Abstract
Background
Dienogest (DNG) improves endometriosis-associated pain (EAP) and patients’ quality of life; however, the modern cornerstone of the management of endometriosis is the long-term adherence of the patient to medical treatment.
Objective
To evaluate DNG as a long-term treatment of endometriosis, focusing on patients’ compliance and side effects, also correlating with different phenotypes of endometriosis.
Methods
This was a cohort study on a group of patients with endometriosis (n = 114) undergoing long-term treatment with DNG. During the follow up visits (12, 24, and 36 months) patients were interviewed: an assessment of EAP was performed by using a visual analogue scale (VAS) and side effects were evaluated by using a specific questionnaire of 15 items.
Results
At 12 months, 81% were continuing the DNG treatment, with a significant reduction of dysmenorrhea, dyspareunia, dyschezia, dysuria and chronic pelvic pain. Of the 19% that discontinued the treatment: 62% was due to spotting, reduced sexual drive, vaginal dryness, and mood disorders. The improvement of EAP was significant for all endometriosis phenotypes, especially in patients with the deep infiltrating type. At 36 months, 73% of patients were continuing the treatment, showing a significant reduction of EAP through the follow up, along with an increase of amenorrhea (from 77% at 12 months to 93% at 36 months). In a subgroup of 18 patients with gastrointestinal disorders, DNG was administered vaginally at the same dosage, showing similar results in terms of efficacy and tolerability.
Conclusions
DNG was an effective long-term treatment for all endometriosis phenotypes, with few side effects that caused the discontinuation of the treatment mainly during the first year. Thus, the course of 1-year treatment is a predictive indicator for long-term treatment adherence.
Introduction
Endometriosis is a benign chronic inflammatory disease of reproductive age women [Citation1]. In the last decade, its prevalence has increased and currently it remains a complex condition, affecting deeply female quality of life (QoL) [Citation2]. Treatment needs to be tailored to each patient, according to their age, pregnancy desire, symptoms and endometriosis phenotypes [Citation3,Citation4].
Medical treatment with hormonal drugs is the first line therapeutical option [Citation5,Citation6]. They block the menstrual cycle and reduce endometriosis related symptoms by inducing a pseudo-pregnancy state and/or reducing estrogen ovarian secretion by blocking the hypothalamic-pituitary-ovary axis. The first-choice treatment are progestins, due to their efficacy and good tolerability despite their low costs: dienogest (DNG), norethisterone acetate and medroxyprogesterone acetate are the compounds approved by different government agencies. By binding to progesterone receptors, progestins act through different mechanisms: they reduce the secretion of follicle stimulating hormone (FSH) and luteinizing hormone (LH), inducing anovulation and endometrial pseudo-decidualization, in addition to inhibiting angiogenesis, decreasing oxidative stress and increasing apoptosis of endometrial cells [Citation7–9].
DNG is a 19-nortestosterone derivative labeled for endometriosis [Citation10–12]. It improves endometriosis related symptoms and patients’ QoL, reducing the size of endometriomas and preventing recurrences after surgery [Citation13–20]. The most frequent reported side effects are abnormal uterine bleeding (AUB) and headache.
The primary aim of the present study was to evaluate DNG as a long-term treatment for endometriosis, from 12 months of follow up to 36 months, focusing on compliance of the patient and reported side effects. A secondary objective was to correlate different phenotypes of endometriosis with the efficacy of the treatment.
Methods
This was a cohort study of prospectively collected data from fertile aged patients who came to our Endometriosis Center (between July 2019 and August 2021) with the diagnosis of endometriosis and undergoing treatment with DNG (2 mg). Our inclusion criteria were reproductive age, presence of endometriosis related symptoms, whereas those with contraindications for hormonal treatment, the desire of pregnancy or seeking hormonal contraception were excluded. A group of 114 patients were included in the study. During the first visit, we collected all the clinical data (age, family history of gynecological disorders, parity, age at menarche, characteristics of the cycle in adolescence, previous hormonal treatment, gastrointestinal or urinary symptoms, past medical history (including questions about headache, autoimmune disorders, psychological disorders), previous surgery, endometriosis related symptoms ().
Table 1. Baseline characteristics of the study population.
The phenotypes of endometriosis were as follows: ovarian endometrioma (OMA, n = 23), deep infiltrating endometriosis (DIE, n = 38) and mixed phenotype with both OMA and DIE (n = 53). According to the patient’s clinical history, symptoms and imaging (transvaginal ultrasound or MRI), we planned the first follow up visit to our clinic after 6 months and then every 12 months. During the follow up visits (12, 24, and 36 months) patients were interviewed.
A visual analog scale (VAS) of 10 points (measured in cm) was used to assess EAP: dysmenorrhea, dyspareunia, chronic pelvic pain (CPP), dysuria, dyschezia. Baseline values were compared to values obtained at each follow up.
Side effects were evaluated by using a specific questionnaire of 15 items, regarding weight gain or hydric retention, moods disorders, libido, gastrointestinal symptoms, headache, dermatological symptoms, hot flushes, breast tenderness, and breakthrough bleeding. Each item can be rated from 0 (absence of symptoms), 1 (few/mild), 2 (often/moderate), 3 (daily/severe, interfering with QoL). Then if the symptom was already present at the beginning of the treatment, at follow-up, if this got worst (four), unchanged (five) or improved (six). The questionnaire was always filled out by the patients with the help of a healthcare professional of the clinic. After the DNG prescription, patients were requested to report side effects by mail or by phone calls; if this was judged as clinically relevant, the consultant could anticipate the follow up visit.
The comparison between EAP and side effects was conducted by evaluating data from the same cohort of patients during subsequent follow-up visits. Specifically, all data collected pertained to patients who remained under DNG treatment at each time point (12, 24, 36 months), adhering to a per-protocol analysis approach.
Ethical approval
The study protocol was approved by the local Ethics Committee (n.14558_oss approved on 28 May 2019). Prior to initiation of treatment, patients were informed that DNG does not offer a definitive cure for the disease, but rather constitutes a long-term medical regimen aimed at improving their QoL. It was emphasized that the treatment may lead to the reduction of symptoms, despite it can be associated with some side effects. Before being enrolled in the study, patients provided informed written consent for their clinical data to be used for scientific research purposes.
Statistical analysis
Collected data were entered in an electronic database and analyzed with SPSS software (Statistical Package for Social Science; IBM SPSS Statistics 23, IBM Corporation). Continuous data were checked for normality by using normal probability plots. A descriptive analysis was conducted with the evaluation of position measures (mean, median) and dispersion indices (standard deviation, range) for the quantitative variables. Binomial variables are described as frequencies n (%). According to normality distribution of data, the Mann Whitney U test or independent-sample T test was used to compare continuous variables. One-way ANOVA was used to compare pain scores according to endometriosis phenotypes. A p value < .05 was considered as statistically significant.
Results
After 12 months of DNG treatment, a statistically significant reduction of dysmenorrhea (p < .05), dyspareunia (p < .05), dyschezia (p < .05), dysuria (p < .05) and CPP (p < .05) was achieved (). Comparing changes of endometriosis associated pain (EAP) according to endometriosis phenotypes, the reduction of dysmenorrhea (p = .014), dyspareunia (p = .025) and dysuria (p = .035) were significantly better in the DIE and mixed phenotypes ().
Figure 1. VAS change of EAP from baseline to 12-months follow up. Data represent in box and whiskers plot.
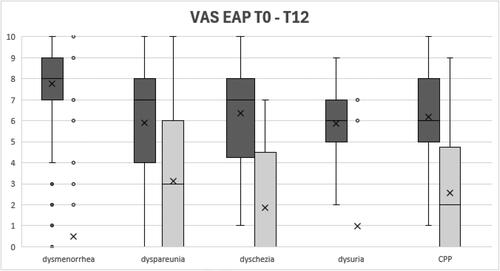
Table 2. Delta Change of VAS for endometriosis-associated pain (EAP) in study population and according to endometriosis phenotypes (mean ± standard deviation). CPP, chronic pelvic pain.
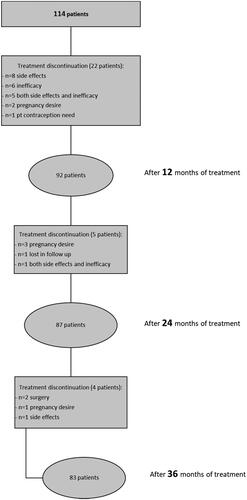
At 12 months, amenorrhea was reached by 77%; meanwhile, 23% reported breakthrough bleeding, of whom 52% was spotting, 38% was mild bleeding and 10% was moderate bleeding. At 12 months follow up, ninety-two patients (81%) were continuing the therapy, whereas twenty-two patients (19%) suspended it. The reported causes of discontinuation were: side effects (36%), inefficacy (27%), both inefficacy and side effects (23%), desire of pregnancy (9%), and one seeking contraception (5%). Among the side effects reported for discontinuing DNG treatment, the most frequent were AUB, reduced sexual drive, vaginal dryness, and mood disorders. The average time for discontinuation was 7.2 months. The overall reported side effects were hydric retention (29.3%), weight gain (26% with a mean value of 2.0 kg), AUB (22.9%), breast tenderness (20.7%), abdominal bloating (18.7%), mood disorders (17.4%), headache (16.3%), reduced sexual drive (15.2%), vaginal dryness (15.2%), hot flushes (10.9%), acne (10.8%), hair loss (9.8%), insomnia (8.7%), seborrhea (6.6%) and hirsutism (6.6%) (). In a subgroup of 18 patients with persistent irregular bleeding and other side effects, along with the presence of preexisting gastrointestinal symptoms potentially impairing the oral adsorption of the drug, DNG was successfully administered vaginally with similar efficacy on EAP.
Table 3. Side effects at 12 months follow up. Data are presented as frequencies n (%).
At 24 months, 76% were continuing the treatment. Three patients suspended DNG due to a desire of pregnancy, one was lost to follow up, and one reported inefficacy and side effects (AUB, headache, alopecia, and hot flushes). At 36 months, 73% were continuing the treatment (). Two patients suspended it to undergo surgery, one due to pregnancy desire and one due to side effects. This last patient interrupted DNG at 34 months despite her great improvement regarding EAP (VAS score 0 for all evaluated aspects) due to an important reduction of sexual drive with consecutive abstention of sexual intercourse, weight gain (+8 kg), headache, and hair loss.
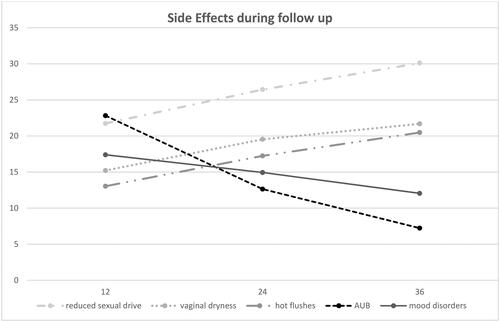
During the follow up, we reported a higher frequency of reduced sexual drive (23/87, 26% at 24 months; 25/83, 30% at 36 months), vaginal dryness (17/87, 20% at 24 months; 18/83, 22% at 36 months), hot flushes (15/87, 17% at 24 months; 17/83, 20% at 36 months). On the other hand, there was a progressive decrease of AUB (11/87 [13%] referred spotting at 24 months; 6/83 [7%] at 36 months) and mood disorders (13/87 [15%] at 24 months; 10/83 [12%] at 36 months) ().
Discussion
The present study shows that DNG is a suitable option for a long-term treatment of endometriosis, with good efficacy in decreasing EAP, independently of endometriosis phenotype. Moreover, the highest reduction of pain scores was observed when DIE lesions were present. Nevertheless, it has already been reported the non-inferiority of DNG compared to GnRH agonists as a post-operative treatment of DIE, with better tolerability indeed [Citation14]. In our study, a significant reduction of dysmenorrhea, dyspareunia, dyschezia, dysuria and CPP was achieved and maintained over the follow up, supporting the increased QoL in patients undergoing DNG treatment [Citation12,Citation21].
The patients’ adherence to the treatment regimen remained high throughout the follow up, with 81% of individuals continuing DNG at 12 months, followed by 76% at 24 months, and 73% at the 36-month visit, confirming recent studies of long-term observation of DNG treatment [Citation11,Citation17]. Furthermore, the course of the first 6 months of treatment resulted crucial, both in terms of response and tolerance, since the average time for treatment discontinuation was 7 months. The rate of amenorrhea increased up 93% at 36 months, showing a progressive reduction in bleeding across time, supporting the importance of counseling patients regarding bleeding irregularities especially during the first months of treatment, to increase the compliance [Citation22].
The primary reason for discontinuation of treatment was due to side effects (including those patients that interrupted the treatment for both side effects and inefficacy of the drug) and overall, nearly 90% of them discontinued the treatment during the first year. Given that the initial year signifies the period with the highest observed prevalence of therapy withdrawal, it could be used as a predictive indicator for long-term treatment tolerance, potentially enabling the assessment of a patient’s likelihood to tolerate the drug over an extended duration [Citation13].
The withdrawal from treatment was notably influenced by specific side effects, including reduced sexual drive, AUB, vaginal dryness, and mood disorders. Throughout the follow-up, these side effects underwent changes in frequency, along with the onset of hot flushes, despite an overall low rate observed in our study as in others [Citation11,Citation23]. Particularly, reduced sexual drive, vaginal dryness, and hot flushes demonstrated an increase during the 36-month follow-up, whereas AUB and mood disorders significantly decreased. Common side effects such as weight gain, hydric retention, headache, breast tenderness remained unchanged in frequency over the study period. In case of symptoms already present before DNG treatment, for instance a preexisting headache, this was improved after initiating DNG therapy. In addition, the observation that the vaginal administration of DNG pill may overcome some of these side effects is of interest for some endometriosis patients who require a long-term treatment. The vagina has previously shown to be a good route of administration for hormones [Citation24], and notably for progestins labeled for the treatment of endometriosis, such as danazol, resulted efficient also by vaginal administration with less systemic side effects [Citation25].
In our cohort, despite the presence of side effects, patients continued DNG treatment due to the significant reduction of EAP in all evaluated aspects, hence, significantly improving their QoL. Besides, all the side effects were from mild to moderate in intensity. Furthermore, the use of a specific questionnaire with 15-items allowed a detailed collection of side effects, representing a strength of the study. Nevertheless, the influence of comorbidities should be considered when evaluating symptoms such as abdominal bloating, headache and mood disorders, as confounding factors for side effects profile. Despite the limited sample size, the long follow up of patients is a strength of the study and provides a reliable overview of real-life experience of DNG treatment.
In conclusion, DNG is an effective long-term treatment for all phenotypes of endometriosis, with few side effects, causing the discontinuation of the treatment mainly during the first year. Thus, the course of 1-year treatment is a predictive indicator for long-term treatment adherence.
Authors contributors
F. La Torre, S. Vannuccini and F. Petraglia were involved in the study conception and design. F. Toscano, E. Gallucci, G. Orlandi and V. Manzi carried out the surveys and collected the data. F. La Torre and S. Vannuccini performed the statistical analysis. F. La Torre and S. Vannuccini performed the drafting of the manuscript. All authors were involved in critically revising the manuscript for its intellectual content, and the final approval of the manuscript was performed by all authors.
Disclosure statement
No potential competing interest was reported by the authors related to the present study.
Data availability statement
The data that support the findings of this study are available from the corresponding author (F.P) upon reasonable request.
Additional information
Funding
References
- Bulun SE, Yilmaz BD, Sison C, et al. Endometriosis. Endocr Rev. 2019;40(4):1–7. doi: 10.1210/er.2018-00242.
- Vannuccini S, Reis FM, Coutinho LM, et al. Surgical treatment of endometriosis: prognostic factors for better quality of life. Gynecol Endocrinol. 2019;35(11):1010–1014. doi: 10.1080/09513590.2019.1616688.
- Saunders PTK, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021;184(11):2807–2824. doi: 10.1016/j.cell.2021.04.041.
- Chapron C, Marcellin L, Borghese B, et al. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol. 2019;15(11):666–682. doi: 10.1038/s41574-019-0245-z.
- Capezzuoli T, Rossi M, La Torre F, et al. Hormonal drugs for the treatment of endometriosis. Curr Opin Pharmacol. 2022;67:102311. doi: 10.1016/j.coph.2022.102311.
- Vannuccini S, Clemenza S, Rossi M, et al. Hormonal treatments for endometriosis: the endocrine background. Rev Endocr Metab Disord. 2022;23(3):333–355. doi: 10.1007/s11154-021-09666-w.
- Reis FM, Coutinho LM, Vannuccini S, et al. Progesterone receptor ligands for the treatment of endometriosis: the mechanisms behind therapeutic success and failure. Hum Reprod Update. 2020;26(4):565–585. doi: 10.1093/humupd/dmaa009.
- Grandi G, Mueller M, Bersinger NA, et al. Does dienogest influence the inflammatory response of endometriotic cells? A systematic review. Inflamm Res. 2016;65(3):183–192. doi: 10.1007/s00011-015-0909-7.
- Miyashita M, Koga K, Takamura M, et al. Dienogest reduces proliferation, aromatase expression and angiogenesis, and increases apoptosis in human endometriosis. Gynecol Endocrinol. 2014;30(9):644–648. doi: 10.3109/09513590.2014.911279.
- Strowitzki T, Marr J, Gerlinger C, et al. Dienogest is as effective as leuprolide acetate in treating the painful symptoms of endometriosis: a 24-week, randomized, multicentre, open-label trial. Hum Reprod. 2010;25(3):633–641. doi: 10.1093/humrep/dep469.
- Cho B, Roh J-W, Park J, et al. Safety and effectiveness of dienogest (visanne®) for treatment of endometriosis: a large prospective cohort study. Reprod Sci. 2020;27(3):905–915. doi: 10.1007/s43032-019-00094-5.
- Petraglia F, Hornung D, Seitz C, et al. Reduced pelvic pain in women with endometriosis: efficacy of long-term dienogest treatment. Arch Gynecol Obstet. 2012;285(1):167–173. doi: 10.1007/s00404-011-1941-7.
- Barra F, Scala C, Leone Roberti Maggiore U, et al. Long-Term administration of dienogest for the treatment of pain and intestinal symptoms in patients with rectosigmoid endometriosis. J Clin Med. 2020;9(1):154. doi: 10.3390/jcm9010154.
- Ceccaroni M, Clarizia R, Liverani S, et al. Dienogest vs GnRH agonists as postoperative therapy after laparoscopic eradication of deep infiltrating endometriosis with bowel and parametrial surgery: a randomized controlled trial. Gynecol Endocrinol. 2021;37(10):930–933. doi: 10.1080/09513590.2021.1929151.
- Uludag SZ, Demirtas E, Sahin Y, et al. Dienogest reduces endometrioma volume and endometriosis-related pain symptoms. J Obstet Gynaecol. 2021;41(8):1246–1251. doi: 10.1080/01443615.2020.1867962.
- Capezzuoli T, Vannuccini S, Mautone D, et al. Long-term hormonal treatment reduces repetitive surgery for endometriosis recurrence. Reprod Biomed Online. 2021;42(2):451–456. doi: 10.1016/j.rbmo.2020.09.018.
- Maiorana A, Maranto M, Restivo V, et al. Evaluation of long-term efficacy and safety of dienogest in patients with chronic cyclic pelvic pain associated with endometriosis. Arch Gynecol Obstet. 2024;309(2):589–597. doi: 10.1007/s00404-023-07271-7.
- Römer T. Long-term treatment of endometriosis with dienogest: retrospective analysis of efficacy and safety in clinical practice. Arch Gynecol Obstet. 2018;298(4):747–753. doi: 10.1007/s00404-018-4864-8.
- Xholli A, Filip G, Previtera F, et al. Modification of endometrioma size during hormone therapy containing dienogest. Gynecol Endocrinol. 2020;36(6):545–549. doi: 10.1080/09513590.2019.1703942.
- Angioni S, Pontis A, Malune ME, et al. Is dienogest the best medical treatment for ovarian endometriomas? Results of a multicentric case control study. Gynecol Endocrinol. 2020;36(1):84–86. doi: 10.1080/09513590.2019.1640674.
- Techatraisak K, Hestiantoro A, Soon R, et al. Impact of long-term dienogest therapy on quality of life in asian women with endometriosis: the prospective non-interventional study ENVISIOeN. Reprod Sci. 2022;29(4):1157–1169. doi: 10.1007/s43032-021-00787-w.
- Murji A, Biberoğlu K, Leng J, et al. Use of dienogest in endometriosis: a narrative literature review and expert commentary. Curr Med Res Opin. 2020;36(5):895–907. doi: 10.1080/03007995.2020.1744120.
- Lee J, Park HJ, Yi KW. Dienogest in endometriosis treatment: a narrative literature review. Clin Exp Reprod Med. 2023;50(4):223–229. doi: 10.5653/cerm.2023.06128.
- Buggio L, Lazzari C, Monti E, et al. “Per vaginam” topical use of hormonal drugs in women with symptomatic deep endometriosis: a narrative literature review. Arch Gynecol Obstet. 2017;296(3):435–444. doi: 10.1007/s00404-017-4448-z.
- Razzi S, Luisi S, Calonaci F, et al. Efficacy of vaginal danazol treatment in women with recurrent deeply infiltrating endometriosis. Fertil Steril. 2007;88(4):789–794. doi: 10.1016/j.fertnstert.2006.12.077.