stopping for 7 days in every menstrual cycle. All patients were treated for 12 weeks.
At baseline and after treatment for 12 weeks, all patients took measurements after an overnight fast for demographic and clinical profile between the second and fifth day of a spontaneous menstrual cycle. The height, weight and waist-hip ratio were measured with light clothes and no shoes. BMI is equal to weight(kg) by the square of the height(m). And visceral fat area and percentage of body fat were measured with bioelectrical impedance analysis. Besides, their blood samples were collected for determination of sex hormone, glucose and lipid metabolism, including follicle-stimulating hormone (FSH), luteinizing hormone (LH), prolactin (PRL), total testosterone(TT), free testosterone (FT), dehydroepiandrosterone (DHEA), sex hormone binding globulin (SHBG), androstenedione, HbA1c, total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), apolipoprotein A1 and apolipoprotein B. The free androgen index (FAI) is calculated as TT(nmol/L)×100/SHBG(nmol/L), we take 0.7–6.4 as the normal range of FAI [Citation24]. Then they did a standard 75 g oral glucose tolerance test (OGTT) and got glucose and insulin concentration at 0/30/60/120/180 min. Insulin resistance was estimated by HOMA-IR score, which was calculated as (fasting glucose [mmol/L] × fasting insulin [mIU/L]/22.5). And HOMA-IR ≥ 2.69 was the cut off for abnormal values [Citation25]. As for safety parameters, we concerned about markers of inflammation, hepatic and renal functions like C-Reactive Protein (CRP), aspartate transaminase (AST), alanine transaminase (ALT), blood urea nitrogen (BUN), serum creatinine (SCR).
Normal data distribution was checked by the Shapiro-Wilk test. We used Student’s paired t-test to compare the differences before and after therapy for normally distributed data, and Wilcoxon rank sum test for non-normal distributed data. The parameters are presented as mean (SD) or median (quartiles), p < 0.05 was considered statistically significant. SPSS 23.0 software package was used to do the data analysis.
Result
Weight loss
Mean age of the 102 patients was 25.83 ± 4.20 years. They had a mean body weight at baseline of 70.75 ± 9.09 kg, and a mean BMI at baseline of 28.03 ± 3.18 kg/m2 (). And 57 of them (55.88%) were overweight, while 45 (44.12%) of them were obese. After therapy for 12 weeks, we observed significant effects on weight change (). The mean weight loss was 7.20 ± 3.42 kg (95%CI: 6.55–7.86, p < 0.001), and the mean reduction of BMI was 2.87 ± 1.36 kg/m2 (95%CI: 0.02–0.27, p < 0.001). Furthermore, there were 19 (18.63%) of them got BMI less than 24, and 41(40.20%) of them were overweight, while 42 (41.18%) of them were still obese. And no one got a higher BMI. More specifically, 90 (88.24%) women lost more than 5% of their baseline of weight, 51 (50.00%) women lost more than 10%, and even 3 (2.94%) women lost more than 20%. The average percentage of weight loss was 10.24%. In general, the majority of people lost 5%–15% of their baseline body weight. Only 1 person gained 1.1 kg in weight, but she had grown by 2 cm, that her BMI decreased 0.2 kg/m2.
Figure 2. Body weight change at baseline and after therapy.
Panel A shows the mean body weight at baseline and after therapy of those patients. Panel B shows the frequency of patients whose weight changed in [−25%, −20%), [−20%, −15%), [−15%, −10%), [−10%, −5%), [−5%, 0), [0, 5%) compared with their baseline. As we can see, almost people got at least 5% weight loss, and a majority of patients had a weight change in −5% to −15%. Panel C shows the cumulative percentage of patients with the change of body weight after 3 months of treatment.
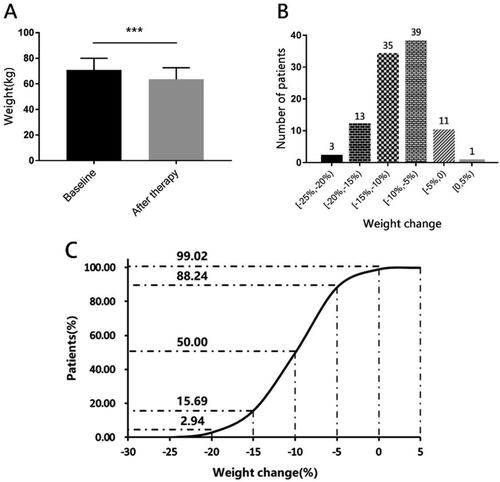
Table 1. Characteristics at baseline and after therapy of low dose of liraglutide combined with metformin for 12 weeks.
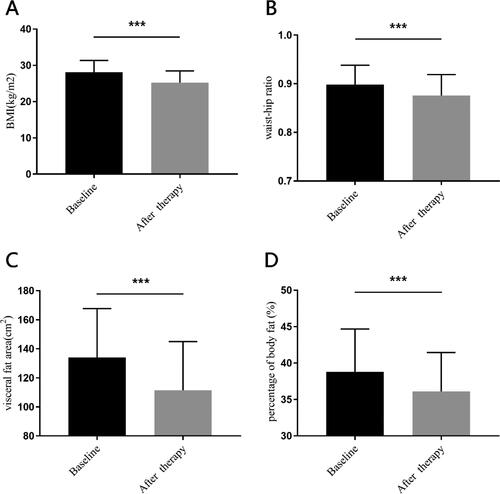
Beyond that, we also found there were significant reductions in waist-hip ratio, visceral fat area and percentage of body fat. The mean reduction of waist-hip ratio was 0.02(0.03) (95%CI: 2.61–3.14, p < 0.001), while the mean reduction of visceral fat area was 24.64(14.88) cm2 (95%CI: 21.70–27.58, p < 0.001), and the mean decrease of body fat decreased was 3.29(3.25) % (95%CI: 2.64–3.93, p < 0.001) ().
Figure 3. The other characteristics change at baseline and after therapy.
Panel A shows body mass index (BMI). Panel B shows waist-hip ratio. Panel C shows visceral fat area. Panel D shows percentage of body fat. All the differences of parameters between baseline and after therapy were analyzed by Student’s paired t-test. ***for p < 0.001.
Endocrine changes
As for endocrine, the total testosterone, free testosterone, androstenedione and FAI decreased significantly after therapy (p < 0.05) (). LH decreased from 10.56(5.99) IU/L to 6.99(3.27) IU/L (p < 0.001), and the LH/FSH decreased from 1.74(0.90) to 1.10(0.53). There were 40 women with LH/FSH ≥ 2 at baseline, after treatment there was only one patient got LH/FSH ≥ 2. Furthermore, PRL and SHBG increased obviously, while FSH and DHEA had no significant change.
Table 2. Endocrine parameters at baseline and after therapy for 12 weeks.
Metabolic changes
As for the serum lipid metabolism, the HDL-C and ApoA1 had improved significantly () [1.27(0.44) mmol/L to 1.40(0.36) mmol/L, p < 0.001; 1.15(0.24) mmol/L to 1.45(0.26) mmol/L, p < 0.001, respectively]. And total cholesterol, triglycerides, LDL-C and ApoB had no significant change. We considered the LDL/HDL ratio and TC/HDL ratio as the parameters for cardiovascular disease risk, and they both had significant decreases [2.33(0.90) to 2.05(0.65), p < 0.001; 3.96(1.16) to 1.27(0.44), p < 0.001, respectively].
Table 3. Metabolic parameters at baseline and after therapy for 12 weeks.
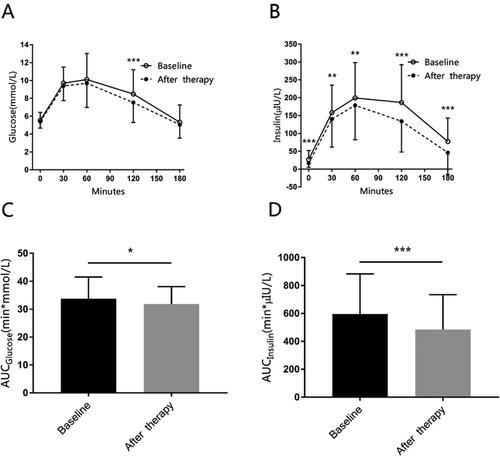
About glucose metabolism, when we collated the data of OGTT, we found there was one patient’s result of OGTT missed. We speculated the side effect of OGTT like nausea and vomiting might be the reason why the patient did not do the test. For the rest 101 patients, the glucose in 120 min during OGTT, fasting insulin, and insulin during OGTT are statistically significant (). At baseline, 83 women were insulin resistant because their HOMA-IR score ≥2.69. After therapy, 17 of them (20.48%) had improved their insulin resistant, for HOMA-IR score <2.69. And the mean of HbA1c for all patients decreased from 5.30(0.55) % to 4.93(0.33) %.
Figure 4. The mean glucose, insulin concentration during OGTT at baseline and after therapy.
Panel A shows glucose concentrations during oral glucose tolerance test (OGTT). Panel B shows insulin concentrations during OGTT. Panel C shows the area under the curve (AUC) of glucose during OGTT. Panel D shows the AUC of insulin during OGTT. The data are presented as means ± SD, and calculated with Student’s paired T-test. *for p < 0.05, ** for p < 0.01, *** for p < 0.001.
Side effects
During the therapy, no one got hypoglycemia reaction. A few patients had mild transitory headache or gastrointestinal reactions, such as diarrhea and nausea. And these symptoms usually happened at the first 4 weeks and alleviated voluntarily. Nobody dropped out because of these mild symptoms. The parameters of hepatic function had no significant change as a whole, only one patient had aspartate transaminase increased fivefold on the 12th week. C-reactive protein of few patients had increased after 12 weeks of treatment, but we cannot rule out other reasons.
Discussion
The pathophysiological mechanism of PCOS is complex and has not yet been elucidated. Obesity, insulin resistance and hyperinsulinemia are considered to be closely related to PCOS. Most women with PCOS are overweight or obese, and obesity plays a key role in the pathophysiological mechanisms of insulin resistance and hyperandrogenism. Too much adipose tissue may cause insulin resistance even hyperinsulinemia and hyperandrogenism, and these will result in acne, hirsutism, menstrual irregularity and infertility. In the other hand, overweight and obesity increase the risks of type II diabetes, coronary heart disease and other metabolic diseases in the long term. Metformin is commonly used in the treatment of PCOS, for reducing insulin resistance and ovulation induction, but its effect on weight loss is often unsatisfactory.
Liraglutide, as an analog of the incretin hormone glucagon-like peptide 1 (GLP-1), has 97% homology with human GLP-1. It is widely used in type 2 diabetes to help control glucose level [Citation18,Citation19]. Moreover, liraglutide can reduce appetite and caloric intake so it was approved for weight management in many countries. As we known, the maximum dose of liraglutide for weight loss is 3.0 mg QD. In 2015, Davies MJ et al. [Citation22] found 3.0 mg of once-daily liraglutide was more effective in weight loss than 1.8 mg once-daily liraglutide, and improved patients’ quality of life significantly. During 56 weeks, among overweight and obese participants with type 2 diabetes, the weight loss was 6.0% (6.4 kg) with liraglutide (3.0 mg dose), while 4.7% (5.0 kg) with liraglutide (1.8 mg dose). But patients used liraglutide (3.0 mg) had gastrointestinal disorders more frequently.
There were other studies evaluated the effect of the combination of metformin and liraglutide. Jensterle Sever et al. [Citation26] reported that in a 12 weeks study of 36 obese women with PCOS, the mean weight loss was 6.5 kg with liraglutide 1.2 mg plus metformin, 3.8 kg with liraglutide 1.2 mg alone and 1.2 kg with metformin monotherapy. And no one in the metformin group lost 5% of their initial weight. Rasmussen CB [Citation27] reported that 84 overweight or obese women with PCOS treated with liraglutide and metformin at least 4 weeks. The dose of liraglutide was started at 0.6 mg and increased to 1.2 mg or 1.8 mg QD finally if there were no effect on weight and no side effect. And the dose of metformin was given due to insulin resistance. They found a mean weight loss of 9.0 kg and a mean decrease in BMI of 3.2 kg/m2. The proportion of women who lost more than 5% of their baseline was 81.7%.
In the present study, a short-term combination therapy with liraglutide 0.6 mg QD and metformin 0.85 g BID in overweight or obese women with PCOS result in a mean weight loss of 7.25(3.48) kg and a reduction of BMI from 28.13(3.24) to 25.24(3.21) kg/m2. And 88.24% participants lost at least 5% of their initial body weight. And no one dropped out because of gastrointestinal symptoms or other side effects. Our results are in accord with these studies in some ways. It seems that a lower dose of liraglutide (0.6 mg) combined with metformin has better effect and less side reactions in weight loss in Chinese Han women. And we suppose there might be two explanations for this result. On the one hand, many studies proved metformin might not only enhance the secretion of GLP-1 by regulating multiple components of the incretin axis [Citation28,Citation29], but also inhibit the activity of DPP-4 thus increase the active GLP-1 concentrations [Citation30,Citation31]. And on the other hand, we suspect that the Chinese Han women may be more sensitive to liraglutide. Therefore, metformin can enhance GLP-1 effect of liraglutide and make a lower dose of liraglutide more efficiently in weight loss.
Otherwise, Rocha et al. [Citation32] found that patients with PCOS usually have abnormal lipid metabolism, and their incidence is twice than general population. The incidence of the reduction of HDL (57.60%) and the increase of TG (28.30%) are the most common. Wing RR et al. [Citation33] showed that weight loss can reduce the risk of cardiovascular disease by improving lipid metabolism and blood pressure. The results of our study suggested there were significant improvements in HDL, ApoA1, LDL/HDL and TC/HDL ratio, while the changes of total cholesterol, TG, LDL, and ApoB were not statistically significant. Combined the obvious reduction in mean of weight loss, the combination of liraglutide and metformin might reduce the risks of cardiovascular disease in overweight or obese women with PCOS.
However, the present study has some limitations, including possible selection bias, recall bias, and the ignorance of influence of lifestyle on weight loss. More large randomized double-blind multicenter controlled clinical trials in overweight and obese Chinese Han women with PCOS are needed to assess the optimal dose of liraglutide in weight loss. And we need a longer follow-up period to observe how long the effect of weight loss would last and the reproductive outcome after this treatment.
Conclusions
Above all, in this study, our data support the effect of the combination of a low dose liraglutide (0.6 mg QD) and metformin (0.85 g BID) as weight loss medication in Chinese Han women with PCOS.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology.
| Abbreviations | ||
| PCOS | = | Polycystic ovary syndrome |
| BMI | = | Body Mass Index |
| GLP-1 | = | glucagon-like peptide-1 |
| QD | = | quaque die |
| BID | = | bis in die |
| FSH | = | follicle-stimulating hormone |
| LH | = | luteinizing hormone |
| PRL | = | prolactin |
| TT | = | total testosterone |
| FT | = | total testosterone |
| DHEA | = | dehydroepiandrosterone |
| SHBG | = | sex hormone binding globulin |
| TC | = | total cholesterol |
| TG | = | triglycerides |
| LDL-C | = | low-density lipoprotein cholesterol |
| HDL-C | = | high-density lipoprotein cholesterol |
| FAI | = | free androgen index |
| OGTT | = | oral glucose tolerance test |
| HOMA-IR | = | homeostasis model assessment of insulin resistance |
| CRP | = | C-Reactive Protein |
| AST | = | aspartate transaminase |
| ALT | = | alanine transaminase |
| BUN | = | blood urea nitrogen |
| SCR | = | serum creatinine |
| SD | = | standard deviation |
| ApoA1 | = | apolipoprotein A1 |
| ApoB | = | apolipoprotein B |
| AUC | = | area under the curve |
Availability of data and materials
The data that support the findings of this study are openly available in [ResMan] at [http://www.medresman.org], reference number [ChiCTR1900024384].
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):1–7. doi: 10.1038/nrendo.2018.24.
- Li R, Zhang Q, Yang D, et al. Prevalence of polycystic ovary syndrome in women in China: a large community-based study. Hum Reprod. 2013;28(9):2562–2569. doi: 10.1093/humrep/det262.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841–2855. doi: 10.1093/humrep/dew218.
- Joham AE, Norman RJ, Stener-Victorin E, et al. Polycystic ovary syndrome. Lancet Diabet Endocrinol. 2022;10(9):668–680. doi: 10.1016/S2213-8587(22)00163-2.
- Glueck CJ, Goldenberg N. Characteristics of obesity in polycystic ovary syndrome: etiology, treatment, and genetics. Metabolism. 2019;92:108–120. doi: 10.1016/j.metabol.2018.11.002.
- Palomba S, Piltonen TT, Giudice LC. Endometrial function in women with polycystic ovary syndrome: a comprehensive review. Hum Reprod Update. 2021;27(3):584–618. doi: 10.1093/humupd/dmaa051.
- Moran L, Gibson-Helm M, Teede H, et al. Polycystic ovary syndrome: a biopsychosocial understanding in young women to improve knowledge and treatment options. J Psychosom Obstet Gynaecol. 2010;31(1):24–31. doi: 10.3109/01674820903477593.
- Jiskoot G, van der Kooi AL, Busschbach J, et al. Cognitive behavioural therapy for depression in women with PCOS: systematic review and meta-analysis. Reproduct Biomed Online. 2022;45(3):599–607. doi: 10.1016/j.rbmo.2022.05.001.
- Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6(1):231.
- Moghetti P, Tosi F. Insulin resistance and PCOS: chicken or egg? J Endocrinol Invest. 2021;44(2):233–244. doi: 10.1007/s40618-020-01351-0.
- Gu Y, Zhou G, Zhou F, et al. Life modifications and PCOS: old story but new tales. Front Endocrinol. 2022;13:808898. doi: 10.3389/fendo.2022.808898.
- Moran LJ, Hutchison SK, Norman RJ, et al. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011;(7):CD007506.
- Nuzzo A, Czernichow S, Hertig A, et al. Prevention and treatment of nutritional complications after bariatric surgery. Lancet Gastroenterol Hepatol. 2021;6(3):238–251. doi: 10.1016/S2468-1253(20)30331-9.
- Tso LO, Costello MF, Albuquerque LET, et al. Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2020;12(12):CD006105. doi: 10.1002/14651858.CD006105.pub4.
- Garzia E, Galiano V, Marfia G, et al. Hyperandrogenism and menstrual imbalance are the best predictors of metformin response in PCOS patients. Reprod Biol Endocrinol. 2022;20(1):6. doi: 10.1186/s12958-021-00876-0.
- Harborne LR, Sattar N, Norman JE, et al. Metformin and weight loss in obese women with polycystic ovary syndrome: comparison of doses. J Clin Endocrinol Metab. 2005;90(8):4593–4598. doi: 10.1210/jc.2004-2283.
- Knudsen LB, Lau J. The discovery and development of liraglutide and semaglutide. Front Endocrinol (Lausanne). 2019;10:155. doi: 10.3389/fendo.2019.00155.
- van Can J, Sloth B, Jensen CB, et al. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond). 2014;38(6):784–793. doi: 10.1038/ijo.2013.162.
- Vilsboll T, Christensen M, Junker AE, et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771. doi: 10.1136/bmj.d7771.
- Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1-5 studies. Diabetes Obes Metab. 2009;11(Suppl 3):26–34. doi: 10.1111/j.1463-1326.2009.01075.x.
- Lundgren JR, Janus C, Jensen SBK, et al. Healthy weight loss maintenance with exercise, liraglutide, or both combined. N Engl J Med. 2021;384(18):1719–1730. doi: 10.1056/NEJMoa2028198.
- Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687–699. doi: 10.1001/jama.2015.9676.
- He L, Wang J, Ping F, et al. Association of Glucagon-Like peptide-1 receptor agonist use with risk of gallbladder and biliary diseases: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2022;182(5):513–519. doi: 10.1001/jamainternmed.2022.0338.
- Zhou Z, Ni R, Hong Y, et al. Defining hyperandrogenaemia according to the free androgen index in chinese women: a cross-sectional study. Clin Endocrinol (Oxf). 2012;77(3):446–452. doi: 10.1111/j.1365-2265.2012.04395.x.
- Xing XY, Yang WY. The diagnostic significance of homeostasis model assessment of insulin resistance in metabolic syndrome among subjects with different glucose tolerance. Chinese J Diabet. 2004;12(3):182–186.
- Jensterle Sever M, Kocjan T, Pfeifer M, et al. Short-term combined treatment with liraglutide and metformin leads to significant weight loss in obese women with polycystic ovary syndrome and previous poor response to metformin. Eur J Endocrinol. 2014;170(3):451–459. doi: 10.1530/EJE-13-0797.
- Rasmussen CB, Lindenberg S. The effect of liraglutide on weight loss in women with polycystic ovary syndrome: an observational study. Front Endocrinol (Lausanne). 2014;5:140. doi: 10.3389/fendo.2014.00140.
- Kappe C, Patrone C, Holst JJ, et al. Metformin protects against lipoapoptosis and enhances GLP-1 secretion from GLP-1-producing cells. J Gastroenterol. 2013;48(3):322–332. doi: 10.1007/s00535-012-0637-5.
- Maida A, Lamont BJ, Cao X, et al. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-α in mice. Diabetologia. 2011;54(2):339–349. doi: 10.1007/s00125-010-1937-z.
- Cuthbertson J, Patterson S, O'Harte FP, et al. Addition of metformin to exogenous glucagon-like peptide-1 results in increased serum glucagon-like peptide-1 concentrations and greater glucose lowering in type 2 diabetes mellitus. Metabolism. 2011;60(1):52–56. doi: 10.1016/j.metabol.2010.01.001.
- Green BD, Irwin N, Duffy NA, et al. Inhibition of dipeptidyl peptidase-IV activity by metformin enhances the antidiabetic effects of glucagon-like peptide-1. Eur J Pharmacol. 2006;547(1–3):192–199. doi: 10.1016/j.ejphar.2006.07.043.
- Rocha MP, Marcondes JA, Barcellos CR, et al. Dyslipidemia in women with polycystic ovary syndrome: incidence, pattern and predictors. Gynecol Endocrinol. 2011;27(10):814–819. doi: 10.3109/09513590.2010.508852.
- Wing RR, Tate DF, Garcia KR, et al. Improvements in cardiovascular risk factors in young adults in a randomized trial of approaches to weight gain prevention. Obesity (Silver Spring). 2017;25(10):1660–1666. doi: 10.1002/oby.21917.