Abstract
Objective
To investigate the target and mechanism of action of Bushen Huoxue Recipe (BSHX) for the treatment of infertility in polycystic ovary syndrome (PCOS), to provide a basis for the development and clinical application of herbal compounds.
Methods
Prediction and validation of active ingredients and targets of BSHX for the treatment of PCOS by using network pharmacology-molecular docking technology. In an animal experiment, the rats were randomly divided into four groups (control group, model group, BSHX group, metformin group, n = 16 in each group), and letrozole combined with high-fat emulsion gavage was used to establish a PCOS rat model. Body weight, vaginal smears, and number of embryos were recorded for each group of rats. Hematoxylin-eosin (HE) staining was used to observe the morphological changes of ovarian and endometrial tissues, and an enzyme-linked immunosorbent assay (ELISA) was used to detect the serum inflammatory factor levels. Expression levels of transforming growth factor-β (TGF-β), transforming growth factor beta activated kinase 1 (TAK1), nuclear factor kappa-B (NF-κB), Vimentin, and E-cadherin proteins were measured by western blot (WB).
Results
Ninety active pharmaceutical ingredients were obtained from BSHX, involving 201 protein targets, of which 160 were potential therapeutic targets. The active ingredients of BSHX exhibited lower binding energy with tumor necrosis factor-α (TNF-α), TGF-β, TAK1, and NF-κB protein receptors (< −5.0 kcal/mol). BSHX significantly reduced serum TNF-α levels in PCOS rats (p < .01), effectively regulated the estrous cycle, restored the pathological changes in the ovary and endometrium, improved the pregnancy rate, and increased the number of embryos. The results of WB suggested that BSHX can down-regulate protein expression levels of TGF-β and NF-κB in endometrial tissue (p < .05), promote the expression level of E-cadherin protein (p < .001), intervene in the endometrial epithelial-mesenchymal transition (EMT) process.
Conclusions
TGF-β, TAK1, NF-κB, and TNF-α are important targets of BSHX for treating infertility in PCOS. BSHX improves the inflammatory state of PCOS, intervenes in the endometrial EMT process through the TGF-β/NF-κB pathway, and restores endometrial pathological changes, further improving the pregnancy outcome in PCOS.
Introduction
Polycystic ovary syndrome (PCOS) is a reproductive endocrine disorder common in gynecology, with a prevalence of 6–20% in premenopausal women [Citation1]. The pathogenesis of PCOS remains unclear, and the long course of the disease makes treatment ineffective, which is the focus and difficulty of research in the field of gynecological endocrinology. Infertility is one of the main features of PCOS. An endometrial component has been suggested to contribute to infertility and poor reproductive outcomes in PCOS women. Clinical studies have shown that when an ideal blastocyst is transferred in vitro, transplant success and live birth rates are lower in patients with polycystic disease than in healthy controls [Citation2]. However, the use of oocytes donated by women with PCOS in assisted reproductive technology (ART) does not reduce the overall success rate [Citation3]. Patients with PCOS infertility have reduced endometrial receptivity. "Endometrial receptivity" refers to the ability of the endometrium to accept embryos for implantation and occurs between days 22 and 24 of the menstrual cycle, a short period known as the "window of implantation" (WOI). This period belongs to the middle and late stages of secretion and is an important period for the endometrium to undergo metamorphosis, which is key to embryo implantation. However, endometrial epithelial-mesenchymal transition (EMT) interferes with endometrial mesenchymal cell ecdysis in PCOS, leading to the downregulation of epithelial cell characteristic proteins, acquisition of mesenchymal cell phenotype and behavior, and inhibition of endometrial stromal cell conversion from mesenchymal-like to epithelial-like, thereby reducing endometrial tolerance. Therefore, endometrial EMT affects embryo implantation and survival, resulting in difficulty conceiving in PCOS patients even after ovulation has resumed.
In recent centuries, Chinese medicine has been used to treat infertility caused by PCOS. The Chinese medicine master's combined theory and practice took "kidney deficiency and blood stasis" as the fundamental pathogenesis of infertility caused by PCOS. They used the treatment principle of "tonifying the kidney and activating blood" to achieve satisfactory results in clinical application. Studies have reported that herbs that tonify the kidneys and invigorate blood can achieve ovulation by regulating sex hormones and local ovarian factors, increasing the expression of endometrial integrin αVβ3 during bedtime, improving endometrial tolerance, and reducing early pregnancy loss [Citation4,Citation5]. The clinical use of the Bushen Huoxue Recipe (BSHX) increases pregnancy rates and improves pregnancy outcomes in PCOS patients. BSHX is composed of Traditional Chinese Medicine Classic Formulas Guizhi Fuling Wan and Shoutai Wan with addition and reduction, which modify the kidney and strengthen the fetus. Guizhi Fuling Wan originated from "Synopsis of the Golden Chamber", which has the power to activate blood circulation to dissipate blood stasis and eliminate dampness and diuresis. Modern findings have shown that Guizhi Fuling Wan can effectively improve the clinical symptoms of PCOS by regulating endocrine levels, correcting insulin resistance, and suppressing inflammatory responses [Citation6]. Shoutai Wan is from "Records of Traditional Chinese and Western Medicine in Combination", which has the effect of nourishing the kidney and replenishing blood, fixing the kidney, and calming the fetus, and is a classic formula for kidney deficiency and fixing the fetus. As previously shown [Citation7,Citation8], Guizhi Fuling Wan can activate the granulosa fine PI3K/Akt/mTOR signaling pathway, down-regulate the protein expression of Beclin1 and LC3II/I, inhibit excessive autophagy in granulosa cells, improve ovarian function and insulin resistance and restore ovulation. In addition, Guizhi Fuling Wan can improve reproductive endocrine disorders in PCOS model rats by inhibiting the secretion of inflammatory factors and remodeling intestinal homeostasis. Currently, however, there is a lack of research on BSHX for PCOS.
Since the treatment of PCOS infertility is mainly focused on ameliorating hyperandrogenemia and ovulation disorders, various adverse effects have been encountered with the currently popular pharmacological treatments [Citation9–11]. Therefore, the search for an effective multi-targeted therapeutic modality with low side effects is imminent. In recent years, the "network pharmacology" drug design method based on systems biology has received great attention from researchers [Citation12]. Network pharmacology, as a drug research methodology, integrates the multiple advantages of pharmacology and biology, and features a drug-component target-pathway network model to provide a research methodology for drug-disease linkage, reflecting the relationship between the action of drugs with multiple targets and pathways [Citation13]. Various traditional herbs, such as Tephrosia purpurea for the treatment of PCOS [Citation14] have been explored by using this approach, which provides a mechanic-based understanding of traditional knowledge and a new approach for the discovery of active compounds in the drug. Although BSHX is effective for treating PCOS-mediated infertility in clinical practice, its composition and mechanism of action have not been fully elucidated. Therefore, this study combined network pharmacology and animal experiments to investigate the mechanism of action of BSHX in the treatment of PCOS.
Materials and methods
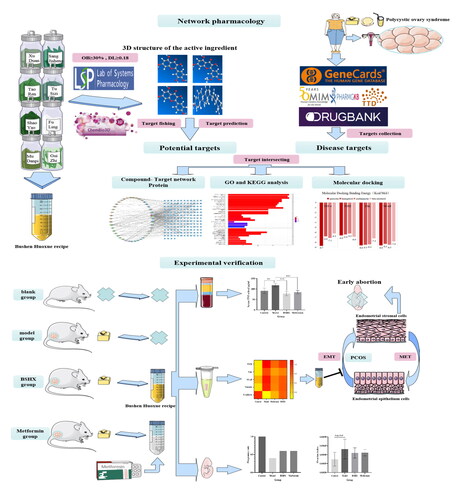
Materials and methods of network pharmacology and molecular docking technology
Establishment of the active ingredient library of the Bushen Huoxue Recipe (BSHX)
The eight herbal ingredients (Cinnamomum cassia(L.) J. Presl, Poria cocos(Schw.) Wolf, Paeonia suffruticosa Andr, Paeonia lactiflora Pall, Prunus persica(L.) Batsch, Cuscuta australis R.Br, Taxillus chinensis(DC.) Danser, and Dipsacus asper walls. ex Henry) of the BSHX were searched in the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, https://tcmsp-e.com/) as keywords, and the related targets were screened and predicted based on the oral bioavailability[Citation15] (OB ≥30%) and drug-like [Citation16] (DL ≥0.18). After obtaining the target information of the active ingredient, the target was converted into a gene symbol, and the gene corresponding to the target protein was determined using the Uni Prot (https://www.uniprot.org/) database.
Screening for polycystic ovary syndrome (PCOS) disease targets
Using "Polycystic ovary syndrome" as the keyword, the differential genes for PCOS were obtained from GeneCards (https://www.genecards.org/), Online Mendelian Inheritance in Man (OMIM, https://mirror.omim.org/), DrugBank (https://www.drugbank.com/), Pharmacogenetics and Pharmacogenomics Knowledge Base (PharmGkB, https://www.pharmgkb.org/) and Therapeutic Target Database (TTD, https://db.idrblab.net/ttd/) databases. Duplicate values were removed after combining the obtained genes to obtain disease targets related to PCOS.
Construction of drug-compound-target network diagram
The Cytoscape 3.9.1 (https://cytoscape.org/download.html) software was applied to construct a drug-compound-target network of the active compounds of BSHX with the above-predicted targets, and the three key topological parameters of degree, betweenness, and closeness were used to screen key active ingredients.
Protein-protein interaction (PPI) network construction
The intersection of the active ingredient targets and PCOS disease targets was taken, and the intersection was entered into the STRING database (https://string-db.org/), the protein species was set to "Homo sapiens", the minimum interaction between the PPI network diagram and the mutual information data table between the target proteins were obtained by setting the protein species as "Homo sapiens", the minimum interaction threshold as "high confidence >0.9", hiding the free targets, and keeping the rest of the parameters as default. Network topology parameters were analyzed using the Network Analyzer plug-in in Cytoscape 3.9.1.
Key target protein gene ontology (GO) enrichment analysis and Kyoto Encyclopaedia of Genes and Genomes (KEGG) pathway enrichment analysis
Gene ontology (GO) enrichment and Kyoto Encyclopaedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed on the targets using R4.2.1. (https://www.r-project.org/). The symbols of drug active ingredient and disease intersection genes were converted into IDs, and the GO enrichment analysis of key target proteins were obtained by filtering the intersection genes with the condition of "P valueFilter =.05, QualueFilter =.05" using the "clusterprofiler" plug-in in R4.2.1 software. Statistical hypergeometric distribution quantification (P-value) was used to assess the significance of the proteins present in each GO annotation and the biological functions of the proteins. The KEGG pathway enrichment analysis of drug-disease intersection target genes were carried out by using the "clusterprofiler" plug-in in R software to obtain the main pathways of BSHX in the treatment of PCOS, and the algorithm of this tool was the same as that of GO analysis algorithm.
Molecular docking verification
The 2D structures of the core components of BSHX were downloaded from the PubChem database (https://Pubchem.ncbi.nlm.nih.gov/) and stored in "SDF" format, which was converted to "MOL2" format using Chem3D software. The 3D structure of the core gene target can be downloaded from the RCSB PDB database (http://www.rcsb.Org/) and stored in "PDB" format, and the protein structure can be pre-processed using PyMol 1.5.7. (https://pymol.org/) software. The Grid Box coordinates and size were set to check the mutual affinity and fusion pattern between the target material and target protein. Docking models with small binding energy equal to zero, which can be considered as a ligand-receptor protein capable of spontaneous fusion, and binding energy ≤-5 kcal/mol (1 cal ≈4.186 J) is usually used as evaluation criteria to detect functional target proteins. Finally, the docking results were compiled and analyzed, and the receptor-ligand pairs were filtered and sorted according to the binding free energy (affinity) and visualized using PyMol 1.5.7.
Materials and methods for animal experiments
Animals
Sixty-four female Sprague-Dawley (SD) rats, 6–7 weeks old, non-pregnant, with a body mass of 170–190 g. Sixteen sexually mature male SD rats, 9–10 weeks old, with a body mass of 300–350 g. Certificate of Conformity No. SCXK (Chuan) 2018-026. Animal use permit number: SYXK (Chuan) 2018–185. The experiments were approved by the Experimental Animal Ethics Committee of the Chengdu University of Traditional Chinese Medicine. Experimental SD rats were purchased from Chengdu Dashuo Biotechnology Co. and housed in the West China Laboratory of Sichuan University, with a controlled room temperature (22 °C ± 2 °C) and relative humidity (55% ± 5%), 12h of light per day, 12h of light avoidance under cyclic feeding conditions, and standard feed and drinking water were freely available.
Animal grouping and modeling
Sixty-four female SD rats were acclimatized for 4-5 days and then numbered, 48 SD rats were selected according to a random number table for PCOS modeling, leaving 16 female rats as a control group. The 48 modeled SD rats were divided into the model, BSHX, and metformin groups according to the random number table method, with 16 rats in each group. PCOS model rats were constructed using letrozole combined with a high-fat emulsion method [Citation7,Citation17]. Rats in the model group were gavaged with letrozole 1 mg/kg bw/day and high-fat emulsion (lard + cholesterol, lard:cholesterol = 10:3), given in a volume of 15 ml/kg bw/day for 21 consecutive days. The control group was administered saline (0.9% NaCl solution) for 21 days. The rats had an estrous cycle of 4–5 days. Starting from day 10, the rats were coated daily and observed for two estrous cycles. The model was considered successful when 50% of the rats in the model group had a disturbed estrous cycle as well as polycystic changes in the ovaries [Citation7].
Treatment group intervention and regrouping
According to the body surface area formula published by the FDA, the equivalent dose of the drug administered to rats was determined as 6.3 times the clinically applied amount in adults (body mass calculated at 60 kg). The volume of each gavage was fixed to regulate the concentration of the drug administered to reach the target dose. The BSHX group was given .33 g/ml of the herbal compound suspension by a gavage of 3 ml per day. The positive drug control group was given 3 ml of metformin at .0275 g/ml daily, and all rats were given free access to water and fed pelleted diets. After 21 days of continuous treatment, the above four groups were further divided into groups A and B, with eight rats in each group. Group A were used to detect various indicators such as inflammatory factor levels and epithelial-mesenchymal transition (EMT)-related proteins. Male SD rats were randomly selected and mated with female rats in group B with a male-to-female ratio of 2:1. Female rats were observed for vaginal and cage excretion at 7:00 am daily after the cages were combined, and female rats were fed separately from male rats after vaginal plugs were found. Both female and male rats in group B were fed water and pellet feed freely during the process.
Observation indexes and methods
Body mass, emotional cycle, ovarian index, and ovarian path morphology of rats
From the beginning of the modeling, the body mass of each group of rats was weighed and recorded at 8 am on the first day of gavage, and measured every 7 days, with the last weighing performed on the day of sampling. The body mass growth rate of rats was calculated and plotted, body mass growth rate = (rat body mass at execution - rat initial body mass)/rat initial body mass. Estrus cycle: vaginal smear analysis was performed daily at 9 am starting from day 10. Vaginal cells were collected via saline lavage and placed on glass slides. After air drying, they were placed under a microscope with a 10x field of view and photographed. The ovarian index was calculated as follows = ovarian weight/body weight. Ovarian sections were stained with hematoxylin-eosin (HE) and observed under a 10x microscope for each group of rat ovarian morphology.
Histomorphology of the uterus observed by hematoxylin-eosin (HE) staining
Under anesthesia, the rat's abdominal cavity was opened, the uterus was aseptically removed, and then the surrounding tissues were carefully peeled off on the ice. The right uterus was fixed in 4% paraformaldehyde for 24 h, routinely dehydrated, paraffin-embedded, and 3-5 μm uterine tissue sections were made, baked at 60 °C for 3 h, hematoxylin nucleation, ethanol hydrochloride fractionation, eosin staining, dehydration, transparency, sealed with neutral gum, and observed under a 10x light microscope. The endometrial blood supply was measured, the diameter of the uterine body, the thickness of the endometrium, the number of glands counted, and the morphology of the endometrial interstitial cells were examined. The sections were placed in a panoramic scanner to observe and collect images and observed under a 200x microscope. The left uterine tissue was placed in a freezing tube, frozen rapidly with liquid nitrogen, and stored at −80 °C refrigerator for backup.
Enzyme-linked immunosorbent assay (ELISA) for serum inflammatory factor levels, and expression levels of related proteins in endometrial tissues by protein immunoblotting
Rats fasted at 8 pm on day 21, and the rats were anesthetized by intraperitoneal injection of 3% sodium pentobarbital (.1 ml/100 g) at 8 am the next day. The abdominal skin was disinfected with alcohol, and the abdominal cavity was opened, blood was collected from the abdominal aorta, and the blood specimens were placed at room temperature for 30 min and then centrifuged at 4 °C, 2000r-min-1 for 10 min, and the upper serum was taken for Enzyme-linked immunosorbent assay (ELISA) detection. The endometrial tissues were prepared under item 2.4.2 and thawed in a constant temperature water bath, the endometrial mesenchymal cell samples were isolated under a microscope, placed into a centrifuge tube, and lysed at a ratio of 20 mg of tissue to plus 200 µl of lysis solution. The mixture was thoroughly homogenized using an electric homogenizer and an ice bath for 1h. After sufficient lysis, centrifuge at 13000r-min-1 for 10 min, and the supernatant was removed in another centrifuge tube. After the samples were diluted and prepared, the expression levels of EMT-associated Vimentih proteins, e-calmodulin proteins, and pathway-associated TGF-β, TAK1, and NF-κB proteins were examined by immunoblotting. Finally, the membrane was placed into the developer, observed, and photographed, and the grayscale of each band was analyzed using the Image J software.
Observation of intrauterine fertilization and embryo implantation in rats
The whole uterus of rats in group B was taken and the number of implantation sites was collected. After removing the fat and connective tissue, the uterus was separated and the gestational sac was removed. The pregnancy rate (number of pregnant females/total number of females) was calculated for each group of rats. The number of implantation sites in pregnant rats was recorded, and the average number of pregnancy sites in each group was calculated.
Statistical methods
IBM SPSS26.0 software (IBM, Armonk, New York, USA) was used for statistical analysis. Data were tested for distribution using the Shapiro-Wilk test (S-W test), which is used to test normality for small sample sizes (n < 50). The measurements are expressed as mean ± standard deviation. T-tests were employed to compare two groups, and a one-way analysis of variance (ANOVA) was utilized for comparisons between multiple groups. Data were analyzed and presented visually in combination with GraphPad Prism 9 (https://www.graphpad-prism.cn/) software. Histograms with marks (*, #, ▲) showed a significant difference (p < .05).
Results
Screening of targets for the treatment of PCOS with Bushen Huoxue Recipe (BSHX)
The study obtained ninety active ingredients () and 201 action targets of drug ingredients for BSHX (). In total, 160 targets for therapy of BSHX for PCOS were obtained in this study ().
Figure 1. Network pharmacology analysis results in presentation. a: Disease target and drug-disease shared target acquisition. b: Bushen Huoxue Recipe (BSHX) " Chemical Composition-Common Targets-Disease Information Network Diagram. c: Protein-protein interaction (PPI) network diagram of key and core targets.

Table 1. Information of active ingredients and predicted targets in the Bushen Huoxue Recipe.
Building drug-component-target networks
The herbal compound regulatory network was constructed using Cytoscape 3.9.1 (), and the nodes were rendered according to degree size, with larger nodes representing greater degrees of freedom and more biological functions. Thirteen components with larger nodes were screened, including seven common active components in the eight herbs ().
Table 2. Information on the ranking of active ingredients in the treatment of polycystic ovary syndrome with the Bushen Huoxue Recipe.
PPI network construction, key target selection, GO and KEGG enrichment analysis
The PPI network diagram was constructed using the STRING database and Cytoscape 3.9.1 software, 34 key targets were screened in the first round and 13 core targets were screened in the second round for the treatment of PCOS with BSHX (). Target enrichment yielded 1478 GO entries, including 1334 biological processes (BP), 45 cell components (CC), and 99 molecular functions (MF) (). KEGG pathway map analysis suggested that BSHX treatment in PCOS was associated with oxidative stress, inflammatory response, infection, endothelial dysfunction, and other pathways (). The MAPK and TNF signaling pathways are of interest. TNF-α and NF-κB are key hubs of these two pathways, which are associated with inflammation and oxidative stress. The docking binding energy and docking animation suggested that the binding sites of dendrobium, kaempferol, isorhamnetin, and B-sitosterol with TNF-α and NF-κB all formed hydrogen bonds, and the binding energies were all less than −5 kcal/mol, with good intermolecular binding activity and stability ().
Figure 3. Molecular docking animation and binding energy demonstration. a: Docking animation of TNF-α (-8.7kcal/mol), NF-κB (-8.6kcal/mol), TAK1 (-8.6kcal/mol), TGFβ (-6.6kcal/mol) and quercetin molecules. b: Docking binding ability of quercetin, kaempferol, isorhamnetin, and beta-sitosterol with TNF-α, TGFβ, TAK1, and NF-κB molecules.

Effects of BSHX on body mass, estrous cycle, ovarian index, and ovarian pathological morphology in PCOS rats
Data are shown as mean ± SD (n = 8), and data between multiple groups were compared by one-way analysis of variance (ANOVA). Body weight gain index and ovarian index were significantly higher (p < .05) in the model group rats after modeling compared to the control group (), and the estrous cycle was disturbed (). The number of cystic and atretic follicles in the ovaries of rats in the model group increased, with a polycystic-like appearance (), suggesting successful PCOS modeling. Rats treated with BSHX and metformin showed a reduction in body weight gain and ovarian index compared with the model group. Compared with the model group, the BSHX and metformin groups had complete and regular estrous cycles, the number of cystic dilated follicles and atretic follicles in the ovaries of rats was significantly reduced, and mature follicles were visible, reflecting the better intervention effect of the drug.
Figure 4. Basic conditions of rats in each group. a: Body mass growth index of rats and ovarian index of rats. Data are shown as mean ± SD (n = 8), and data between multiple groups were compared by one-way analysis of variance (ANOVA). b: Rat ovary sections (HE staining). c: Vaginal exfoliated cell map of rats in the control group. d: Estrus cycle curves diagram for each group of rats.

Effect of BSHX on pregnancy and histological changes in the endometrium in PCOS rats
According to , the control group: the endometrial epithelium was columnar with transparent cytoplasm, the stromal cells of the lamina propria were arranged in an orderly manner, and the blood vessels were abundant and mildly dilated. Model group: the uterus was significantly reduced in size, the endometrial epithelium was cuboidal, the myometrial cells were very densely arranged, and no stromal cells were seen in the lamina propria, only a small number of capillaries. BSHX group: the uterus was reduced in size, the endometrial epithelium was short and columnar, the cytoplasm was transparent, the myometrial cells were densely arranged, and stromal cells and abundant capillaries could be seen in the lamina propria. Metformin group: uterine volume decreased, epithelium was short and columnar, myometrial cells were densely arranged, and stromal cells and abundant capillaries could be seen in the lamina propria. The statistical results () showed the uterine diameter, endometrial thickness, and the number of endometrial glands of the model group (p < .05) compared to the control group. The uterine diameter, endometrial thickness, and number of endometrial glands in the model group of rats were not statistically different from those in the BSHX and the metformin groups but differed significantly during pregnancy (p < .05). Different results were observed for pregnancy in different groups of female rats (, ). Data are shown as mean ± SD, and data between multiple groups were compared by one-way analysis of variance (ANOVA).
Figure 5. Uterine tissue morphology and pregnancy in rats of four groups. a: Rat endometrial tissue (HE staining). b: Pregnancy loci of rats.

Table 3. Diameter of the rat uterus, thickness of the endometrium, and number of endometrial glands in different groups.
Table 4. Pregnancy rate and the number of embryos in different groups.
Effects of BSHX on inflammatory factor levels and expression of EMT-related protein levels of PCOS rats
The serum TNF-α levels of rats in the model group were significantly higher than those in the control, BSHX, and metformin groups (p < .01, p < .001, p < .001). Compared to the control group, the relative protein expression levels of TGF-β, TAK, NF-κB, and vimentin were significantly increased in the model group (***p < .001, ***p < .001, **p < .01, ***p < .001), and the relative protein expression levels of E-cadherin were significantly decreased (***p < .001). Compared to the model group, the relative protein expression levels of TGF-β and NF-κB in the BSHX and metformin groups were significantly decreased (#p < .05, ##p < .01). The relative protein expression levels of TAK and Vimentin in the BSHX group tended to decrease compared with those in the model group. Compared to the model group, the relative protein expression levels of E-cadherin were significantly higher in the BSHX and metformin groups (###p < .001, ##p < .01). Notably, the relative protein expression level of E-cadherin was significantly increased in the BSHX group compared with the metformin group (▲▲▲p < .001), and there was no significant difference compared to the control group (). Data are shown as mean ± SD (n = 5), and data between multiple groups were compared by one-way analysis of variance (ANOVA).
Figure 6. Serum TNF-α levels, and levels of target proteins and EMT-related proteins in rat endometrial tissue. a: Endometrial TGF-β, TAK, NF-κB, E-cadherin, and Vimentin WB results. b: Endometrial TGF-β, TAK, NF-κB, Vimentin Heatmap Display. c: Serum TNF-α levels in rats (*p < .05, **p < .01, ***p < .001). Levels of TGFβ, TAK, and NF-κB in rat endometrial tissue and levels of EMT-related proteins Vimentin and E-cadherin in the endometrium. Data are shown as mean ± SD (n = 5), and data between multiple groups were compared by one-way ANOVA. Compared with the control group: *p < .05, **p < .01, ***p < .001; compared with the model group: #p < .05, ##p < .01, ###p < .001; compared with the metformin group: ▲p < .05, ▲▲p < .01, ▲▲▲p < .001.

Discussion
This study investigated the potential targets and pathways of BSHX in PCOS and identified four potential drug targets: TNF-α, TGF-β, TAK1, and NF-κB. Molecular docking and animal experiments verified that BSHX could target TNF-α, TGF-β, TAK1, and NF- NF-κB. BSHX treatment regulated the estrous cycle, restored ovarian morphologic damage, restored endometrial pathological changes, elevated the pregnancy rate, and increased the number of embryos in PCOS rats. The further experiment revealed that BSHX down-regulated TGF-β, TAK1, and NF-κB, up-regulated E-cadherin, inhibited inflammation, and interfered in the endometrial EMT process.
Among the core active ingredients of Chinese medicine, quercetin (derived from Cuscuta australis R.Br., Taxillus chinensis (DC.) Danser, and Paeonia suffruticosa Andr.) is a flavonol compound with multiple biological activities, which iswidely distributed in the plant kingdom. Modern pharmacological studies have shown that quercetin has antioxidant, anticancer, antiallergic, anti-inflammatory, anti-obesity, and neuroprotective properties and exhibits functions such as inhibition of platelet aggregation, enhancement of mitochondrial synthesis, and regulation of intestinal microorganisms [Citation18–20]. Pourteymour et al. [Citation21] reported that quercetin could treat PCOS by reducing serum androgen (T) and luteinizing hormone (LH) levels in patients with antioxidant and anti-inflammatory effects. Wang, et al. [Citation22] found that quercetin could alleviate chronic inflammation in PCOS model rats by mimicking the effects of estrogen, lowering insulin (INS) levels, and improving INS resistance. Kaempferol (derived from Cuscuta australis R.Br, Paeonia Suffruticosa Andr., and Paeonia lactiflora Pall.), a flavonol widely found in natural medicines, is one of the important natural anti-inflammatory compounds that reduce the risk of inflammation-related diseases and play an ameliorating role in cancer, cardiovascular, and other diseases [Citation23,Citation24]. Kaempferol exhibits strong anti-inflammatory effects through the modulation of NF-κB and MAPK pathways [Citation25,Citation26]. Isorhamnetin (derived from Cuscuta australis R.Br.) is one of the main compounds of flavonols, which have antioxidant, anti-microbial [Citation27] as well as anti-hepatic steatosis effects [Citation28]. In addition, isorhamnetin exhibits comprehensive antitumor activity by inhibiting cell proliferation and migration, and activating apoptosis [Citation29]. Beta-sitosterol (derived from Cuscuta australis R.Br., Prunus persica (L.) Batsch, Dipsacus asper Wall. ex Henry, Cinnamomum cassia (L.) J. Presl, and Paeonia lactiflora Pall.) is highly effective in anti-inflammatory [Citation30], anti-diabetic [Citation31], and antitumor effects [Citation32]. Beta-sitosterol has been shown to effectively inhibit the excessive reduction of integral protein αvβ3, LIF, and HOXA10 expression and COX-2 overexpression in the endometrium of PCOS-like mice. It can alter the imbalance of gut microbiota and harmonize sex hormone homeostasis in the pathogenesis of PCOS [Citation33]. In addition, sesamin, hederagenin, gentisin, and taxifolin have varying degrees of anti-inflammatory and antioxidant effects. These active components and the effects of herbal medicines are the basis of the pharmacological efficacy of BSHX for the treatment of PCOS, providing support for further experimental studies ().
In animal experiments, the male rats selected for the experiment were consistent in breed, age, body mass, health status, and husbandry environment, and male rats were excluded as the cause. However, the intervention of BSHX and metformin in PCOS pregnancy is not related to whether they improve uterine diameter, endometrial thickness, and endometrial gland count. Nevertheless, there was a trend toward higher pregnancy rates in the BSHX group, and BSHX improved embryonic development in PCOS rats. This condition may correlate with the shape of endometrial epithelial cells, and the number of endometrial stromal cells and capillaries. Transformation of the endometrium to be receptive to an embryo and progesterone-induced differentiation (decidualization) of the stromal fibroblasts to orchestrate the immune repertoire for embryo implantation are key components of pregnancy success [Citation34]. BSHX may be involved at the molecular level in the repair of endometrial pathology during the secretory phase in PCOS rats, thereby improving pregnancy outcomes.
The inflammatory state is an important environmental factor in EMT induction. TGF-β is not only a transforming growth factor but also a pro-inflammatory factor. TGF-β up-regulates the transcription factors Snail1, Snail2, and Twist1, and Snail1 and Snail2 bind directly to the E-cadherin promoter of the E-box sequence to inhibit its expression downregulates the epithelial cell marker E-cadherin and induce EMT [Citation35,Citation36]. Epithelial cells have apical-basal polarity and a low migration potential [Citation37]. In contrast, mesenchymal cells have anterior-posterior polarity and a stronger migratory phenotype, which can be identified by cell surface markers such as N-calmodulin, fibronectin, and vimentin proteins [Citation38,Citation39]. Downregulation of E-cadherin is the most obvious marker of epithelial-mesenchymal transformation. Huber-MA [Citation40] found that NF-κB signaling is required for TGF-β to drive and maintain EMT in breast cancer, which is consistent with the induction of EMT in non-small cell lung cancer mesenchymal cells with TGF-β to show sustained activation of NF-κB [Citation41]. Asgarova et al. [Citation42] found that activation of the NF-κB pathway by TNFα increased the ability of TGF-β to induce EMT by promoting PD-L1 expression. In this study, molecular docking of quercetin, kaempferol, isorhamnetin, and β-sitosterol with TNFα, TGF-β, TAK1, and NF-κB () showed lower binding energy and higher binding activity. TGF-β, TAK1, NF-κB, and vimentin levels in rat endometrial tissues showed a decreasing trend and E-cadherin levels increased significantly under BSHX intervention (). Since TGF-β and NF-κB can individually and/or interact to induce endometrial EMT. In future clinical and experimental studies, time course experiments will help to demonstrate the temporal sequence of molecular events after drug treatment.
A previous study found that the endometrium of PCOS patients exhibited elevated expression of Vimentin and Slug proteins and decreased expression of Claudin 1, suggesting that EMT contributes to the switch from a healthy state to a PCOS state in the endometrium [Citation43]. Our study found that the development of EMT is associated with the TGF-β/NF-kB pathway, which induces an inflammatory response, and forms a vicious circle with the chronic inflammatory process in PCOS, leading to pathologic changes in the endometrium, interfering with PCOS embryo implantation. This study initially investigated the mechanism of BSHX for the treatment of PCOS-mediated infertility. This is also the first study to investigate the mechanism of herbal compounds to improve pregnancy outcomes in PCOS from the perspective of endometrial EMT. It aims to continue in-depth research on reproductive endocrine disorders in PCOS in the future, to provide a scientific basis for the development and application of drugs, and to provide a favorable alternative treatment option for low-fertility individuals with PCOS. However, this study is only a preliminary investigation of the mechanism of BSHX treatment of PCOS-mediated infertility and lacks evidence from clinical studies, which needs to be demonstrated by clinical trials that include time-course experiments. In addition, future relevant studies will use techniques such as immunohistochemistry to identify specific proteins or markers associated with inflammation and reproductive processes.
Conclusions
Current measures to treat infertility in PCOS patients are limited. BSHX can inhibit inflammation, intervene in the endometrial EMT process, and improve the pregnancy outcome in PCOS. This study provides a new therapeutic strategy for PCOS-mediated infertility and a basis for the development and application of herbal compounding.
Author's contributions
Hongqiu Zhu: Project management, designed the study and guided the experiments. Hanxue Wu: Data compilation, writing the original manuscript, data analysis, and plotting. Peijuan Wu, Junjie Li, Ying Zhu, and Haiyan Chen assisted with experiments and reviewed the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
We are open to providing our research data. The data that support the findings of this study are openly available in 10.6084/m9.figshare.24156384.
Additional information
Funding
References
- Yildiz BO, Bozdag G, Yapici Z, et al. Prevalence, phenotype, and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod. 2012; 27(10):1–12. doi: 10.1093/humrep/des232.
- Steiner N, Ates S, Shaulov T, et al. Comparison of IVF outcomes transferring a single ideal blastocyst in women with polycystic ovary syndrome and normal ovulatory controls. Arch Gynecol Obstet. 2020;302(6):1479–1486. doi: 10.1007/s00404-020-05699-9.
- Ashkenazi J, Farhi J, Orvieto R, et al. Polycystic ovary syndrome patients as oocyte donors: the effect of ovarian stimulation protocol on the implantation rate of the recipient. Fertil Steril. 1995;64(3):564–567. doi: 10.1016/s0015-0282(16)57793-0.
- Liu L. Effect of kidney tonic and blood stasis formula on the expression of integrin α_Vβ_3 in patients with PCOS with kidney deficiency and blood stasis evidence. Hunan University of Traditional Chinese Medicine;2013. https://kns.cnki.net/KCMS/detail/detail.aspx?filename=1013183069.nh&dbcode=CDMD
- Zhao SX, Chen L, Zhan ZQ, et al. Progress in Chinese and Western medicine research on polycystic ovary syndrome-insulin resistance-reduced endothelial tolerance-early pregnancy loss. Jilin Chin Med. 2018;38(08):984–988. doi: 10.13463/j.cnki.jlzyy.2018.08.035.
- Ye Y, Zhou W, Ren Y, et al. The ameliorating effects of Guizhi Fuling Wan combined with rosiglitazone in a rat ovarian model of polycystic ovary syndrome by the PI3K/AKT/NF-κB and Nrf2/HO-1 pathways. Gynecol Endocrinol. 2023; 39(1):2254848. doi: 10.1080/09513590.2023.2254848.
- Liu M, Zhu H, Zhu Y, et al. Guizhi Fuling Wan reduces autophagy of granulosa cell in rats with polycystic ovary syndrome via restoring the PI3K/AKT/mTOR signaling pathway. J Ethnopharmacol. 2021; 270:113821. doi: 10.1016/j.jep.2021.113821.
- Zhu Y, Li Y, Liu M, et al. Guizhi fuling wan, Chinese herbal medicine, ameliorates insulin sensitivity in PCOS model rats with insulin resistance by remodeling intestinal homeostasis. Front Endocrinol . 2020;11:575. doi: 10.3389/fendo.2020.00575.
- Manzoor S, Ganie MA, Amin S, et al. Oral contraceptive use increases risk of inflammatory and coagulatory disorders in women with polycystic ovarian syndrome: an observational study. Sci Rep. 2019; 9(1):10182. doi: 10.1038/s41598-019-46644-4.
- Rossing MA, Daling JR, Weiss NS, et al. Ovarian tumors in a cohort of infertile women . N Engl J Med. 1994;331(12):771–776. doi: 10.1056/NEJM199409223311204.
- Li L, Li G, Chen L, et al. Risk signal mining for letrozole based on the FDA adverse event reporting system database. J Adverse Drug React. 2020;22(9):511–517. doi: 10.3760/cma.j.cn114015-20190910-00752.
- Li S, Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin J Nat Med. 2013;11(2):110–120. doi: 10.1016/S1875-5364(13)60037-0.PMID: 23787177.
- Yan R, Yanjun D, En M, et al. Research progress and challenges of network pharmacology in field of traditional Chinese medicine. J Chin Herb Med. 2020;51(18):4789–4797. doi: 10.7501/j.issn.0253-2670.2020.18.024.
- Choudhary N, Choudhary S, Kumar A, et al. Deciphering the multi-scale mechanisms of Tephrosia purpurea against polycystic ovarian syndrome (PCOS) and its major psychiatric comorbidities: studies from network pharmacological perspective. Gene. 2021; 773:145385. doi: 10.1016/j.gene.2020.145385.
- Xu X, Zhang W, Huang C, et al. A novel chemometric method for the prediction of human oral bioavailability. Int J Mol Sci. 2012;13(6):6964–6982. doi: 10.3390/ijms13066964.
- Tao W, Xu X, Wang X, et al. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal radix Curcumae formula for application to cardiovascular disease. J Ethnopharmacol. 2013;145(1):1–10. doi: 10.1016/j.jep.2012.09.051.
- Xiaojuan X, Lijuan Y, Zhang W, et al. Study on morphological changes of ovaries in a modified PCOS animal model simulating information on the characteristics of kidney deficiency phlegm and dampness. J Chengdu Univ Trad Chin Med. 2016;39(02):5–9 + 14. doi: 10.13593/j.cnki.51-1501/r.2016.02.005.
- Yang D, Wang T, Long M, et al. Quercetin: it is main pharmacological activity and potential application in clinical medicine. Oxid Med Cell Longev. 2020;2020:8825387–8825313. doi: 10.1155/2020/8825387.
- Azeem M, Hanif M, Mahmood K, et al. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: a review. Polym Bull. 2023;80(1):241–262. doi: 10.1007/s00289-022-04091-8.
- Eisvand F, Tajbakhsh A, Seidel V, et al. Quercetin and its role in modulating endoplasmic reticulum stress: a review. Phytother Res. 2022;36(1):73–84. doi: 10.1002/ptr.7283.
- Pourteymour Fard Tabrizi F, Hajizadeh-Sharafabad F, Vaezi M, et al. Quercetin and polycystic ovary syndrome, current evidence, and future directions: a systematic review. J Ovarian Res. 2020;13(1):11. doi: 10.1186/s13048-020-0616-z.
- Wang Z, Zhai D, Zhang D, et al. Quercetin decreases insulin resistance in a polycystic ovary syndrome rat model by improving inflammatory microenvironment. Reprod Sci. 2017;24(5):682–690. doi: 10.1177/1933719116667218.
- Imran M, Salehi B, Sharifi-Rad J, et al. Kaempferol: a key emphasis to its anticancer potential. Molecules. 2019;24(12):2277. doi: 10.3390/molecules24122277.
- Devi KP, Malar DS, Nabavi SF, et al. Kaempferol and inflammation: from chemistry to medicine. Pharmacol Res. 2015;99:1–10. doi: 10.1016/j.phrs.2015.05.002.
- Zhuang Z, Ye G, Huang B. Kaempferol alleviates the interleukin-1β-Induced inflammation in rat osteoarthritis chondrocytes via suppression of NF-κB. Med Sci Monit. 2017;23:3925–3931. doi: 10.12659/msm.902491.
- Huang X, Pan Q, Mao Z, et al. Kaempferol inhibits interleukin‑1β stimulated matrix metalloproteinases by suppressing the MAPK‑associated ERK and P38 signaling pathways. Mol Med Rep. 2018;18(3):2697–2704. doi: 10.3892/mmr.2018.9280.
- Yang JH, Shin BY, Han JY, et al. Isorhamnetin protects against oxidative stress by activating Nrf2 and inducing the expression of its target genes. Toxicol Appl Pharmacol. 2014;274(2):293–301. doi: 10.1016/j.taap.2013.10.026.
- Ganbold M, Owada Y, Ozawa Y, et al. Isorhamnetin alleviates steatosis and fibrosis in mice with nonalcoholic steatohepatitis. Sci Rep. 2019;9(1):16210. doi: 10.1038/s41598-019-52736-y.
- Cai F, Zhang Y, Li J, et al. Isorhamnetin inhibited the proliferation and metastasis of androgen-independent prostate cancer cells by targeting the mitochondrion-dependent intrinsic apoptotic and PI3K/akt/mTOR pathway. Biosci Rep. 2020; 40(3):BSR20192826. doi: 10.1042/BSR20192826.
- Zhang F, Liu Z, He X, et al. β-sitosterol-loaded solid lipid nanoparticles ameliorate complete Freund's adjuvant-induced arthritis in rats: involvement of NF-кB and HO-1/nrf-2 pathway. Drug Deliv. 2020;27(1):1329–1341. doi: 10.1080/10717544.2020.1818883.
- Babu S, Jayaraman S. An update on β-sitosterol: a potential herbal nutraceutical for diabetic management. Biomed Pharmacother. 2020; 131:110702. doi: 10.1016/j.biopha.2020.110702.
- Ju YH, Clausen LM, Allred KF, et al. beta-sitosterol, beta-sitosterol glucoside, and a mixture of beta-sitosterol and beta-sitosterol glucoside modulate the growth of estrogen-responsive breast cancer cells in vitro and in ovariectomized athymic mice. J Nutr. 2004;134(5):1145–1151. doi: 10.1093/jn/134.5.1145.
- Yu Y, Cao Y, Huang W, et al. β-sitosterol ameliorates endometrium receptivity in PCOS-Like mice: the mediation of gut microbiota. Front Nutr. 2021;8:667130. doi: 10.3389/fnut.2021.667130.
- Evans J, Salamonsen LA, Winship A, et al. Fertile ground: human endometrial programming and lessons in health and disease. Nat Rev Endocrinol. 2016;12(11):654–667. doi: 10.1038/nrendo.2016.116.
- Su J, Morgani SM, David CJ, et al. TGF-βorchestrates fibrogenic and developmental EMTs via the RAS effector RREB1. Nature. 2020;577(7791):566–571. doi: 10.1038/s41586-019-1897-5.
- Park M-J, Lee D-E, Shim MK, et al. Piperlongumine inhibits TGF-β-induced epithelial-to-mesenchymal transition by modulating the expression of E-cadherin, Snail1, and Twist1. Eur J Pharmacol. 2017;812:243–249. doi: 10.1016/j.ejphar.2017.07.036.
- Whitby S, Zhou W, Dimitriadis E. Alterations in epithelial cell polarity during endometrial receptivity: a systematic review. Front Endocrinol. 2020;11:596324. doi: 10.3389/fendo.2020.596324.
- Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007.
- Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119(6):1429–1437. doi: 10.1172/JCI36183.
- Huber MA, Azoitei N, Baumann B, et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114(4):569–581. doi: 10.1172/JCI21358.
- Kumar M, Allison DF, Baranova NN, et al. NF-κB regulates mesenchymal transition for the induction of non-small cell lung cancer-initiating cells. PLOS One. 2013;8(7):e68597. doi: 10.1371/journal.pone.0068597.
- Asgarova A, Asgarov K, Godet Y, et al. PD-L1 expression is regulated by both DNA methylation and NF-kB during EMT signaling in non-small cell lung carcinoma. Oncoimmunology. 2018; 17(5):e1423170. doi: 10.1080/2162402X.2017.1423170.
- Hu M, Zhang Y, Li X, et al. Alterations of endometrial epithelial-mesenchymal transition and MAPK signaling components in women with PCOS are partially modulated by metformin in vitro. Mol Hum Reprod. 2020; 26(5):312–326. doi: 10.1093/molehr/gaaa023.