Abstract
Objective
This study aims to examine the short-term effects of oral metformin (MET) on serum anti-müllerian hormone (AMH) levels and to verify its impact on AMH concentrations in women with polycystic ovary syndrome (PCOS).
Methods
The literature search, extending from January 2000 to April 2023, was conducted using databases such as PubMed, Embase, and the Cochrane Central, resulting in the inclusion of 20 studies. These selected studies, evaluated for quality using the Newcastle–Ottawa Scale, investigated changes in AMH levels before and after treatment, with durations ranging from less than three months to over six months. The reported outcomes were quantified as standardized mean differences (SMD) with 95% confidence intervals (CI). This comprehensive systematic review and meta-analysis was registered with the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42023420705. The statistical analyses were performed using Review Manager 5.4.1.
Results
① The study incorporated 20 articles, consisting of 12 prospective studies, 7 randomized controlled trials (RCT), and 1 cross-sectional study. ② Serum AMH levels in patients with PCOS diminish subsequent to the oral administration of MET. ③ Across the spectrum of studies analyzed, a pronounced degree of heterogeneity is evident, potentially ascribed to differential parameters including body mass index (BMI), daily pharmacological dosages, the temporal extent of treatment regimens, criteria of PCOS, and detection Methods. ④ The impact of MET on AMH levels exhibits a dose-responsive trend, with escalating doses of MET being associated with progressively greater declines in AMH concentrations in the patient population. ⑤ For women with PCOS receiving MET therapy, a minimum treatment duration of three months may be necessary to observe a reduction in serum AMH levels.
Conclusions
The results of this meta-analysis indicate that MET treatment exerts a suppressive effect on serum AMH levels in women with PCOS. It appears that a treatment duration of at least three months is required to achieve a significant decrease in AMH concentrations. Furthermore, the influence of MET on AMH is dose-dependent, with higher doses correlating with more pronounced reductions in AMH levels among the patients studied.
Introduction
With a prevalence of about 5% to 10%, Polycystic ovary syndrome (PCOS) is the most common gynaecological endocrine disorder in adolescent and childbearing women [Citation1]. PCOS is characterized by three hallmark features: hyperandrogenism (HA), ovulatory dysfunction (OD), and polycystic ovarian morphology (PCOM), with the presence of at least two of these features required for diagnosis [Citation2]. Reproductive concerns such as irregular menstrual cycles and infertility are common among women with PCOS and are often the reasons for seeking medical care. Beyond its reproductive implications, PCOS is associated with a range of metabolic issues. Women with the condition are at increased risk for insulin resistance, disturbances in glucose metabolism, dyslipidemia, and an elevated risk of cardiovascular disease [Citation2]. The impact of PCOS on both metabolic and reproductive health underscores its significance as a comprehensive women’s health concern.
Metformin (MET), widely acknowledged as a foundational therapy for insulin resistance, has proven its worth by ameliorating insulin sensitivity, diminishing androgen levels, and promoting ovulatory regularity. Its therapeutic benefits in PCOS patients are particularly noteworthy, encompassing the regulation of menstrual cycles, induction of ovulation, and enhancement of fertility prospects [Citation3]. The research has found that lower doses of MET may be sufficient to improve biochemical markers and the morphology of polycystic ovaries [Citation4].
As a member of the transforming growth factor β family, AMH is named for its role in male sex differentiation, during which it induces the regression of the Müllerian ducts [Citation5]. In women, AMH, secreted by the antral follicles and small antral follicles in the ovary, plays a crucial role in the early recruitment, growth, regulation of follicle selection, and final maturation during the follicular growth process [Citation6]. Cross-sectional studies have shown that serum AMH levels are generally higher in patients with PCOS, often 2–4 times higher than in the normal population [Citation7]. The typical clinical feature of PCOS is the presence of multiple small follicles with arrested growth and developmental arrest, which may be associated with excessive levels of AMH in the body [Citation6]. The implications of AMH in the pathogenesis of PCOS are profound, not only for understanding the etiology of the syndrome but also for tailoring interventions like MET treatment. Monitoring AMH levels could thus offer valuable insights for managing PCOS, including the optimization of therapeutic strategies and the assessment of treatment response [Citation8].
The relationship between MET and serum AMH levels in women with PCOS remains an area of active debate within the scientific community. This is due to the heterogeneous nature of PCOS and the variable outcomes reported in clinical studies. On one hand, there are studies suggesting that MET has a diminishing effect on serum AMH concentrations in reproductive-aged women diagnosed with PCOS [Citation9]. These findings are significant as they imply that MET may exert a direct or indirect influence on ovarian function, potentially through the restoration of insulin sensitivity and subsequent hormonal regulation. On the other hand, several prospective studies have reported a different outcome, indicating that MET treatment, even over a period of 2–6 months, don’t alter AMH levels. These studies, however, do consistently report improvements in body weight and insulin resistance, which are beneficial effects of MET that can indirectly influence reproductive health.
The objective of this study is to explore the short-term variations in serum AMH levels in patients with PCOS undergoing MET. By focusing on short-term AMH level variations, the study seeks to contribute to a better understanding of the immediate impact of MET on ovarian function and its broader implications for reproductive endocrinology.
Materials and methods
Literature and search strategy
To ensure the rigor and reliability of our systematic review, we meticulously followed the guidelines set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) [Citation10]. Our review protocol was prospectively registered with PROSPERO (registration number: CRD42023420705). We executed a comprehensive literature search across PubMed, Embase, and the Cochrane Central Register of Controlled Trials, targeting publications from the year 2000 up to April 2023. The search was restricted to human studies published in English. We utilized a strategic combination of Medical Subject Headings (MeSH) and keyword searches to maximize the retrieval of pertinent studies. Following the initial search, two independent reviewers undertook a meticulous screening and data extraction process for all identified studies. To augment the thoroughness of our search and ensure no significant studies were omitted, a third reviewer performed an additional check of the references of all selected articles. This layered approach to study selection and data extraction aimed to minimize bias and enhance the validity of our findings.
Inclusion and exclusion criteria
Studies were included in this meta-analysis if they met the following criteria: (1) the study population included reproductive-age women, (2) serum AMH were measured in all study participants at least once before and after medication, (3) the association between MET and AMH levels was described and quantitative information was provided.
Studies were excluded if: (1) Clinical case report, review, meta-analysis or cell, animal model. (2) Evidence-based information comes from books, conferences, notes, thesis, case series, letters, or unpublished studies. (3) Unreliable extracted data, overlapped data sets, and paragraphs only abstract available.
Data extraction and Quality evaluation
In line with the principles of Design, Measurement, and Evaluation in Clinical Research (DME) and evidence-based medicine, our meta-analysis process involved a rigorous independent review of each article by two evaluators proficient in evidence-based practices. Data were meticulously extracted on key study characteristics including authorship, publication year, region, study design, population size, PCOS criteria, participant age, BMI, treatment duration, metformin dosage, AMH detection methods, and study conclusions. Discrepancies between reviewers were resolved via consensus with the involvement of all authors. Efforts to obtain missing information from study authors were unsuccessful. We applied the Newcastle-Ottawa Scale (NOS) for quality assessment, with any differences in evaluation resolved by revisiting the original articles [Citation11].
Statistical analysis
To perform the meta-analysis, we utilized the RevMan software (Review Manager, version 5.4; Cochrane Collaboration), focusing on the AMH as our primary outcome measure. Initially, we extracted the mean and standard deviation (MSD) of AMH levels from each selected study. For consistency across data sets, we converted the units based on the molecular weight of AMH, which is 140 kDa, with the conversion being 1 ng/mL = 7.14 pmol/L [Citation12]. After standardizing the data, we conducted a comparative analysis of AMH levels pre- and post-metformin intervention. To quantify the impact of MET on these hormonal concentrations, we computed the weighted mean difference (WMD) and associated 95% confidence intervals (CI) for the continuous variables. To assess the consistency of our findings across the studies, we evaluated heterogeneity using the I2 statistic. A p-value of less than 0.05 was considered statistically significant. Noticing variations among study results, we conducted further subgroup and sensitivity analyses to identify potential sources of heterogeneity and to ensure the stability and accuracy of our meta-analysis conclusions.
Results
Literature search
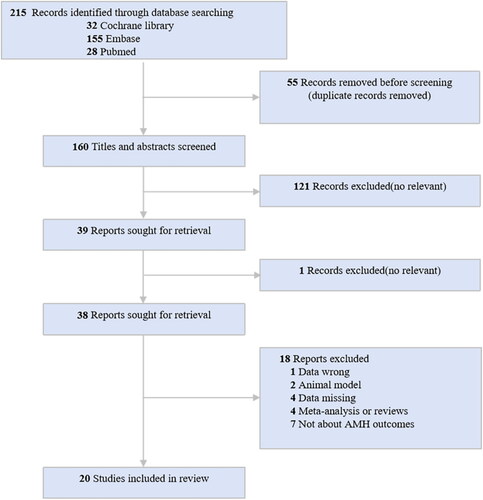
The systematic literature search retrieved 215 articles. summarizes the quantity of articles initially retrieved during the search process, as well as the number of studies ultimately included in the analysis after appropriate exclusions were applied. Ultimately, a total of 20 studies met the inclusion criteria for the systematic review.
Characteristics of the included studies
concisely summarizes the characteristics of the studies included in the systematic review. Published between 2000 and 2023, the body of literature encompasses 12 prospective studies, 7 randomized controlled trials, and 1 cross-sectional study, totaling 542 participants with PCOS. Diagnostic criteria varied, with the Rotterdam criteria being employed in 12 studies, the NIH criteria in two, and the AE-PCOS criteria in one. Some of the studies documented changes in hormone levels or follicular counts before and after MET treatment, as presented in .
Table 1. Characteristics of the studies included in the meta-analysis.
Table 2. Changes in LH and FSH hormones and number of follicles before and after medication.
While six studies found no significant change in AMH levels with MET therapy [Citation13,Citation15–18,Citation23], nine others observed a notable decrease in serum AMH after treatment [Citation14,Citation19–22,Citation24–27]. Additionally, three studies detected a downward trend in AMH levels following MET administration, but the results didn’t achieve statistical significance [Citation15, Citation28, Citation29]. Conversely, four studies specifically identified that MET effectively lowers AMH levels in obese PCOS patients [Citation19,Citation29,Citation30,Citation31].
Quality of included studies
The methodological quality of the studies incorporated into our analysis was appraised independently by two reviewers, Liu and Tian, utilizing the Newcastle-Ottawa Scale (NOS). This scale assesses three critical domains—selection, comparability, and outcome—awarding a total of zero to nine stars to each study in correlation with its perceived risk of bias. The assignment of a greater number of stars on the NOS signifies a study of higher methodological quality. Disagreement among the reviewers was resolved by discussion ().
Table 3. Quality Assessment of meta-analysis based on the Newcastle-Ottawa Scale judgment.
Publication bias
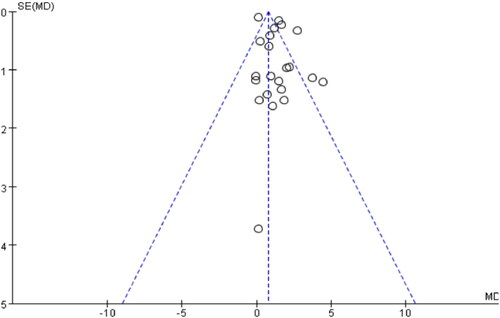
The funnel plot analysis displayed a near symmetrical distribution of published literature on either side of the graph, however the meta-funnel plot analysis conducted in this study revealed a potential presence of publication bias .
Meta-Analysis results
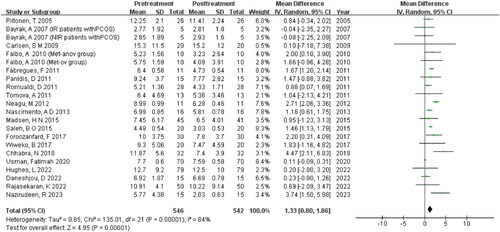
The meta-analysis comprised 542 literature samples. As shown in , significant heterogeneity was found for the included studies (I2 = 84%, p < 0.00001). Thus, a random-effects model was applied to combine effect size. The analysis demonstrated a decrease in the serum AMH levels in patients with PCOS after MET treatment [MD = 1.33, 95%CI (0.80, 1.86), p < 0.00001].
In order to delve into the origin of heterogeneity, a sensitivity analysis employing the ‘single division method’ was conducted. Notably, upon the exclusion of Usman and Fatimah’s papers, a substantial alteration in heterogeneity was observed (I2 = 51%, p = 0.004) .
Table 4. Sensitivity analysis of serum AMH Changes before and after MET treatment.
The observed heterogeneity may arise from the divergent units of measurement utilized for the AMH index, specifically the use of pmol/L in the present study compared to ng/mL in other literature, which could potentially account for the incongruity observed in the findings.
To investigate the underlying factors contributing to heterogeneity, a subgroup meta-analysis was conducted. The parameters for this subgroup analysis encompassed diagnostic criteria for AMH levels, AMH detection methods, body mass index (BMI) categories, daily medication dosages, and the duration of the treatment.
Subgroup analysis of criteria of PCOS
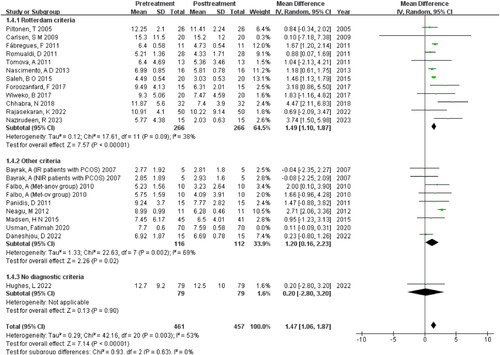
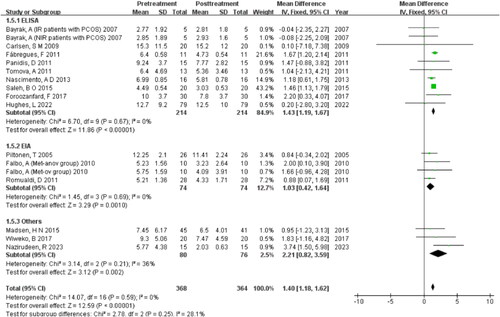
The study findings reveal that the subgroup analysis of Rotterdam criteria (I2 = 38%, p = 0.09) demonstrates lower within-group heterogeneity compared to other criteria (I2 = 69%, p =0 .002), and there’s statistically significant difference between the two groups (Z = 7.14, p <0 .00001) .
Subgroup analysis of detection methods
The study utilized enzyme-linked immunosorbent assay (ELISA), enzyme immunoassay (EIA), Beckman Coulter Gen II ELISA (Coulter Gen II), and electrochemiluminescence immunoassay (ECLIA) for AMH detection. Analysis of subgroups revealed low heterogeneity both within and between groups, indicating that the variation in AMH detection methods may be the primary cause of heterogeneity (I2 = 0%, p =0 .59) .
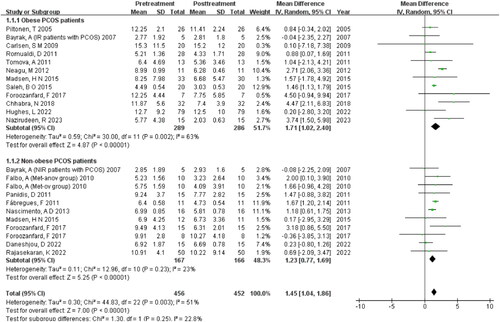
Subgroup analysis of BMI
The subgroup analysis of BMI reveal that MET has a greater effect in reducing serum AMH levels in obese women with PCOS (MD = 1.71, 95% CI [1.02, 2.40], Z = 4.87) compared to non-obese women with PCOS (MD = 1.23, 95% CI [0.77, 1.69], Z = 5.25) .
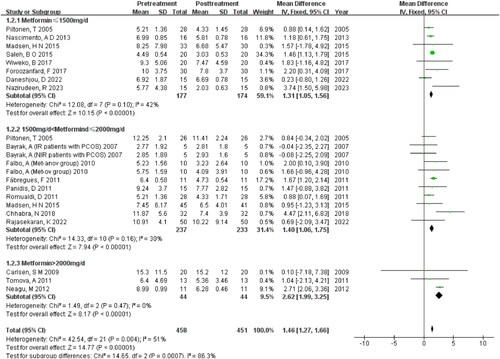
Subgroup analysis of daily dosage
The three subgroups exhibit low heterogeneity within each group, while the between-group heterogeneity is moderate (I2=51%, p = 0.004). Findings indicated that higher doses of MET were associated with a greater reduction in AMH levels among patients. Especially, when patients with PCOS were administered doses exceeding 2000 mg/d, an even more pronounced decrease in AMH was observed (MD = 2.62, 95% CI (1.99, 3.25), Z = 8.17, p < 0.00001) .
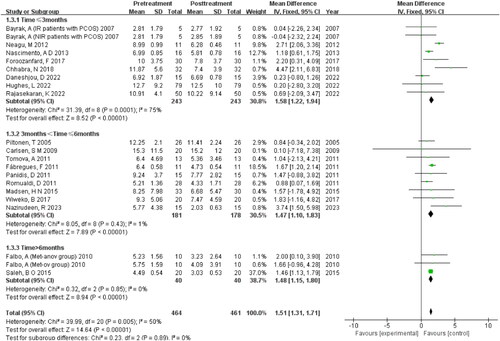
Subgroup analysis of treatment duration
The subgroup analysis, which focused on treatment duration, revealed low heterogeneity between the different duration groups, yet it showed high heterogeneity within subgroup 1, which comprises treatments of three months or less. These findings suggest that treatment duration may contribute to the variability in outcomes .
Discussion
PCOS is one of the most common endocrine disorders of reproductive age group women with rising prevalence of obesity, gestational and type 2 diabetes mellitus globally. Due to its progressive nature, untreated PCOS results in long-term metabolic and fertility consequences as well as distressing symptoms to affected women, lead to an important public health issues.
The findings of this meta-analysis support that MET treatment in women with PCOS has a downregulation effect on serum AMH. Recent studies have illuminated that the administration of MET, an activator of AMP-activated protein kinase (AMPK), can effectively reduce AMH levels and enhance ovarian follicle development. Utilizing α1AMPK-deficient transgenic murine models, studies have demonstrated the manifestation of irregular estrous cycles and ovulatory impairments, coupled with aberrant follicular maturation, heightened AMH concentrations, and increased ovarian androgen synthesis. A study suggests that MET may exert its therapeutic effects on PCOS by suppressing the expression of the α1AMPK gene, potentially ameliorating the PCOS phenotype [Citation32]. Nevertheless, further research is necessary to completely elucidate the molecular mechanisms underlying MET’s role in the pathophysiology of PCOS.
The meta results showed that the AMH value decreased more significantly in obese PCOS women than in non-obese individuals when high doses of MET were used. Insulin can promote the recruitment of primordial follicles and increase the number of small antral follicles, which in turn can lead to an increase in AMH levels. MET may affect AMH levels in obese PCOS patients by improving insulin resistance and high insulin levels. In obese patients with PCOS, it has been demonstrated that MET can reduce fasting and glucose-stimulated insulin levels, as well as decrease ovarian cytochrome P450c17 activity, leading to a reduction in serum free testosterone concentration [Citation23]. This suggests that MET may be effective in treating the hormonal imbalances associated with PCOS, particularly in obese patients.
While there’s a proposal that the dosage of MET, particularly for obese patients with PCOS, may significantly impact treatment outcomes and serve as a crucial determinant of treatment success, there remains a lack of consensus on the optimal dosage of MET [Citation33].
The meta-analysis demonstrated a dose-response relationship between MET and AMH levels in patients with PCOS. A greater reduction in AMH levels was observed with increasing doses of MET, with the most significant decreases occurring when the daily dosage surpassed 2000 mg. Moreover, it appears that the duration of MET therapy is a critical factor in reducing AMH levels in women with PCOS. The analysis suggests that a minimum treatment period of three months is necessary to achieve a noticeable reduction in serum AMH levels.
This temporal requirement for MET’s effect on AMH levels was further supported by a longitudinal study spanning eight months [Citation34]. In this study, no significant changes in AMH levels were observed during the initial four months of MET therapy. However, a statistically significant reduction in AMH concentrations became evident in the latter four months. This finding aligns with the notion that prolonged MET treatment is required to exert a significant therapeutic impact on AMH levels in PCOS patients.
The presence of previous literature, including the review and meta-analysis cited as reference [Citation35], don’t overshadow the unique contributions of our current study to the field. This research advances the understanding of MET’s effects on serum AMH levels in PCOS patients through several significant enhancements and novel insights: (1) Inclusion of Recent Studies: This meta-analysis extends the existing body of knowledge by including eight additional studies than those meta-analysis [Citation35], which only covered literature up until 2018. (2) Expanded Sample Size: By encompassing a sample size twice as large as the one used in the previous study, our research offers greater statistical power and external validity. (3) Dose-Response Relationship: This results confirm the significant reduction in serum AMH levels following MET treatment and, crucially, elucidate a dose-response relationship. This finding is particularly valuable as it provides guidance on dosing that can be translated into clinical practice, optimizing therapeutic outcomes for patients with PCOS. (4) Identification of Sources of Heterogeneity: We have identified and analyzed potential sources of heterogeneity. These factors are critical for interpreting the results and for designing future research.
The combination of these elements underscores the originality and importance of this study. By addressing gaps in the existing research and providing new insights into the management of PCOS with MET, this study not only complements but also significantly expands upon the findings of previous analyses.
Given the current state of evidence, it may be premature to draw definitive conclusions regarding the impact of MET on AMH levels in patients with PCOS. The primary limitations of this study include a limited understanding of the optimal dosage of MET, its long-term therapeutic effects, and its variable effects across different patient. Furthermore, AMH detection methods heterogeneity poses a significant challenge to the integrative analysis of data, potentially affecting the clarity and generalizability of the results.
The quantification of AMH has become increasingly pivotal in clinical diagnostics and reproductive research. Yet, inter-laboratory variability in AMH measurements has emerged as a significant concern. These variations may stem from a multitude of factors within the assay methodologies, such as the selection of antibodies, reagent composition, calibration protocols, data interpretation, and sample processing [Citation36].
Considering these discrepancies, and the fact that serum AMH levels naturally decline with age, it is crucial to establish a method-independent international standard for AMH measurement. Such a standard must be informed by rigorous, large-scale, multicenter studies that include samples from various demographic backgrounds, age groups, and physiological conditions, particularly when using AMH as a prognostic biomarker for PCOS treatment [Citation37].
Therefore, future research should focus on these areas to refine treatment protocols and improve clinical outcomes. Further research is needed ideally in the form of large-scale, randomized controlled trials with standardized protocols for MET administration and AMH measurement, to elucidate the role of MET on this important biomarker and its implications for the management of PCOS.
Conclusion
This meta-analysis provides evidence that MET therapy exerts an inhibitory effect on serum AMH levels in women diagnosed with PCOS. The findings indicate that a treatment duration of at least three months is required to achieve a significant reduction in AMH concentrations. Furthermore, the impact of MET on AMH levels is dose-dependent, as higher dosages lead to more pronounced decreases in AMH.
Consent for publication
The author assures that the described work has not been published before, and is not under consideration for publication elsewhere. The publication has received explicit or implicit approval from all coauthors, if applicable, and from the appropriate authorities at the research institution.
Authors’ contributions
Hongcen Liu: Conceptualization, Methodology, Software, Investigation, Formal Analysis, Writing - Original Draft, Data Curation.
Li Mo: Conceptualization, Methodology, Software, Investigation, Formal Analysis, Writing - Original Draft.
Xiaofang Tian: Conceptualization, Software, Formal Analysis, Writing - Original Draft, Data Curation, Visualization.
Shizhen Fan: Conceptualization, Methodology, Software, Investigation, Formal Analysis, Writing - Original Draft.
Jiayi Hu: Methodology, Software, Investigation, Formal Analysis.
Lin Zhang: Validation, Writing - Review & Editing.
Bohai Yu Conceptualization, Funding Acquisition, Resources, Supervision, Writing - Review & Editing.
Acknowledgements
We would like to express our gratitude for the valuable discussion with Shijun Xia, Shenzhen Hospital (Futian) of Guangzhou University of Chinese Medicine, in how to approach system analysis and use Review Manager.
Disclosure statement
The authors declare no competing interests.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Joham AE, Norman RJ, Stener-Victorin E, et al. Polycystic ovary syndrome. Lancet. Diabetes Endocrinol. 2022;10(9):668–680. doi:10.1016/S2213-8587(22)00163-2.
- Goodman NF, Cobin RH, Futterweit W, et al. American association of clinical endocrinologists, American college of endocrinology, and androgen excess and PCOS society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome–part 1. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2015;21(11):1291–1300.
- Harborne L, Fleming R, Lyall H, et al. Descriptive review of the evidence for the use of metformin in polycystic ovary syndrome. Lancet. 2003;361(9372):1894–1901. doi: 10.1016/S0140-6736(03)13493-9.
- Laura P, Laurine H, Mark B, et al. Granulosa cell production of anti-müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92(1):240–245. doi:10.1210/jc.2006-1582.
- van Rooij JAI, Broekmans MJF, te Velde, ER, et al. Serum anti-müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod (Oxford, England). 2002;17(12):3065–3071. doi:10.1093/humrep/17.12.3065.
- Webber L, Stubbs S, Stark J, et al. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362(9389):1017–1021. doi: 10.1016/s0140-6736(03)14410-8.
- Carlsen SM, Vanky E, Fleming R. Anti-Mullerian hormone concentrations in androgen-suppressed women with polycystic ovary syndrome. Hum Reprod. 2009;24(7):1732–1738. doi:10.1093/humrep/dep074.
- Piltonen TT, Komsi E, Morin-Papunen LC, et al. AMH as part of the diagnostic PCOS workup in large epidemiological studies. Euro J Endocrinol. 2023;188(6):547–554. doi:10.1093/ejendo/lvad065.
- Wei WY, Chang CH, Ru YC, et al. The effect of medication on serum anti-müllerian hormone (AMH) levels in women of reproductive age: a meta-analysis. BMC Endocr Disord. 2022;22(1):158. doi:10.1186/s12902-022-01065-9.
- Rethlefsen ML, Kirtley S, Waffenschmidt S, et al. PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. J Med Lib Assoc JMLA. 2021;109(2):174–200. doi:10.5195/jmla.2021.962.
- Claudio L, Nicola V, Alessia N, et al. Assessing the quality of studies in meta-research: review/guidelines on the most important quality assessment tools. Pharm Stat. 2020;20(1):185–195. doi:10.1002/pst.2068.
- Yumiko T, Yoshikazu K, Yuko H, et al. Anti-müllerian hormone levels in the diagnosis of adolescent polycystic ovarian syndrome: a systematic review and meta-analysis. Endocrine Journal. 2022;69(8):897–906. doi:10.1507/endocrj.EJ22-0081.
- Hughes L, Komorowski AS, Aaby DA, et al. The effect of clomiphene, metformin, or both on anti- müllerian hormone (amh) levels: a new look at the pregnancy in polycystic ovarian syndrome trial (ppcos I). Fertil Steril. 2022;118(4S):e193–e193, doi:10.1016/j.fertnstert.2022.08.549.
- Fatemeh F, Mansooreh S, Hosseini KA, et al. Effect of metformin on the anti-müllerian hormone level in infertile women with polycystic ovarian syndrome. Electron Phys. 2017;9(12):5969–5973. doi:10.19082/5969.
- Panidis D, Georgopoulos NA, Piouka A, et al. The impact of oral contraceptives and metformin on anti-müllerian hormone serum levels in women with polycystic ovary syndrome and biochemical hyperandrogenemia. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2011;27(8):587–592. doi:10.3109/09513590.2010.507283.
- Romualdi D, De Cicco S, Tagliaferri V, et al. The metabolic status modulates the effect of metformin on the antimullerian hormone-androgens-insulin interplay in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2011;96(5):E821–E824. doi:10.1210/jc.2010-1725.
- Carlsen SM, Vanky E, Fleming R. Anti-Müllerian hormone concentrations in androgen-suppressed women with polycystic ovary syndrome. Hum Reprod. 2009;24(7):1732–1738. doi: 10.1093/humrep/dep074.
- Aykut B, Heather T, Rebecca U, et al. Acute effects of metformin therapy include improvement of insulin resistance and ovarian morphology. Fert Steril. 2007;87(4):870–875. doi: 10.1016/j.fertnstert.2006.08.096.
- Nazirudeen R, Sridhar S, Priyanka R, et al. A randomized controlled trial comparing myoinositol with metformin versus metformin monotherapy in polycystic ovary syndrome. Clin Endocrinol (Oxf). 2023;99(2):198–205. doi: 10.1111/cen.14931.
- Delbar D, Soleimani MM, Shahrzad Z, et al. Sitagliptin/metformin improves the fertilization rate and embryo quality in polycystic ovary syndrome patients through increasing the expression of GDF9 and BMP15: a new alternative to metformin (a randomized trial.). J Reprod Immunol. 2022;150(prepublish):103499.
- Keerthana R, Neena M, Reeta M, et al. Myoinositol versus metformin pretreatment in GnRH-antagonist cycle for women with PCOS undergoing IVF: a double-blinded randomized controlled study. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2021;38(2):1–8.
- Chhabra N, Malik S. Effect of insulin sensitizers on raised serum anti-mullerian hormone levels in infertile women with polycystic ovarian syndrome. J Hum Reprod Sci. 2018;11(4):348–352. doi: 10.4103/jhrs.JHRS_59_17.
- Diogo AN, Alves LLS, Carolina ARSDJ, et al. Effects of metformin on serum insulin and anti-mullerian hormone levels and on hyperandrogenism in patients with polycystic ovary syndrome. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2013;29(3):246–249. doi:10.3109/09513590.2012.736563.
- Wiweko B, Susanto AC. The effect of metformin and cinnamon on serum anti-mullerian hormone in women having PCOS: a Double-Blind, randomized, controlled trial. J Hum Reprod Sci. 2017;10(1):31–36. doi:10.4103/jhrs.JHRS_90_16.
- Neagu M, Cristescu C. Anti-Műllerian hormone–a prognostic marker for metformin therapy efficiency in the treatment of women with infertility and polycystic ovary syndrome. J Med Life. 2012;5(4):462–464.
- Angela F, Morena R, Tiziana R, et al. Serum and follicular anti-mullerian hormone levels in women with polycystic ovary syndrome (PCOS) under metformin. J Ovarian Res. 2010;3(1):16. doi:10.1186/1757-2215-3-16.
- Terhi P, Laure M, Riitta K, et al. Serum anti-müllerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod (Oxford, England). 2005;20(7):1820–1826. doi:10.1093/humrep/deh850.
- Francisco F, Camil C, Francisco C, et al. The effect of different hormone therapies on anti-müllerian hormone serum levels in anovulatory women of reproductive age. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2011;27(4):216–224. doi:10.3109/09513590.2010.487595.
- Tomova A, Deepinder F, Robeva R, et al. Anti-Mullerian hormone in women with polycystic ovary syndrome before and after therapy with metformin. Horm Metab Res. 2011;43(10):723–727. doi:10.1055/s-0031-1286307.
- Madsen HN, Lauszus FF, Trolle B, et al. Impact of metformin on anti-müllerian hormone in women with polycystic ovary syndrome: a secondary analysis of a randomized controlled trial. Acta Obstet Gynecol Scand. 2015;94(5):547–551. doi: 10.1111/aogs.12605.
- Saleh BO, Ibraheem WF, Ameen NS. The role of anti-Mullerian hormone and inhibin B in the assessment of metformin therapy in women with polycystic ovarian syndrome. Saudi Med J. 2015;36(5):562–567. doi: 10.15537/smj.2015.5.11112.
- Froment P, Plotton I, Giulivi C, et al. At the crossroads of fertility and metabolism: the importance of AMPK-dependent signaling in female infertility associated with hyperandrogenism. Hum Reprod. 2022;37(6):1207–1228. doi: 10.1093/humrep/deac067.
- Harborne LR, Sattar N, Norman JE, et al. Metformin and weight loss in obese women with polycystic ovary syndrome: comparison of doses. J Clin Endocrinol Metab. 2005;90(8):4593–4598. doi:10.1210/jc.2004-2283.
- Fleming R, Harborne L, MacLaughlin DT, et al. Metformin reduces serum mullerian-inhibiting substance levels in women with polycystic ovary syndrome after protracted treatment. Fert Steril. 2005;83(1):130–136. doi:10.1016/j.fertnstert.2004.05.098.
- Zhijiao Z, Hongzhi C, Ling C, et al. The effects of metformin on anti-müllerian hormone levels in patients with polycystic ovary syndrome: a systematic review and meta-analysis. J Ovarian Res. 2023;16(1):123. doi: 10.1186/s13048-023-01195-1.
- Moolhuijsen LME, Visser JA. Anti-müllerian hormone and ovarian reserve: update on assessing ovarian function. J Clin Endocrinol Metab. 2020;105(11):3361–3373. doi: 10.1210/clinem/dgaa513.
- Tunc HT, Dilek C, Ozlem DG, et al. Determining the age group-based cut-off values of serum anti-mullerian hormone concentrations to diagnose polycystic ovary syndrome. Curr Med Res Opin. 2023;39(6):855–863. doi:10.1080/03007995.2023.2204768.