Abstract
This paper provides an overview of the various types of microfluidic devices that are employed to study the complex processes of platelet activation and blood coagulation in whole blood under flow conditions. We elaborate on how these devices are used to detect impaired platelet-dependent fibrin formation in blood from mice or patients with specific bleeding disorders. We provide a practical guide on how to assess formation of a platelet–fibrin thrombus under flow, using equipment that is present in most laboratories. In addition, we describe current insights on how blood flow and shear rate alter the location of platelet populations, von Willebrand factor, coagulation factors, and fibrin in a growing thrombus. Finally, we discuss possibilities and limitations for the clinical use of microfluidic devices to evaluate a hemostatic or prothrombotic tendency in patient blood samples.
Introduction
Platelets play a crucial role in thrombosis and hemostasis by their ability to adhere at sites of vascular injury and then aggregate and provide a procoagulant surface for thrombin generation and fibrin formation. In a damaged vessel wall, several subendothelial matrix components form a potent surface for platelet adhesion, including exposed collagen, laminin, and fibrinogen, while other subendothelial components in particular tissue factor (TF) trigger the coagulation process [1] (). Together, exposed TF and the adhered and activated platelets propagate the formation of a platelet–fibrin thrombus, in a way influenced by the local hemodynamic environment. An important factor herein is the wall shear rate of flowing blood, which varies from low (<500 s−1) in the veins and venules to higher values (>1500 s−1) in the arteries and arterioles [2]. Typical for the adhesion of platelets at high wall shear rate is that it is greatly enhanced by the interaction with von Willebrand factor (VWF), which by itself is captured by collagen, laminin, and other matrix proteins. The VWF is produced as a multimeric protein by endothelial cells and, after partial degradation, circulates in the blood plasma at high concentrations.
Figure 1. Platelet-based coagulation processes in a thrombus. (A) Key adhesive platelet ligands and coagulation proteins during the buildup of a platelet–fibrin thrombus. Indicated are platelet adhesion to and activation on collagen/VWF and triggering of the extrinsic (via TF) and intrinsic (via collagen) coagulation pathways. (B) Microscopic images (scale bar represents 20 μm) showing distinct location of platelets and coagulation factors in a thrombus generated at lower shear rate (200 s−1). Note the difference between the aggregated and single, PS-exposing platelets, and the highly similar label pattern of fibrin and thrombin. (C) Later stages of platelet–fibrin thrombus formation with (1) extension of an intra-thrombus fibrin network around contracted platelets with a likely role of FXII and fibrin-bound VWF/FVIII and (2) fibrin extension outside of the platelet thrombus with roles of fibrin-bound thrombin and plasmin. For further description, see text.
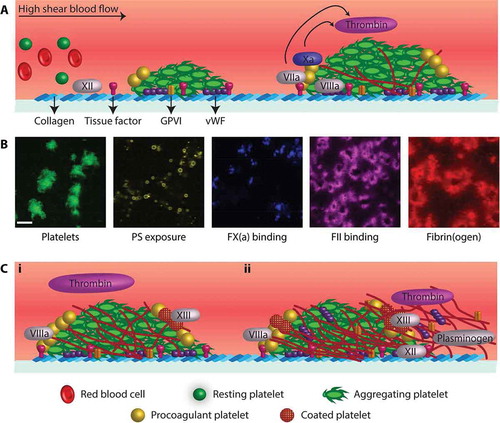
In the majority of in vivo models of hemostasis and thrombosis, platelet aggregation is accompanied by prominent fibrin formation, and both processes are considered to be required for maximal, occlusive thrombus formation [3]. The importance of coagulation is also apparent from the fact that the majority of dissected thrombi in human arteries as well as veins contain both platelet-rich and fibrin-rich regions [4]. Herein another hemodynamic factor is provided by a flow-dependent control of the coagulation process [5]. Current evidence indicates that the relative extent of fibrin deposition decreases with increased blood flow rate, which is explained by a increased dilution of the formed thrombin at higher blood flow [5]. This can explain why in the venous blood compartment a “red” thrombus is formed, consisting of red blood cells and small platelet clumps encapsulated in an extensive fibrin network. In contrast, in the arterial system with high blood flow, mostly a “white” thrombus is generated that is highly enriched in platelet aggregates but also contains fibrin fibers.
Based on flow studies and on autopsies of such arterial and venous thrombi, there is no doubt that feedforward interactions between platelets and coagulation are important in physiology. This has led to the concept of platelet-based coagulation [1,3,6]. A key intermediary process herein is that platelets under specific conditions (e.g., prolonged intercellular Ca2+ rises) are capable to form a procoagulant surface by exposing the negatively charged phospholipid phosphatidylserine (PS). The PS exposure greatly promotes coagulation factor binding and, thereby, enforces the processes of thrombin generation and fibrin formation [7–9]. Detailed studies indicate that the blood flow rate is an important controlling factor, as it determines the transport rates of coagulation factors to and from the procoagulant surface and, thereby, the extent of fibrin polymerization [5,10]. Studies also indicate that higher blood flow stimulates fibrin degradation, by enhancing activity of the fibrinolysis activator plasmin [11,12].
These and other studies have led to the development of assays in which both platelet function and coagulation activity can be examined. Static tube or well plate assays have been employed, such as whole blood thromboelastography and thrombin generation, but these have a disadvantage that massive amounts of thrombin are generated. The high thrombin, being a strong platelet agonist, makes it difficult to detect subtle changes in the functional activities of platelets. To overcome this limitation, tests using small-scale microfluidic devices have been developed, in general with a higher sensitivity toward alterations in both platelet and coagulation activation. Such microfluidic devices provide a well-controlled three-dimensional environment, operating with whole blood flowed at defined, physiological (venous or arterial) or pathological (stenotic) wall shear rates. Their small size allows use of only small amounts of blood. In combination with standard fluorescence microscopy, most microfluidic devices can operate in real time at high resolution, observing the adhesion of single platelets and formation of individual fibrin fibers [13,14].
Microfluidic flow devices and technical aspects to study platelet-based coagulation
Applications of microfluidic devices for studying platelet activation in combination with coagulation are relatively new. At present, only a limited set of studies is published, in which, provisions are made for a precise control of the coagulation system. provides an overview of typical devices and methodologies that are being used to assess platelet-dependent coagulation under flow by measurement of thrombin or fibrin formation.
Table I. Microfluidic devices and methodologies used to study platelet-based control of coagulation.
The devices employed usually have flow channel dimensions of 50–100 µm depth (although some papers describe even smaller channels), in this case requiring less than 500 µl of whole blood, depending on the experimental endpoint [15] (). The flow channels can be fabricated from different blood-compatible materials, ranging from (albumin-blocked or siliconized) glass and hard plastics to soft polymers such as polydimethylsiloxane (PDMS). The latter PDMS channels can be custom-made in a cost-effective way with easily adaptable dimensions [13]. However, when using homemade polymerizing and contracting PDMS material, the channels should be checked for microscopic surface irregularities, which can cause local changes in flow dynamics affecting platelet deposition and/or roof-top fibrin formation (unpublished data). Extensive polishing of the PDMS channel overcomes these shortcomings.
Commonly used microfluidic devices contain rectangular or square channels (opposed to the round glass capillaries formerly used), in order to provide a laminar blood flow pattern through the channel [16], although the flow profile near the channel’s corners is disturbed [14]. In order to minimize wall effects, the advice is that rectangular chambers have a minimal width-to-depth ratio of ≥10 [13]. The Maastricht parallel-plate flow chamber commonly used in our lab (depth 50 μm, width 3 mm) fulfills this requirement [15]. Specific multichannel designs have been used, in which several flow channels run in parallel or are connected to the same inlet or the same outlet, such as reviewed elsewhere [13].
Various strategies can be employed in microfluidic studies to control the coagulation process, e.g., the use of specific blood anticoagulants, a selected thrombogenic surface, and adaptation of the blood flow rate (). Most commonly, (human or mouse) blood is anticoagulated with trisodium citrate or corn trypsin inhibitor (CTI). While trisodium citrate blood requires recalcification to allow the clotting, the addition of CaCl2 is not needed with CTI-treated blood. Critical in either case is to prevent residual thrombin activity by drawing the blood without constraints, mixing it well with the anticoagulant medium, and limit the time for experimentation. The most widely used thrombogenic surface for flow perfusion studies is collagen type I [17], due to its prominent platelet activation and ability to trigger the intrinsic coagulation system via factor (F)XII [18]. In recent years, several studies have used surfaces consisting of both collagen and TF, specifically to trigger the “fast” extrinsic coagulation system of FVII, which enhances the dynamic formation of a platelet–fibrin thrombus [19]. As summarized in , each of the various anticoagulants has specific advantages and disadvantages, when targeting the intrinsic or extrinsic coagulation pathways.
Table II. Technical aspects to consider when using microfluidic flow devices in combination with microscopy to measure platelet and coagulation activity under flow. For technical details, see Ref. [22].
As a key modulator of thrombus formation, the blood flow rate is usually varied to monitor platelet-dependent coagulation either at venous, arterial, or pathological (e.g., stenotic) shear conditions (). The blood flow rate affects platelet–fibrin clot formation in different ways. First, there is the well-known effect of higher wall shear rate on VWF-dependent platelet deposition. Second, higher blood flow (independent of the shear rate) causes a higher dilution of the locally formed thrombin. Fibrin formation under flow hence is a compromise between high platelet deposition on the one hand and limited thrombin dilution on the other hand [5,20]. Of note, as a technical artifact, flow stagnation may occur during the experiment, causing fibrin formation independently of the platelet surface, likely due to thrombin diffusion into the surrounding plasma (unpublished data). Common for most microfluidic devices is that they can be used for kinetic measurements or endpoint assessment of the buildup of platelet–fibrin thrombi or clots.
In a specific approach, the so-called side view, leaky microfluidic device, has been developed by the Philadelphia group operating at a relatively low shear rate, thus mimicking that of vascular leakage or hemostasis [21]. This device allows a separate control of the trans-thrombus pressure gradient independently of the wall shear rate. It can simulate physiological circumstances, where interstitial permeation of a vessel plays a critical role.
When combined with fluorescence microscopy [22], a whole range of fluorescent labels is available (alone or in combination) to assess: platelet adhesion; granule secretion; integrin activation; exposure of procoagulant phosphatidylserine; binding of fibrinogen, coagulation factors, or plasminogen; and/or formation of fibrin fibers (). For adequate measurement of the clot-forming process, high-quality images need to be captured providing sufficiently detailed information. Given the small size of platelets and fibrin fibers, it is essential to maximize both the resolution and the sensitivity of the image capture system. This can be achieved by a highly transparent 40–60× oil immersion objective in combination with a sensitive high-pixel density camera (). Confocal microscopy or equivalent is essential to achieve three-dimensional information, e.g., for volumetric analyses or co-localization measurements of thrombus components, such as (activated) platelets and fibrin fibers, and for distinction between the thrombus core and shell [20–22]. If three-dimensional stacks are imaged at multiple colors in time [20], the collected amount of data can be quite extensive. For complete characterization of thrombi formed in microfluidic chambers, advantage can be taken from the morphometric images obtained by enhanced-contrast, transmission illumination in combination with fluorescence imaging [15].
Collected trans-illumination and fluorescence microscopic images can be analyzed in different ways (). Morphology and specific features of the platelet–fibrin thrombi formed can be assessed by visual inspection of brightfield images. Fluorescence images – whether or not as time series – can precisely be quantified for platelet adhesion, platelet activation markers, and fibrin formation, depending on the probes used [22].
A very different approach for the assessment of platelet–fibrin thrombi is employed in the total thrombus-formation assay (T-TAS) device [23,24]. This microfluidic device is used for the continuous monitoring of blood flow pressure in time. It allows precise recording of the pressure buildup upon capillary occlusion. The T-TAS device further provides information on thrombus stability, by calculating the area under the flow pressure curve for a given time period.
Practical approach of microfluidic flow devices to assess platelet-based control of coagulation
The few studies published so far to assess platelet-based coagulation using microfluidic devices already provide valuable insight into the molecular determinants of this process. As summarized in , main applications are the testing of blood from genetically transformed mice, of pharmacological agents, and of blood from patients with hemostatic insufficiencies.
Table III. Different applications of microfluidic devices to study platelet activation and coagulation under flow conditions.
Using blood from knockout mice, microfluidic devices allowed evaluation of the roles of single genes and proteins in thrombus and fibrin formation in vitro. Typical findings are that murine deficiency in the P2Y12 receptor for ADP caused substantial instability of platelet–fibrin thrombi [25], and that deficiency in FVIII or FIX led to reduced platelet procoagulant activity (PS exposure) as well as low fibrin formation. The latter result implicated a key role of the tenase complex in feedforward mechanisms of platelet activation, thrombin generation, and fibrin fiber formation [26]. In the presence of TF, it was confirmed that the extrinsic coagulation factor VII had a rate-limiting role in the buildup of fibrin thrombi [27]. In the absence of TF, several groups established that the intrinsic coagulation factors FXII and FXI enhanced the kinetics of fibrin formation and thrombus stabilization [18,27,28].
Pharmacological agents with supposed antiplatelet or anticoagulant effects have been tested in flow assays in the presence of coagulation. At high shear rate, inhibition of the thrombin receptor protease activated receptor (PAR) 1, but not of the isoform PAR4, was found to play a role in the buildup of occlusive platelet–fibrin thrombi, an inhibitory effect that was enhanced by aspirin and P2Y12 antagonism [23]. Suppression of the intrinsic coagulation pathway with an antibody against FXI lowered platelet activation and aggregation, in particular at the downstream site of formed thrombi [28]. Concerning the extrinsic pathway, in vitro supplementation of recombinant FVIIa resulted in a restoration of platelet deposition when using blood from patients with FVIIa deficiency but, surprisingly, did not rescue fibrin formation [29]. In recent work, direct FXa inhibitors rivaroxaban and apixaban suppressed fibrin-rich thrombus formation but left platelet accumulation unaffected [30]. Along the same line, the direct thrombin inhibitor argatroban was found prolong the occlusion time and cause clot instability [23].
Microfluidic flow devices can detect hemostatic insufficiencies in blood from patients with a variety of bleeding disorders (). Using the T-TAS device, it was demonstrated that in blood from patients with hemodilution, the addition of fibrinogen concentrate normalized thrombus formation, and especially the formation of fibrin fibers [31]. The same device detected impaired thrombus formation in patients with von Willebrand disease, an effect that was partly restored by patient treatment with the VWF-releasing agent desmopressin [32]. Using the Maastricht flow chamber, fibrin formation was shown to be impaired in blood from patients with several bleeding symptoms, including FIX deficiency [20]. Other experiments demonstrated the requirements of platelet adhesion, PS exposure, and thrombin generation for appreciable fibrin formation under flow. In patients with unstable coronary syndrome, treatment with the antiplatelet agent abciximab (integrin αIIbβ3 antagonist) suppressed both platelet and fibrin accumulation [33]. Even antidepressant treatment targeting serotonin signaling reduced fibrin formation [34] and suppressed thrombus formation on plaque material [35]. Blood flow was an important factor in the lysis of fibrin fibers around thrombi, in which PS-exposing platelets had a key role in plasminogen activation (the main fibrinolysis mediator) [12].
In the majority of these cases, the thrombogenic surface consisted of collagen with or without TF, and recalcified-citrated blood was used to allow coagulation (). Blood flow was performed at variable shear rates and, frequently, both platelet deposition and fibrin accumulation are measured in time. These studies indicate that as long as the flow rate is high enough (i.e., the thrombin formed is sufficiently diluted), adhered and activated platelets are required for a local buildup of fibrin fibers. Interestingly, inability of platelet PS exposure (e.g., blood from Scott patients or anoctamin-6 deficient mice) severely delayed the formation of fibrin [20,36]. This corresponds with in vivo studies [37,38] and supports the concept of platelet-based coagulation. The various studies furthermore point out that the precise wall shear and flow rates are important variables for the proper assessment of hemostatic defects.
Mechanisms of platelet-based control of coagulation under flow conditions
Obviously, microfluidic flow devices provide detailed insight into mechanisms of clotting occurring under flow conditions. In a platelet thrombus formed at arterial shear rate, several coagulation factors completely co-localized with PS-exposing platelets, i.e., (activated) FV, FIX, FX, and uncleaved prothrombin [26,37,39]. This indicated that these major components of the tenase and prothrombinase complexes assemble at PS-exposing platelets and facilitate the local activation of FX into FXa and of prothrombin into thrombin [9]. Interestingly, the location of (activated) FVIII appeared to be different, in that immunological staining showed a transient presence near PS-exposing membranes, whereas the majority of FVIII co-localized with VWF [26]. It was concluded that the VWF in a thrombus acts as a supply site of FVIII, which after its activation (by thrombin) releases and can incorporate into the tenase complex.
Re-localization has also been observed for prothrombin: time series of images of fluorescent-labeled prothrombin indicated a gradual shift from PS-exposing platelets to fibrin fibers, once the prothrombin was cleaved into thrombin [39]. This is particularly prominent at lower flow rates, when fibrin fibers grow outside the platelet aggregates. This co-localization of (cleaved) thrombin with fibrin fibers agrees well with earlier evidence that fibrin can act as a main sink for thrombin. A similar rearrangement to fibrin fibers was found for plasminogen, once cleaved into plasmin [12]. Typical fluorescent images obtained with labeled platelets and coagulation factors are shown in .
Recently, the Philadelphia group has shown that the tightly packed platelet thrombi formed at high shear rates also contain a pool of core-localized thrombin, which enhances platelet activation and granule secretion [40,41]. This was observed from in vivo models of thrombus formation as well as from in vitro flow studies using the side view microfluidic device [41]. The porous fibrin structures in the thrombus core were found to obstruct the internal transport of thrombin and provide a trans-clot pressure differential. Recent findings from our group indicate that the so-called coated platelets, with high transglutaminase (FXIII) activity – forming a subpopulation of PS-exposing platelets – play an important role in platelet-dependent fibrin formation [42].
The method of nano-indentation was applied to assess the physical properties of a fibrin thrombus produced under coagulant conditions in a flow device [20]. The type of thrombogenic surface (i.e., with different densities of collagen and TF) greatly influenced the micro-elasticity of thrombi in a way depending on the shear rate. It could be established that both the fibrin content and the fibrin distribution through a thrombus determined its elastic properties, with luminal-oriented fibrin fibers being most elastic. In vitro studies performed under stasis point to a more dense network of highly branched thin fibrin fibers under conditions where platelets are present and thrombin levels are high [43]. How this finding extends to thrombi formed under flow conditions is still unclear.
Insight is increasing that fibrin formation is not the end of thrombus buildup (Figure 1C). Various pieces of evidence suggest that fibrin fibers can act as a scaffold for a new cycle of platelet adhesion and activation, and subsequent thrombin generation. For instance, platelet recruitment can be promoted by the binding of VWF to fibrin fibers [44]. Incorporation of platelets is also enforced by their ability to bind to fibrin via the glycoprotein (GP) VI receptors, with ensuing increased procoagulant activity [45,46]. In a developing thrombus, also FXII co-localizes in part with fibrin fibers, pointing to a fibrin-dependent progression of the intrinsic coagulation pathway [27,47]. An implication of these findings is that during thrombus buildup with activated platelets and first fibrin fibers, cross-stimulation with fibrin-dependent platelet stimulation can result in expansion of the fibrin network until stabilization of the mature thrombus.
Both in vitro flow chamber and in vivo studies indicate that, mostly, the thrombus-forming process halts within 10–20 min. Various processes may contribute to the stopped thrombus growth: the biorheological (flow) conditions, anticoagulation pathways, and fibrinolytic activity. In vivo, the endothelium is considered to act as a main suppressant of platelet activation and coagulation; this recognition would require inclusion of the relevant endothelial processes in microfluidic measurements. Activated platelets also can support fibrinolysis [12], although this process is limited by the density of the fibrin network [48]. Unclear is still which of the possible thrombus termination pathways is most important.
Toward clinical use
There are a number of challenges regarding the use of microfluidic devices under coagulant conditions for clinical assessment of a hemostatic or prothrombotic tendency. One drawback is the still limitedly controlled coagulation – due to continuous thrombin accumulation and its relocation to fibrin fibers – suggesting that suppressant regulatory mechanisms need to be better incorporated into the flow assays, for instance by mimicking relevant endothelial processes.
The endless combination of variables that can be applied in coagulant flow assays is both strength and weakness. This makes it possible to tailor a flow assay for specific clinical questions, namely by changing: (1) the type, size, and strength of the platelet-activating and coagulation-triggering surface; (2) the flow chamber dimensions; (3) the flow chamber design (e.g., incorporating a shunt or leak out); (4) the wall shear rate determining platelet deposition; (5) the flow rate determining coagulation factor transport; and (6) the flow pattern over time (steadily or pulsatile). Currently, for specific sets of conditions, clinical and research laboratories have been collecting normal values of thrombus parameters with blood samples from healthy volunteers for comparison with patient samples (). The challenge will be to define for each specific clinical question those conditions, where the intraindividual variation of thrombus buildup is limited [49], while the difference between normal and the concerning patient group is maximal [50]. For instance, assessment of thrombus formation in blood from patients with an inherited disease such as hemophilia likely requires an assay setup where fibrin formation is less limited than such measurement in blood from patients with hyperlipidemia or atherothrombosis.
From the current state of the art, it is clear that microfluidic flow devices can assess platelet and coagulant functions at the same time under defined flow conditions. In principle, this is of advantage in comparison to the clinically used hemostatic assays, which determine either platelet or coagulation functions or do not operate under flow. In part, proof of principle so far comes from the studies of , showing that microfluidic flow devices can detect the impaired hemostatic activity in blood from patients with platelet defects, coagulation disorders, or von Willebrand disease [26,31,32,50]. Furthermore, microfluidics can be used in the monitoring and treatment of dilution coagulopathy, for instance caused by fluid infusion upon major surgery. Studies indicate that the diminished thrombus formation at dilution can be normalized at high shear rate by VWF replacement and at low shear rate by fibrinogen concentrate, the latter specifically restoring fibrin formation [31]. Another relevant application is the use of “coagulation” microfluidics for the monitoring of (combined) anticoagulant and antiplatelet therapy, such as the direct thrombin inhibitor argatroban and the integrin αIIbβ3 antagonist abciximab [23,33]. This work is promising for introducing such devices in the clinical laboratory. Yet, the precise assay conditions still need to be defined and subsequently, important assay parameters such as cutoff values, selectivity, and sensitivity need to be determined.
Declaration of interest
The authors declare that no conflicts of interest exist.
Funding
This study was supported by CTTM/MICRO-BAT and F.S. was supported by the Alexander von Humboldt Foundation.
Additional information
Funding
References
- Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev 2013;93:327–358.
- Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol Rev 2011;91:327–387.
- Mazepa M, Hoffman M, Monroe D. Superactivated platelets: Thrombus regulators, thrombin generators, and potential clinical targets. Arterioscler Thromb Vasc Biol 2013;33:1747–1752.
- Rittersma SZ, van der Wal AC, Koch KT, Piek JJ, Henriques JP, Mulder KJ, Ploegmakers JP, Meesterman M, De Winter RJ. Plaque instability frequently occurs days or weeks before occlusive coronary thrombosis: A pathological thrombectomy study in primary percutaneous coronary intervention. Circulation 2005;111:1160–1165.
- Neeves KB, Illing DA, Diamond SL. Thrombin flux and wall shear rate regulate fibrin fiber deposition state during polymerization under flow. Biophys J 2010;98:1344–1352.
- De Witt SM, Swieringa F, Cavill R, Lamers MM, van Kruchten R, Mastenbroek T, Baaten C, Coort S, Pugh N, Schulz A, et al. Identification of platelet function defects by multi-parameter assessment of thrombus formation. Nat Commun 2014;5:4257.
- Monroe DM, Hoffman M, Roberts HR. Platelets and thrombin generation. Arterioscler Thromb Vasc Biol 2002;22:1381–1389.
- Heemskerk JW, Kuijpers MJ, Munnix IC, Siljander PR. Platelet collagen receptors and coagulation. A characteristic platelet response as possible target for antithrombotic treatment. Trends Cardiovasc Med 2005;15:86–92.
- Heemskerk JW, Mattheij NJ, Cosemans JM. Platelet-based coagulation: Different populations, different functions. J Thromb Haemost 2013;11:2–16.
- Shen F, Kastrup CJ, Liu Y, Ismagilov RF. Threshold response of initiation of blood coagulation by tissue factor in patterned microfluidic capillaries is controlled by shear rate. Arterioscler Thromb Vasc Biol 2008;28:2035–2041.
- Dejouvencel T, Doeuvre L, Lacroix R, Plawinski L, Dignat-George F, Lijnen HR, Angles-Cano E. Fibrinolytic cross-talk: A new mechanism for plasmin formation. Blood 2010;115:2048–2056.
- Whyte CS, Swieringa F, Mastenbroek TG, Lionikiene AS, Lance MD, Van der Meijden PE, Heemskerk JW, Mutch NJ. Plasminogen associates with phosphatidylserine-exposing platelets and contributes to thrombus lysis under flow. Blood 2015;125:2568–2578.
- Westein E, de Witt S, Lamers M, Cosemans JM, Heemskerk JW. Monitoring in vitro thrombus formation with novel microfluidic devices. Platelets 2012;23:501–509.
- Zhu S, Herbig BA, Li R, Colace TV, Muthard RW, Neeves KB, Diamond SL. In microfluidico: Recreating in vivo hemodynamics using miniaturized devices. Biorheology 2015;52:303–318.
- Van Kruchten R, Cosemans JM, Heemskerk JW. Measurement of whole blood thrombus formation using parallel-plate flow chambers - A practical guide. Platelets 2012;23:229–242.
- Sarvepalli DP, Schmidtke DW, Nollert MU. Design considerations for a microfluidic device to quantify the platelet adhesion to collagen at physiological shear rates. Ann Biomed Eng 2009;37:1331–1341.
- Roest M, Reininger A, Zwaginga JJ, King MR, Heemskerk JW. Flow chamber-based assays to measure thrombus formation in vitro: Requirements for standardization. J Thromb Haemost 2011;9:2322–2324.
- Van der Meijden PE, Munnix IC, Auger JM, Govers-Riemslag JW, Cosemans JM, Kuijpers MJ, Spronk HM, Watson SP, Renne T, Heemskerk JW. Dual role of collagen in factor XII-dependent thrombus formation. Blood 2009;114:881–890.
- Colace TV, Jobson J, Diamond SL. Relipidated tissue factor linked to collagen surfaces potentiates platelet adhesion and fibrin formation in a microfluidic model of vessel injury. Bioconjug Chem 2011;22:2104–2109.
- Swieringa F, Baaten CC, Verdoold R, Mastenbroek TG, Rijnveld N, van der Laan KO, Breel EJ, Collins PW, Lance MD, Henskens YM, et al. Platelet control of fibrin distribution and microelasticity in thrombus formation under flow. Arterioscler Thromb Vasc Biol 2016;36:692–699.
- Muthard RW, Diamond SL. Side view thrombosis microfluidic device with controllable wall shear rate and transthrombus pressure gradient. Lab Chip 2013;13:1883–1891.
- De Witt S, Swieringa F, Cosemans JM, Heemskerk JW. Multi-parameter assessment of thrombus formation on microspotted arrays of thrombogenic surfaces. Nat Protocol Exchange 2014;2014:3309#.
- Hosokawa K, Ohnishi T, Kondo T, Fukasawa M, Koide T, Maruyama I, Tanaka KA. A novel automated microchip flow-chamber system to quantitatively evaluate thrombus formation and antithrombotic agents under blood flow conditions. J Thromb Haemost 2011;9:2029–2037.
- Yamaguchi Y, Moriki T, Igari A, Matsubara Y, Ohnishi T, Hosokawa K, Murata M. Studies of a microchip flow-chamber system to characterize whole blood thrombogenicity in healthy individuals. Thromb Res 2013;132:263–270.
- Nergiz-Unal R, Cosemans JM, Feijge MA, van der Meijden PE, Storey RF, van Giezen JJ, oude Egbrink MG, Heemskerk JW, Kuijpers MJ. Stabilizing role of platelet P2Y12 receptors in shear-dependent thrombus formation on ruptured plaques. PLoS One 2010;5:e10130.
- Swieringa F, Kuijpers MJ, Lamers MM, Van der Meijden PE, Heemskerk JW. Rate-limiting roles of the tenase complex of factors VIII and IX in platelet procoagulant activity and formation of platelet-fibrin thrombi under flow. Haematologica 2015;100:748–756.
- Kuijpers MJ, van der Meijden PE, Feijge MA, Mattheij NJ, May F, Govers-Riemslag J, Meijers JC, Heemskerk JW, Renne T, Cosemans JM. Factor XII regulates the pathological process of thrombus formation on ruptured plaques. Arterioscler Thromb Vasc Biol 2014;34:1674–1680.
- Zilberman-Rudenko J, Itakura A, Wiesenekker CP, Vetter R, Maas C, Gailani D, Tucker EI, Gruber A, Gerdes C, McCarty OJ. Coagulation factor XI promotes distal platelet activation and single platelet consumption in the bloodstream under shear flow. Arterioscler Thromb Vasc Biol 2016;36:510–517.
- Li R, Panckeri KA, Fogarty PF, Diamond SL. Recombinant factor VIIa enhances platelet deposition from flowing haemophilic blood but requires the contact pathway to promote fibrin deposition. Haemophilia 2015;21:266–274.
- Sugihara H, Idemoto Y, Kuwano T, Nagata Y, Morii J, Sugihara M, Ogawa M, Miura SI, Saku K. Evaluation of the antithrombotic effects of rivaroxaban and apixaban using the total thrombus-formation analysis system: In vitro and ex vivo studies. J Clin Med Res 2016;8:899–907.
- Ogawa S, Ohnishi T, Hosokawa K, Szlam F, Chen EP, Tanaka KA. Haemodilution-induced changes in coagulation and effects of haemostatic components under flow conditions. Br J Anaesth 2013;111:1013–1023.
- Ogiwara K, Nogami K, Hosokawa K, Ohnishi T, Matsumoto T, Shima M. Comprehensive evaluation of haemostatic function in von Willebrand disease patients using a microchip-based flow chamber system. Haemophilia 2015;21:71–80.
- Dangas G, Badimon JJ, Coller BS, Fallon JT, Sharma SK, Hayes RM, Meraj P, Ambrose JA, Marmur JD. Administration of abciximab during percutaneous coronary intervention reduces both ex vivo platelet thrombus formation and fibrin deposition: Implications for a potential anticoagulant effect of abciximab. Arterioscler Thromb Vasc Biol 1998;18:1342–1349.
- Lopez-Vilchez I, Serra-Millas M, Navarro V, Rosa Hernandez M, Villalta J, Diaz-Ricart M, Gasto C, Escolar G, Galan AM. Prothrombotic platelet phenotype in major depression: Downregulation by antidepressant treatment. J Affect Disord 2014;159:39–45.
- Bampalis VG, Dwivedi S, Shai E, Brandl R, Varon D, Siess W. Effect of 5-HT2A receptor antagonists on human platelet activation in blood exposed to physiologic stimuli and atherosclerotic plaque. J Thromb Haemost 2011;9:2112–2115.
- Baig AA, Haining EJ, Geuss E, Beck S, Swieringa F, Wanitchakool P, Schuhmann MK, Stegner D, Kunzelmann K, Kleinschnitz C, et al. TMEM16F-mediated platelet membrane phospholipid scrambling is critical for hemostasis and thrombosis but not thromboinflammation in mice. Arterioscler Thromb Vasc Biol 2016;36:2152–2157.
- Munnix IC, Kuijpers MJ, Auger J, Thomassen CM, Panizzi P, van Zandvoort MA, Rosing J, Bock PE, Watson SP, Heemskerk JW. Segregation of platelet aggregatory and procoagulant microdomains in thrombus formation: Regulation by transient integrin activation. Arterioscler Thromb Vasc Biol 2007;27:2484–2490.
- Kuijpers MJ, Munnix IC, Cosemans JM, Vlijmen BV, Reutelingsperger CP, Egbrink MO, Heemskerk JW. Key role of platelet procoagulant activity in tissue factor-and collagen-dependent thrombus formation in arterioles and venules in vivo differential sensitivity to thrombin inhibition. Microcirculation 2008;15:269–282.
- Berny MA, Munnix IC, Auger JM, Schols SE, Cosemans JM, Panizzi P, Bock PE, Watson SP, McCarty OJ, Heemskerk JW. Spatial distribution of factor Xa, thrombin, and fibrin(ogen) on thrombi at venous shear. PLoS One 2010;5:e10415.
- Stalker TJ, Welsh JD, Tomaiuolo M, Wu J, Colace TV, Diamond SL, Brass LF. A systems approach to hemostasis: 3. Thrombus consolidation regulates intrathrombus solute transport and local thrombin activity. Blood 2014;124:1824–1831.
- Muthard RW, Welsh JD, Brass LF, Diamond SL. Fibrin, gamma’-fibrinogen, and transclot pressure gradient control hemostatic clot growth during human blood flow over a collagen/tissue factor wound. Arterioscler Thromb Vasc Biol 2015;35:645–654.
- Mattheij NJ, Swieringa F, Mastenbroek TG, Berny-Lang MA, May F, Baaten CC, van der Meijden PE, Henskens YM, Beckers EA, Suylen DP, et al. Coated platelets function in platelet-dependent fibrin formation via integrin aIIbb3 and transglutaminase factor XIII. Haematologica 2016;101:427–436.
- Wolberg AS, Aleman MM, Leiderman K, Machlus KR. Procoagulant activity in hemostasis and thrombosis: Virchow’s triad revisited. Anesth Analg 2012;114:275–285.
- Miszta A, Pelkmans L, Lindhout T, Krishnamoorthy G, De Groot PG, Hemker CH, Heemskerk JW, Kelchtermans H, de Laat B. Thrombin-dependent incorporation of von Willebrandf factor into a fibrin network. J Biol Chem 2014;289:35979–35986.
- Alshehri OM, Hughes CE, Montague S, Watson SK, Frampton J, Bender M, Watson SP. Fibrin activates GPVI in human and mouse platelets. Blood 2015;126:1601–1608.
- Mammadova-Bach E, Ollivier V, Loyau S, Schaff M, Dumont B, Favier R, Freyburger G, Latger-Cannard V, Nieswandt B, Gachet C, et al. Platelet glycoprotein VI binds to polymerized fibrin and promotes thrombin generation. Blood 2015;126:683–691.
- Zhu S, Travers RJ, Morrissey JH, Diamond SL. FXIa and platelet polyphosphate as therapeutic targets during human blood clotting on collagen/tissue factor surfaces under flow. Blood 2015;126:1494–1502.
- Collet JP, Park D, Lesty C, Soria J, Soria C, Montalescot G, Weisel JW. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: Dynamic and structural approaches by confocal microscopy. Arterioscler Thromb Vasc Biol 2000;20:1354–1361.
- Kunicki TJ, Nugent DJ. The genetics of normal platelet reactivity. Blood 2010;116:2627–2634.
- Neeves KB, Onasoga AA, Hansen RR, Lilly JJ, Venckunaite D, Sumner MB, Irish AT, Brodsky G, Manco-Johnson MJ, Di Paola JA. Sources of variability in platelet accumulation on type 1 fibrillar collagen in microfluidic flow assays. PLoS One 2013;8:e54680.
- Reininger AJ, Bernlochner I, Penz SM, Ravanat C, Smethurst P, Farndale RW, Gachet C, Brandl R, Siess W. A 2-step mechanism of arterial thrombus formation induced by human atherosclerotic plaques. J Am Coll Cardiol 2010;55:1147–1158.
- Onasoga-Jarvis AA, Leiderman K, Fogelson AL, Wang M, Manco-Johnson MJ, Di Paola JA, Neeves KB. The effect of factor VIII deficiencies and replacement and bypass therapies on thrombus formation under venous flow conditions in microfluidic and computational models. PLoS One 2013;8:e78732.
- Zhu S, Tomaiuolo M, Diamond SL. Minimum wound size for clotting: Flowing blood coagulates on a single collagen fiber presenting tissue factor and von Willebrand factor. Integr Biol (Camb) 2016;8:813–820.
- Colace TV, Fogarty PF, Panckeri KA, Li R, Diamond SL. Microfluidic assay of hemophilic blood clotting: Distinct deficits in platelet and fibrin deposition at low factor levels. J Thromb Haemost 2014;12:147–158.
- Zhu S, Diamond SL. Contact activation of blood coagulation on a defined kaolin/collagen surface in a microfluidic assay. Thromb Res 2014;134:1335–1343.
- Welsh JD, Colace TV, Muthard RW, Stalker TJ, Brass LF, Diamond SL. Platelet-targeting sensor reveals thrombin gradients within blood clots forming in microfluidic assays and in mouse. J Thromb Haemost 2012;10:2344–2353.
- Nogami K, Ogiwara K, Yada K, Shida Y, Takeyama M, Yaoi H, Minami H, Furukawa S, Hosokawa K, Shima M. Assessing the clinical severity of type 1 von Willebrand disease patients with a microchip flow-chamber system. J Thromb Haemost 2016;14:667–674.
- Onasoga-Jarvis AA, Puls TJ, O’Brien SK, Kuang L, Liang HJ, Neeves KB. Thrombin generation and fibrin formation under flow on biomimetic tissue factor-rich surfaces. J Thromb Haemost 2014;12:373–382.
- Jain A, Graveline A, Waterhouse A, Vernet A, Flaumenhaft R, Ingber DE. A shear gradient-activated microfluidic device for automated monitoring of whole blood haemostasis and platelet function. Nat Commun 2016;7:10176.
- Muthard RW, Diamond SL. Rapid on-chip recalcification and drug dosing of citrated whole blood using microfluidic buffer sheath flow. Biorheology 2014;51:227–237.
- Hosokawa K, Ohnishi T, Miura N, Sameshima H, Koide T, Tanaka KA, Maruyama I. Antithrombotic effects of PAR1 and PAR4 antagonists evaluated under flow and static conditions. Thromb Res 2014;133:66–72.

