Abstract
Objective
To evaluate the effects of alpha lipoic acid (ALA) on hormonal and metabolic parameters in a group of overweight/obese Polycystic Ovary Syndrome (PCOS) patients.
Methods
This was a retrospective study in which thirty-two overweight/obese patients with PCOS (n = 32) not requiring hormonal treatment were selected from the database of the ambulatory clinic of the Gynecological Endocrinology Center at the University of Modena and Reggio Emilia, Italy. The hormonal profile, routine exams and insulin and C-peptide response to oral glucose tolerance test (OGTT) were evaluated before and after 12 weeks of complementary treatment with ALA (400 mg/day). Hepatic Insulin Extraction (HIE) index was also calculated.
Results
ALA administration significantly improved insulin sensitivity and decreased ALT and AST plasma levels in all subjects, though no changes were observed on reproductive hormones. When PCOS patients were subdivided according to the presence or absence of familial diabetes background, the higher effects of ALA were observed in the former group that showed AST and ALT reduction and greater HIE index decrease.
Conclusion
ALA administration improved insulin sensitivity in overweight/obese PCOS patients, especially in those with familial predisposition to diabetes. ALA administration improved both peripheral sensitivity to insulin and liver clearance of insulin. Such effects potentially decrease the risk of nonalcoholic fat liver disease and diabetes in PCOS patients.
Introduction
Polycystic ovary syndrome (PCOS) is a frequent reproductive disorder that affects up to 25% of women of reproductive age depending mainly from the ethnicity [Citation1,Citation2]. The presence of two out of three of the criteria established at the consensus meeting in Rotterdam [Citation3] allows the diagnosis of PCOS. However, in this last decade the issue of a dismetabolic state, that is insulin resistance (IR) and the correlated compensatory hyperinsulinemia, has demonstrated to be relevant and very frequent in PCOS patients [Citation4–6]. In fact, other than the well-known clinical symptoms, PCOS frequently show metabolic problems, such as obesity (up to 50% of the patients), IR and compensatory hyperinsulinemia. Such hyperinsulinemic conditions due to IR has been considered a putative factor triggering PCOS together with the hyperandrogenic conditions [Citation7].
Such compensatory hyperinsulinemia has been observed in up to 50–70% of women with PCOS and obesity and in 15–30% of PCOS women with normal weight [Citation5,Citation8]. Even though such hyperinsulinemia is a biological attempt to keep circulating glucose under control, it represents the putative trigger of incurring in metabolic complications such as metabolic syndrome (MS) [Citation9,Citation10] and nonalcoholic fatty liver disease (NAFLD) and/or liver fibrosis very frequent in PCOS patients [Citation11].
As the liver is deeply involved in the clearance process of insulin, a new index, HIE (Hepatic Insulin Extraction), has recently been applied to obese PCOS subjects, demonstrating to be tightly linked with IR [Citation12,Citation13]. Briefly, HIE index is the ratio between insulin and C-peptide plasma levels and reflects the balance between the kinetics of synthesis and the clearance of the two peptides. HIE is usually calculated as the ratio between the area under the curve (AUC) of insulin and the AUC of C-peptide (AUC Ins/AUC C-Pept) [Citation12,Citation14] but also as the ratio between insulin and C-peptide plasma levels after overnight fasting [Citation15]. HIE has been reported to increase with the increase of IR of muscle and adipose tissues and also when there is a reduced liver ability to clear insulin [Citation12,Citation16]. Liver degrades insulin through the action of a specific enzyme, i.e. the insulin degrading enzyme (IDE) [Citation17]. Additionally, HIE index has been considered an important index for the prevention of nonalcoholic fat liver disease (NAFLD) and hepatic steatosis [Citation11,Citation12]. Since the HIE index reflects insulin kinetics as a balance between pancreatic insulin synthesis and its hepatic clearance, C-peptide almost exclusively reflects pancreatic synthesis because hepatic C-peptide clearance is minimal [Citation12,Citation13,Citation18]. HIE index represents the link between hepatic functions and the risk of NAFLD not only in the normal population but especially in PCOS because these patients show a greater incidence of NAFLD than healthy women due to the high occurrence of IR [Citation19,Citation20]. Recent studies have reported that the HIE index is higher in obese PCOS patients with familial diabetes than in those with no familial predisposition [Citation15,Citation21]. In addition, it has been observed that IR and NAFLD are frequently combined with elevation, within the higher limits of normality, of ALT and AST even though such elevation has not considered a marker of liver dysfunction [Citation19]. Interestingly, our group recently observed that ALA administration significantly decreased ALT and AST plasma concentrations in obese PCOS treated for 12 weeks, thus suggesting a putative role of the anti-oxidant role of ALA on the hepatocyte function [Citation22]. On such basis, we aimed to evaluate the HIE index in a group of overweight/obese PCOS patients before and after 12 weeks of integrative administration of alpha lipoic acid (ALA), exploring also HIE index behavior under oral glucose tolerance test (OGTT).
Methods
Study design and subjects
This was a retrospective study in which we analyzed the outpatients’ database of PCOS subjects attending the ambulatory clinic of the Gynecological Endocrinology Center at the University of Modena and Reggio Emilia, Italy between January 2022 and April 2023. For this, a group of overweight/obese PCOS patients (n = 32) were selected among those not requiring hormonal treatment. On such basis, we selected the patients that received an integrative approach with ALA (400 mg/day) and that had completed the baseline screening and the first clinical check after 3 months of treatment. All these patients (n = 32) were selected according to the criteria established by the American Society for Reproductive Medicine and the European Society for Human Reproduction and Embryology for diagnosing the presence of PCOS [Citation3], and at least two of the following criteria had to be present: (a) oligomenorrhea with inter-menstrual intervals longer than 45 days, (b) clinical (acne, hirsutism) or biochemical signs of hyperandrogenism, (c) presence of micro-polycystic ovaries, i.e. ≥ 20 follicles per ovary and/or an ovarian volume ≥ 10 ml on either ovary (detected using ultrasound transducers with a frequency bandwidth that includes 8 MHz) [Citation4]. In addition, patients had to fulfill the following criteria: (a) absence of enzymatic adrenal deficiency and/or other endocrine disease, including diabetes, (b) normal prolactin (PRL) levels (range 5–25 ng/mL), (c) no hormonal treatment during a period of at least 6 months prior to the study, (d) body mass index (BMI) above 26. None of the subjects was taking medications and/or steroids, or other drugs (i.e. contraceptives or metformin) within the 3 months prior to the evaluation.
From the database, specific anamnestic information was extracted such as whether or not the patients had one or more first-degree relative (parents and/or grandparents) with diabetes. On such basis, 20 out of 32 (62.5%) PCOS subjects resulted to have familial predisposition to diabetes, and 12 patients (37.5%) did not.
Typically, our center screens PCOS subjects before and after at least 3 months (12 weeks) of treatment as follows: on day 3–6 of the menstrual cycle and on day 3–6 of the first bleeding occurring after the 12th week of treatment. All patients were evaluated for luteinizing hormone (LH), follicle stimulating hormone (FSH), thyroid stimulating hormone (TSH), estradiol (E2), progesterone (P), PRL, androstenedione (A), 17-hydroxyprogesterone (17OHP), dehydroepiandrosterone sulfate (DHEAS), cortisol, insulin, glutamic aspartate amino transferase (AST) and alanine amino transferase (ALT). Total cholesterol, HDL-C, LDL-C, triglyceride (TG) plasma levels were also evaluated. Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) index was computed to estimate sensitivity to insulin [Citation23].
An OGTT was performed by determining levels of blood glucose, in addition to insulin to C-peptide, at fasting baseline and at 30, 60, 90, 120, 180 and 240 min after the oral assumption of 75 g of glucose. This was performed before and after the 12 weeks of ALA treatment. A hyperinsulinemic response is recognized when insulin plasma levels were above 50 μU/mL within 90 min of glucose load [Citation24].
Informed consent was obtained from all individual referring to our out-patient ambulatory clinic of the Gynecological Endocrinology Center as a standard procedure of the University of Modena and Reggio Emilia, Italy, before proceeding to the diagnostic investigation. The study was approved by the local Ethical Committee as a retrospective observational study.
Statistical analysis
Statistical analysis was performed with QuickStatCalculations (https://www.socscistatistics.com/). Data are presented as mean ± standard error of the mean (SEM). Analysis of variance (one-way ANOVA) was performed to test for statistically significant differences between the groups (before and after the treatment) and then Student’s T test was applied for paired and unpaired data, as appropriate. The HOMA-IR index was computed to estimate sensitivity to insulin [Citation23] and calculated as: fasting insulin mU/L) × (fasting glucose mmol/Ll)/22.5 [Citation23]. The cutoff value we used to define IR was 2.71, as previously stated [Citation23,Citation24]. The HIE index was calculated as the ratio between insulin and C-peptide plasma concentrations (insulin/C-peptide), as previously reported [Citation15,Citation17]. This index was determined upon on all sampling of the OGTT.
Blood assays
To minimize errors, all samples from each subject (baseline and after treatment) were run in the same assay. Plasma LH and FSH concentrations were determined using a previously described immunofluorometric assay [Citation25]. The sensitivity of the assay, expressed as the minimal detectable dose, was 0.1 IU/mL. Intra-assay and inter-assay coefficients of variation were 4.2% and 6.1%, respectively. Plasma PRL, E2, P, A, TSH, 17OHP, DHEAS, cortisol as well as glycemic and lipid profiles (Triglyceride, total cholesterol, HDL-C, and LDL-C) were determined by standard routine procedure by the Modena Hospital Central Laboratory. Plasma insulin and C-peptide concentrations were determined using an immunoradiometric assay (Biosource Europa S.A., Nivelles, Belgium) [Citation15]. Based on two quality control samples, the average within- and between-assay coefficients of variation were 4.1% and 10.1%.
Results
summarizes hormonal characteristics of subjects under study. Glucose, insulin, AST, and ALT plasma levels were significantly reduced by the integrative administration of ALA. As a result of the decreased insulin plasma levels, HIE index decreased significantly after the treatment interval as well as HOMA-IR index after the overnight fasting.
Table 1. Hormonal and metabolic parameters of PCOS under study (n = 32).
According to the anamnestic investigation, 62.5% of patients reported having a history of familial diabetes. When subdividing the group of PCOS patients according to such aspect (), PCOS patients with familial diabetes predisposition at baseline, before integrative treatment, resulted to have higher ALT and AST plasma levels than the other group. After the treatment interval the former group of patients showed a significant reduction of AST, ALT, insulin plasma levels and of HOMA-IR and HIE indices, while subject with no family history of diabetes showed a significant decrease of glucose, insulin plasma levels and HOMA-IR with no changes for ALT and AST (). It is interesting to underline that the differences observed between the two groups in baseline conditions disappeared after the treatment interval ().
Table 2. Hormonal and metabolic parameters of PCOS patients under study, subdivided according the presence or absence of familial diabetes background.
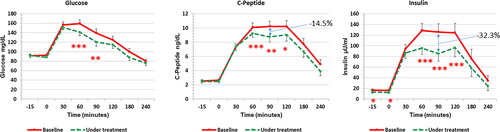
When OGTT was considered, glucose, insulin and C-peptide responses to glucose load decreased within 60–90 min from the glucose load (). Interestingly, the decrease of insulin resulted greater than that of C-peptide within 90 min after the glucose load, −32.3% and −14.5% respectively (). In addition, glucose profile showed a significant improvement ().
Figure 1. Response of glucose (left panel), insulin (middle panel) and insulin (right panel) to OGTT before and after 12 weeks of complementary treatment with ALA (400 mg/day) in all PCOS patients (n = 32). ALA treatment significantly decreases all responses. Left panel: ** p < .01, *** p < .007; middle panel: * p < .02, ** p < .006, *** p < .005; right panel: * p < .01, *** p < .0002.
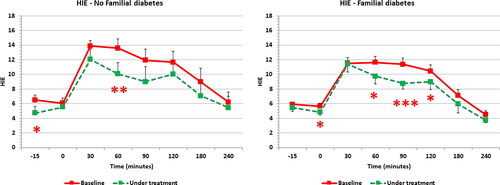
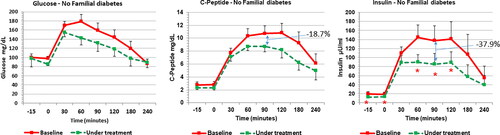
shows the HIE index profile computed along the OGTT. HIE index demonstrated the significant reduction at several points of the OGTT curve as result of a greater reduction of insulin plasma levels than those of C-peptide. When PCOS patients were subdivided according to the presence or not of familial diabetes background, both groups showed significant changes induced by the integrative treatment ( and ). Interestingly, after the integrative treatment, subjects with familial diabetes predisposition showed a reduction of C-peptide and insulin response (-11.9% and −29.9%, respectively) ( central and right panel); but only insulin resulted significantly reduced than in baseline condition (, right panel). Contrary to this, PCOS subjects without family diabetes predisposition showed only a minimal change of glucose response (, left panel) and a non-significant reduction of C-peptide profile (-18.7%) (, central panel); while insulin response resulted significantly decreased after the glucose load (up to −37.9%) ( right panel).
Figure 2. Mean calculated HIE index all along the OGTT. HIE index significantly decreased after ALA treatment interval. * p < .03, ** p < .003, *** p < .001.
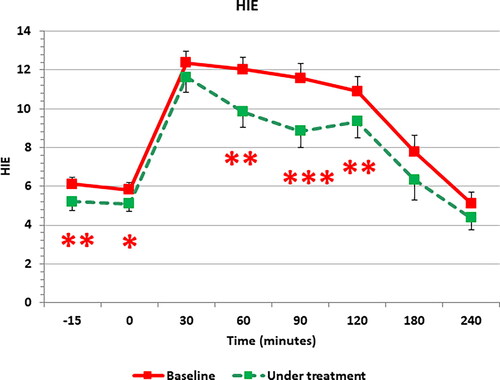
Figure 3. Response of glucose (left panel), insulin (middle panel) and insulin (right panel) to OGTT before and after 12 weeks of complementary treatment with ALA (400 mg/day) in PCOS patients with a history of familial diabetes (n = 20). ALA treatment significantly decreases insulin response but not those of glucose and C-peptide. *** p < .001.

Figure 4. Response of glucose (left panel), insulin (middle panel) and insulin (right panel) to OGTT before and after 12 weeks of complementary treatment with ALA (400 mg/day) in PCOS patients with a familial diabetes background (n = 12). ALA treatment significantly decreases insulin response but not those of glucose and C-peptide. * p < .05.
When computing HIE index in OGTT for both group of patients (), subjects with familial diabetes background showed a consistent change in the shape of the HIE index curve with significant decreases in the time range of 60–120 min (, right panel). The other group showed a change only at time 60 min (, left panel).
Discussion
The present study demonstrated that the integrative approach with ALA improved the HIE index in PCOS patients, especially in those with familial diabetes background, reducing also AST and ALT plasma levels, thus, ameliorating liver function and liver ability to clear insulin.
Our data are in agreement with what has previously been reported by our group [Citation22]. In fact, when treating PCOS patients with ALA, insulin sensitivity was improved and IR was decreased with no changes on the reproductive hormones. Our data show that insulin plasma levels decreased after 3 months of treatment together with HOMA-IR index and AST and ALT plasma levels. Such data confirm our previous work [Citation22], supporting the fact that familial diabetes background is a predisposing factor for the elevation of liver enzymes. In fact, in baseline conditions ALT and AST resulted at the upper limit of normality in PCOS patients with a history of familial diabetes and were higher than in subject without this familial background.
In recent years it has been reported that PCOS patients with a familial diabetes background have various impairments predisposing to IR. Such impairments deal with a reduced expression/function of specific enzymes deeply involved in the metabolic pathways such as epimerase, that transforms MYO-inositol (MYO) to D-chiro inositol (DCI) [Citation26,Citation27] and lipoic acid synthase (LASY) that inside the mitochondria synthetizes ALA [Citation26,Citation27]. The presence of IR in those PCOS patients with familial diabetes predisposition has been reported to be overcome when treating with a combination of DCI or MYO with ALA or using ALA alone [Citation28,Citation29], being ALA the main effector of AST and ALT decrease [Citation25], as confirmed by the present data. In fact, ALA administration significantly decreased these liver enzymes, mainly in PCOS patients with a background of family diabetes as our present data report. This observation is important since it is well known that any increase in transaminases is an index of putative hepatic impairment that, as a matter of time, might induce risk of steatosis, especially if combined with IR and compensatory hyperinsulinemia, frequent in PCOS [Citation12,Citation15]. In addition, all the differences observed between the two groups of PCOS patients in baseline conditions disappeared after the interval of ALA administration, thus supporting the effective action of ALA on the metabolic profile in both groups of PCOS patients.
For the first time, we determined the HIE index in patients undergoing ALA integrative administration to assess whether there is any link between the improved liver function, expressed as decreased transaminase circulating plasma levels, and hepatic efficiency in degrading insulin. It is well known that the liver is in charge of clearing up to 50% of the circulating insulin [Citation13,Citation18].
Our data clearly show that after ALA administration, the calculated overnight fasting HIE index resulted significantly decrease in the whole group of PCOS patients under study. Since the HIE index depends on the ratio between circulating insulin and C-peptide, a reduction of the HIE index reflects a greater decrease of insulin plasma levels. As a consequence, this observation clearly supports that the reduction of such ratio is not due to a decreased secretory ability of Langerhans islets but from the increased insulin clearance, greatly in charge of the liver that clears higher amounts of insulin from the blood circulation [Citation30,Citation31]. According to our present data, the compensatory hyperinsulinemia due to peripheral IR depends not only from a compensatory increased insulin and C-peptide secretion but also from a decreased hepatic insulin clearance, thus, maintaining elevated circulating insulin plasma levels [Citation18].
Regarding the OGTT, well defined changes of insulin and C-peptide responses were observed in all PCOS patients after ALA administration. Interestingly the highest C-peptide decrease under glucose load was observed in PCOS without familial diabetes background. Since C-peptide clearance by the liver is almost negligible, C-peptide can be intended to reflect the pancreatic synthesis of insulin [Citation13]. PCOS without familial diabetes background showed a greater reduction of both C-peptide and insulin in response to glucose load, thus indicating a greater decrease of pancreatic secretion. Contrary to this, patients with familial background showed a slight reduction of C-peptide and a greater reduction of insulin in response to OGTT. This indicates that only a small part of insulin reduction is due to the decreased pancreatic function. According to such considerations, it comes clear that any decrease of the HIE index in these patients reflect a higher amount of degraded insulin by liver function.
After the interval of treatment with ALA, PCOS patients with familial predisposition to diabetes showed greater changes in the HIE index along the OGTT profile. Such data clearly support that these patients had a higher ALA-induced improvement of the hepatic clearance of insulin. Such event can be ascribed to a higher protective effect of ALA on the liver, demonstrated by the higher decrease of transaminase plasma levels, and at the same time to the recovered expression/function of insulin degrading enzyme (IDE). The effect of ALA seems to depend on its antioxidant and anti- inflammatory actions, as previously reported [Citation32], similarly to other complementary treatment such as carnitine L-arginine and N-acetyl cysteine [Citation14]. In fact, these integrative substances have been reported to reduce the HIE index in PCOS patients thanks to the recovery of endogenous glutathione activity as anti-oxidant, improving insulin degradation [Citation14]. However, ALA seems to be greatly effective since it not only reduced the HIE index but also decreases ALT and AST levels.
Considering the differences between the two groups of PCOS patients, our data support the hypothesis that the familial predisposition to diabetes somehow participates, impairs not only the peripheral insulin sensitivity through the lower/defective expression/synthesis of both epimerase and LASY [Citation26,Citation27], but also reduces the hepatic ability to clear insulin for a reduced expression/function of IDE. Our present data highlights the clinical relevance of the integrative use of ALA, especially in PCOS patients with familial diabetes background and IR, contradicting a recent review [Citation33] that mainly focuses on the absence of reproductive improvements under ALA administration, a point we have already reported [Citation22].
In the absence of familial predisposition to diabetes, only overweight or obesity are responsible for the presence of decreased insulin sensitivity, thus inducing a compensatory hyperinsulinemia due to both higher insulin and C-peptide secretion. On the contrary, in case of the presence of familial diabetes, other than overweight/obesity, the reduced insulin sensitivity is dependent also by the impaired function/synthesis of epimerase and LASY [Citation26,Citation27,Citation32] and, additionally, to the impaired hepatic clearance of insulin, due to a reduced/abnormal expression of IDE [Citation13]. The combination of these events determines a higher amount of circulating insulin, only in part due to the overproduction but also due to a reduced clearance in PCOS patients with familial diabetes background.
Our present data support that the presence of elevated ALT and AST levels should be considered as suspicious and a signal of hepatic impairment. In fact, the combination of hyperinsulinemia and elevated transaminase has been considered a trigger for NAFLD, which occurs more frequently in patients with PCOS than in the normal population. NAFLD frequently shows elevated ALT and AST levels [Citation19] which are considered relative indicators of the presence of hepatic disease being eventually close to the upper range of normality [Citation34,Citation35]. There is growing evidence that NAFLD and PCOS share the same metabolic triggering factors [Citation36], and that NAFLD is related more to IR than to liver fat content [Citation12,Citation20]. The incidence of NAFLD in fertile women with PCOS has shown to be correlated with IR, altered lipid profiles and androgen plasma levels [Citation37,Citation38]. On such basis ALA seems to be specifically effective in the defense of the liver function.
In conclusion our study demonstrated that the integrative treatment with ALA at the daily dosage of 400 mg improves insulin sensitivity both acting peripherally on insulin sensitivity and on hepatic function, restoring not only normal transaminase levels but also IDE expression/function. The decrease of the HIE index reflects a consistent reduction of circulating insulin. Though not improving reproductive parameters, ALA represents a valid integrative approach for PCOS patients that together with an appropriate life style and diet might avoid the onset of relevant health issues that these patients might develop with passing of time.
Author contributions
A.D.G., C.B., and T.P. participated in the study design and protocol development; L.R., G.P., C.A., F.R., M.F., A.S., and E.S. performed data collection; A.D.G. formal analysis; A.D.G. writing and preparation of the original draft; A.D.G. and C.B. reviewing and editing. All authors provided input into data interpretation, and critical review and have read and approved the final manuscript.
Acknowledgments
We are grateful to Dr. Peter Chedraui, Universidad Espíritu Santo (UEES), Samborondón, Ecuador, for the helpful critical comments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article.
Additional information
Funding
References
- Azziz R, Woods KS, Reyna R, et al. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745–2749. doi:10.1210/jc.2003-032046.
- Hahn S, Bering van Halteren W, Kimmig R, et al. Diagnostik des polycystischen ovarsyndroms/diagnostic procedures in polycystic ovary syndrome. J Lab Med. 2003;27(1-2):53–59. doi:10.1515/LabMed.2003.009.
- Fauser BC, Tarlatzis BC, Rebar RW, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the amsterdam ESHRE/ASRM-Sponsored 3rd PCOS consensus workshop group. Fertil Steril. 2012;97(1):28–38.e25. doi:10.1016/j.fertnstert.2011.09.024.
- Teede HJ, Misso ML, Costello MF, International PCOS Network., et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602–1618. doi:10.1093/humrep/dey256.
- Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33(6):981–1030. doi:10.1210/er.2011-1034.
- Teede HJ, Tay CT, Laven JJE, et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. J Clin Endocrinol Metab. 2023;108(10):2447–2469. doi:10.1210/clinem/dgad463.
- Zhao H, Zhang J, Cheng X, et al. Insulin resistance in polycystic ovary syndrome across various tissues: an updated review of pathogenesis, evaluation, and treatment. J Ovarian Res. 2023;16(1):9. doi:10.1186/s13048-022-01091-0.
- Prati A, Genazzani AR, Genazzani AD. In Clinical Management of Infertility. Pathogenesis of PCOS: from metabolic and neuroendocrine implications to the choice of the therapeutic strategy. In: Genazzani AR, Ibáñez L, Milewicz A, Shah D, editors. Impact of polycystic ovary, metabolic syndrome and obesity on women health: volume 8: frontiers in gynecological endocrinology. Berlin/Heidelberg: Springer International Publishing; 2021. pp. 43–66.
- Srikanthan K, Feyh A, Visweshwar H, et al. Systematic review of metabolic syndrome biomarkers: a panel for early detection, management, and risk stratification in the west virginian population. Int J Med Sci. 2016;13(1):25–38. doi:10.7150/ijms.13800.
- Pérez-Martínez P, Mikhailidis DP, Athyros VG, et al. Lifestyle recommendations for the prevention and management of metabolic syndrome: an international panel recommendation. Nutr Rev. 2017;75(5):307–326. doi:10.1093/nutrit/nux014.
- Polyzos SA, Goulis DG, Kountouras J, et al. Non-alcoholic fatty liver disease in women with polycystic ovary syndrome: assessment of non-invasive indices predicting hepatic steatosis and fibrosis. Hormones. 2014;13(4):519–531.
- Utzschneider KM, Kahn SE, Polidori D. Hepatic insulin extraction in NAFLD is related to insulin resistance rather than liver fat content. J Clin Endocrinol Metab. 2019;104(5):1855–1865. doi:10.1210/jc.2018-01808.
- Fosam A, Sikder S, Abel BS, et al. Reduced insulin clearance and insulin-degrading enzyme activity contribute to hyperinsulinemia in african americans. J Clin Endocrinol Metab. 2020;105(4):e1835–1846–e1846. doi:10.1210/clinem/dgaa070.
- Genazzani AD, Prati A, Genazzani AR, et al. Synergistic effects of the integrative administration of acetyl-L-carnitine, L-carnitine, L-arginine and N-acetyl-cysteine on metabolic dynamics and on hepatic insulin extraction in overweight/obese patients with PCOS. Gynecol Reprod Endocrinol Metab. 2020;1(1):56–63.
- Genazzani AD, Battipaglia C, Semprini E, et al. Familial diabetes in obese PCOS predisposes individuals to compensatory hyperinsulinemia and insulin resistance (IR) also for reduced hepatic insulin extraction (HIE). Endocrines. 2022;3(2):296–302. doi:10.3390/endocrines3020024.
- Finucane FM, Sharp SJ, Hatunic M, et al. Liver fat accumulation is associated with reduced hepatic insulin extraction and beta cell dysfunction in healthy older individuals. Diabetol Metab Syndr. 2014;6(1):43. doi:10.1186/1758-5996-6-43.
- Leissring MA, González-Casimiro CM, Merino B, et al. Targeting insulin-degrading enzyme in insulin clearance. Int J Mol Sci. 2021;22(5):2235. doi:10.3390/ijms22052235.
- Genazzani AD, Genazzani AR. Polycystic ovary syndrome as metabolic disease: new insights on insulin resistance. touchREV Endocrinol. 2023;19(1):71–77. doi:10.17925/EE.2023.19.1.71.
- Macut D, Božić-Antić I, Bjekić-Macut J, et al. Management of endocrine disease: polycystic ovary syndrome and nonalcoholic fatty liver disease. Eur J Endocrinol. 2017;177(3):R145–R158. doi:10.1530/EJE-16-1063.
- Macut D, Tziomalos K, Božić-Antić I, et al. Non-alcoholic fatty liver disease is associated with insulin resistance and lipid accumulation product in women with polycystic ovary syndrome. Hum Reprod. 2016;31(6):1347–1353. doi:10.1093/humrep/dew076.
- Genazzani AD, Battipaglia C, Petrillo T, et al. HIE (hepatic insulin extraction) index in overweight/obese. PCOS patients with or without familial diabetes. Gynecol Reprod Endocrinol Metab. 2022;3(1):57–68.
- Genazzani AD, Shefer K, Della Casa D, et al. Modulatory effects of alpha-lipoic acid (ALA) administration on insulin sensitivity in obese PCOS patients. J Endocrinol Invest. 2018;41(5):583–590. doi:10.1007/s40618-017-0782-z.
- Madeira IR, Carvalho CN, Gazolla FM, et al. [Cut-off point for homeostatic model assessment for insulin resistance (HOMA-IR) index established from receiver operating characteristic (ROC) curve in the detection of metabolic syndrome in overweight pre-pubertal children]. Arq Bras Endocrinol Metabol. 2008;52(9):1466–1473. doi:10.1590/s0004-27302008000900010.
- Genazzani AD, Prati A, Santagni S, et al. Differential insulin response to myo-inositol administration in obese polycystic ovary syndrome patients. Gynecol Endocrinol. 2012;28(12):969–973. doi:10.3109/09513590.2012.685205.
- Genazzani AD, Petraglia F, Fabbri G, et al. Evidence of luteinizing hormone secretion in hypothalamic amenorrhea associated with weight loss. Fertil Steril. 1990;54(2):222–226. doi:10.1016/s0015-0282(16)53693-0.
- Genazzani AD. Inositol as putative integrative treatment for PCOS. Reprod Biomed Online. 2016;33(6):770–780. doi:10.1016/j.rbmo.2016.08.024.
- Genazzani AD. Expert’s opinion: integrative treatment with inositols and lipoic acid for insulin resistance of PCOS. Gynecol Reprod Endocrinol Metab. 2020;1(3):146–157.
- Genazzani AD, Prati A, Simoncini T, et al. Modulatory role of D-chiro-inositol and alpha lipoic acid combination on hormonal and metabolic parameters of overweight/obese PCOS patients. Euro Gynecol Obstet. 2019;1(1):29–33.
- Genazzani AD, Prati A, Marchini F, et al. Differential insulin response to oral glucose tolerance test (OGTT) in overweight/obese polycystic ovary syndrome patients undergoing to myo-inositol (MYO), alpha lipoic acid (ALA), or combination of both. Gynecol Endocrinol. 2019;35(12):1088–1093. doi:10.1080/09513590.2019.1640200.
- Tura A, Ludvik B, Nolan JJ, et al. Insulin and C-peptide secretion and kinetics in humans: direct and model-based measurements during OGTT. Am J Physiol Endocrinol Metab. 2001;281(5):E966–E974. doi:10.1152/ajpendo.2001.281.5.E966.
- Caumo A, Luzi L. First-phase insulin secretion: does it exist in real life! considerations on shape and function. Am J Physiol Endocrinol Metab. 2003;287(3):E371–E385. doi:10.1152/ajpendo.00139.2003.
- Padmalayam I, Hasham S, Saxena U, et al. Lipoic acid synthase (LASY) a novel role in inflammation, mitochondrial function, and insulin resistance. Diabetes. 2009;58(3):600–608. doi:10.2337/db08-0473.
- Laganà AS, Monti N, Fedeli V, et al. Does alpha-lipoic acid improve effects on polycystic ovary syndrome? Eur Rev Med Pharmacol Sci. 2022;26(4):1241–1247. doi:10.26355/eurrev_202202_28116.
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American association for the study of liver diseases, American college of gastroenterology, and the American gastroenterological association. Hepatology. 2012;55(6):2005–2023. doi:10.1002/hep.25762.
- Loria P, Adinolfi LE, Bellentani S, NAFLD Expert Committee of the Associazione Italiana per lo studio del Fegato., et al. Practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease. A decalogue from the italian association for the study of the liver (AISF) expert committee. Dig Liver Dis. 2010;42(4):272–282. doi:10.1016/j.dld.2010.01.021.
- Bae JC, Rhee EJ, Lee WY, et al. Combined effect of nonalcoholic fatty liver disease and impaired fasting glucose on the development of type 2 diabetes. Diabetes Care. 2011;34(3):727–729. doi:10.2337/dc10-1991.
- Jones H, Sprung VS, Pugh CJA, et al. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97(10):3709–3716. doi:10.1210/jc.2012-1382.
- Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology. 2014;59(2):713–723. doi:10.1002/hep.26672.