To the Editor
Platelet-derived microvesicles (PMV) that are released from activated platelets have a high procoagulant potential, and this is believed to be the result of surface exposure of functional receptors of coagulation factors, i.e. negatively charged phospholipids and tissue factor [Citation[1–3]]. The number of circulating PMV has been shown to increase in various clinical conditions with increased thrombotic risk including coronary artery disease (CAD) [Citation[1], Citation[4]]. Thrombin generation in cell-free plasma that can be easily measured using chromogenic or fluorogenic substrates correlates with the number of circulating microvesicles, including PMV [Citation[5], Citation[6]]. Thrombin generation has been shown to be accelerated and enhanced in CAD Citation[7]. To get further evidence for the procoagulant activity of PMV, in particular that of TF-positive PMV, we measured their concentration as well as thrombin generation in plasma samples obtained from CAD patients prior to elective cardiovascular surgery.
After approval by the local ethical committee and written informed consent 23 patients (mean age 63.6 ± 10.8 years, six females and 17 males) who were elected for coronary artery bypass grafting, aortic valve or aortic aneurysm surgery were included in the study. Seven patients had received anti-platelet drugs (acetyl salicylic acid or clopidogrel during the past 7 days prior to surgery). Blood samples were collected using a 10 ml Sarstedt monovette (Sarstedt GmbH, Nümbrecht, Germany) containing 1 ml of 0.106 M sodium citrate solution. The samples were centrifuged within 15 min for 5 minutes at 5,000 × g, and the supernatant plasma was kept at −80°C until analysis. Thrombin generation was determined using the Technothrombin® TGA (Technoclone GmbH, Vienna, Austria) according to the manufacturer's instructions. Platelet derived microvesicles were detected by flow cytometry (FACScan with CellQuest Pro software, Becton Dickinson GmbH, Heidelberg, Germany) after staining with a PE-labelled anti-CD42a antibody (Becton Dickinson) as well as a FITC-labelled anti-tissue factor antibody (American Diagnostica GmbH, Pfungstadt, Germany) or anti CD62P antibody (Becton Dickinson). They were identified according to their light scatter characteristics and quantified using FlowCount® fluorospheres (Beckman Coulter GmbH, Krefeld, Germany) Citation[8]. Other laboratory parameters were determined using standard procedures.
The number of PMV measured as CD42a-positive events with a size of less then 1 µm in diameter, varied between 0.1 × 10−6/ml and 1.4 × 10−6/ml with a mean (±sd) of 0.60 ± 0.37 × 10−6/ml. Only a part of the CD42a-positive PMV were also positive for tissue factor (0.93 ± 0.59 × 10−5/ml) or CD62P (1.12 ± 0.78 × 10−5/ml), i.e. only about 15% to 20% of the CD42a-positive PMV expressed TF and/or CD62P.
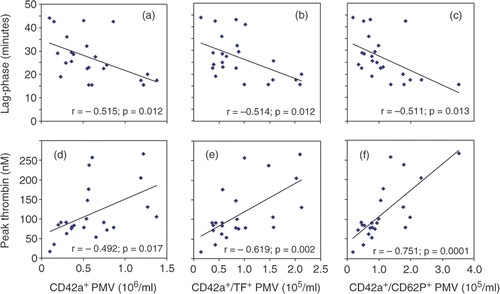
As shown in , we found significant correlations between the number of circulating PMV and parameters of in vitro thrombin generation. PMV were negatively correlated with the lag-phase of thrombin generation and positively correlated with peak thrombin generation. Interestingly, the numbers of TF-positive or CD62P positive PMV were more closely correlated with peak thrombin when compared to all CD42a-positive PMV. With respect to the lag-phase of thrombin generation we could not observe such difference between the different PMV species ().
Figure 1. Correlation between the number of circulating PMV and thrombin generation in cell-free plasma. Pearson correlation coefficients were calculated using SPSS 13.0 for Windows (SPSS Inc., Chicago, Illinois, USA).
In all measured parameters of PMV or TGA we did not observe any significant differences between patients with and without anti-platelet drugs during the past week prior to blood sampling (data not shown).
Our data further support a substantial role of PMV in thrombin formation. In this context, TF- and CD62P-positive PMV seem to have a higher impact on the peak thrombin concentration when compared to PMV that lack these receptors.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Diamant M, Tushuizen ME, Sturk A, Nieuwland R. Cellular microparticles: New players in the field of vascular disease?. Eur J Clin Invest 2004; 34: 392–401
- Joop K, Berckmans RJ, Nieuwland R, Berkhout J, Romijn FP, Hack CE, et al. Microparticles from patients with multiple organ dysfunction syndrome and sepsis support coagulation through multiple mechanisms. Thromb Haemost 2001; 85: 810–820
- Lösche W. Platelets and tissue factor. Platelets 2005; 16: 313–319
- Mallat Z, Benamer H, Hugel B, Benessiano J, Steg PG, Freyssinet JM, et al. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation 2000; 101: 841–843
- Pereira J, Alfaro G, Goycoolea M, Quiroga T, Ocqueteau M, Massardo L, et al. Circulating platelet-derived microparticles in systemic lupus erythematosus. Association with increased thrombin generation and procoagulant state. Thromb Haemost 2006; 95: 94–99
- Bidot L, Jy W, Bidot C, Jr, Jimenez JJ, Fontana V, Horstman LL, et al. Microparticle-mediated thrombin generation assay: Increased activity in patients with recurrent thrombosis. J Thromb Haemost 2008; 6: 913–919, doi:10.1111/j.1538-7836.2008.02963.x
- Orbe J, Zudaire M, Serrano R, Coma-Canella I, Martinez de Sizarrondo S, et al. Increased thrombin generation after acute versus chronic coronary disease as assessed by the thrombin generation test. Thromb Haemost 2008; 99: 382–387
- Scholz T, Temmler U, Krause S, Heptinstall S, Lösche W. Transfer of tissue factor from platelets to monocytes: Role of platelet-derived microvesicles and CD62P. Thromb Haemost 2002; 88: 1033–1038
