Abstract
To establish pan-European consensus on tapering and discontinuing thrombopoietin receptor agonists (TPO-RAs) in patients with immune thrombocytopenia (ITP), we applied a three-step Delphi technique consisting of a one-to-one interview round and two online survey rounds. Three healthcare professionals (HCPs) from Italy, Spain, and the United Kingdom formed the Steering Committee (SC), which advised on study design, panelist selection, and survey development. A literature review also informed the development of the consensus statements. Likert scales were used to collect quantitative data on panelists’ level of agreement. Twelve hematologists representing nine European countries assessed 121 statements spanning three categories: (1) patient selection; (2) tapering and discontinuation strategies; (3) post-discontinuation management. Consensus was reached on approximately half of the statements in each category (32.2%; 44.6%; 66%). Panelists agreed on patients’ main selection criteria, patients’ involvement in decision-making, tapering strategies, and follow-up criteria. Areas not reaching consensus were risk factors and predictors of successful discontinuation, monitoring intervals, and rates of successful discontinuation or relapse. This lack of consensus signals knowledge and practice gaps among European countries and suggests the need for the development of clinical practice guidelines that outline a pan-European, evidence-based approach to tapering and discontinuing TPO-RAs.
Plain Language Summary
Immune thrombocytopenia (ITP) is a condition that may cause extensive bruising and excessive bleeding. Another sign is a pattern of small reddish-purple dots resembling a rash. ITP is treated with a class of medications known as thrombopoietin receptor agonists (TPO-RAs), which include eltrombopag, avatrombopag, and romiplostim. Sometimes the beneficial effects of the medication last even after the patient stops taking it, which means that some patients can be tapered off it. This paper presents the results of a Delphi panel—a method of research that gathers insights from experts—about tapering and discontinuing TPO-RAs. There were 12 physicians from nine European countries on the Delphi panel, all practicing hematologists with expertise in tapering and discontinuing TPO-RAs in patients with ITP. Panelists were presented with a total of 130 statements over three survey rounds. At the end of Round Three, 52 statements (40%) achieved consensus (response pattern of ≥80% “Agree”), and six statements (4.6%) achieved dissensus (response pattern of ≥80% “Disagree”). Consensus was achieved on the appropriateness of tapering the dose of the TPO-RA for two to three months prior to attempting discontinuation. The panel also reached consensus on considering tapering in a slower fashion (six to 12 months) for patients showing suboptimal response to TPO-RAs. More than half the survey’s statements did not achieve consensus or dissensus. This signals that knowledge gaps exist and highlights the importance of conducting prospective, real-world evidence studies to identify best practices and develop pan-European guidelines for tapering and discontinuing TPO-RAs.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune bleeding disorder characterized by accelerated platelet destruction and inadequate platelet production due to autoimmune targeting of platelets and their precursors [Citation1]. The main clinical manifestations of ITP are bleeding symptoms. Bleedings may be mild, including petechiae, purpura, and epistaxis, or severe, including cases of intracranial hemorrhage and serious gastrointestinal or urinary tract bleeding [Citation2]. Health-related quality of life (HRQoL) data have identified significant morbidity in patients with ITP, including fatigue and fear of bleeding, and its negative impact on social and work activities [Citation3].
In Europe, adult ITP has an incidence of 1.6 to 3.9 cases per 100,000 per year [Citation4]. The incidence is slightly higher in females between 45 and 49 years of age [Citation1] and peaks in males after 60 years of age [Citation5]. Whereas adults generally present a chronic course of disease (it lasts >1 year in 80% of adult patients), children usually suffer acute forms of ITP [Citation6].
Thrombopoietin receptor agonists (TPO-RAs), such as eltrombopag, avatrombopag, and romiplostim, have been shown to induce proliferation and differentiation of megakaryocytes, thereby improving the patient’s platelet count and preventing severe bleedings [Citation7]. The goal of TPO-RA treatment is to increase the patient’s platelet count to a level that minimizes the risk of bleeding (>50 × 109/L) [Citation8–10]. Many patients require continuous TPO-RA treatment to maintain a safe platelet count [Citation8]; however, evidence indicates that up to 30% of patients receiving romiplostim or eltrombopag maintain a sustained response for months after treatment is discontinued [Citation8,Citation11]. This may be due to a drug-independent mechanism that persists after treatment discontinuation [Citation12].
To date, randomized studies on the tapering and discontinuation of TPO-RAs are lacking. Country-specific expert consensus regarding tapering and discontinuing TPO-RAs has been developed in Italy [Citation13], the United Kingdom (UK) [Citation7], and the United States (US) [Citation14]. Key opinion leaders (KOLs) in these countries have confirmed that TPO-RAs may be successfully discontinued in some patients who have responded to treatment [Citation7,Citation13,Citation14]. Experts in Italy and in the UK determined that it is usually appropriate to consider tapering TPO-RAs after six to 12 months for patients with adequate response (defined as platelet count >50 × 109/L [Citation7] or >100 × 109/L [Citation13]). In the US, experts agreed on the appropriateness of considering tapering in patients with normal or above normal platelet count (>150 × 109/L); for patients with no history of bleeding, just an adequate platelet count (>50 × 109/L) is sufficient to consider tapering [Citation14]. Consensus on other patient characteristics to be considered by the clinician prior to tapering and on how to taper have also been identified [Citation7,Citation14]; however, questions remain on the selection of suitable patients, tapering regimens, clinical and/or treatment characteristics that may predict a sustained response after discontinuation, and methods for re-initiating therapy.
The present study aimed at validating and extending findings from previous consensus studies in a broader European context. The objectives are to (1) develop consensus on clinical practices for tapering and discontinuing TPO-RAs among experts from different European countries and (2) identify knowledge gaps and clinical practice discrepancies in tapering and discontinuing TPO-RAs to highlight areas where further evidence-based research is needed.
Methods
Study design
To meet the objectives, a three-round Delphi panel was conducted. A Delphi panel allows for anonymous, iterative collection and statistical aggregation of informed judgments from experts; it is characterized by repeated rounds of controlled feedback until consensus is achieved [Citation15,Citation16]. The Delphi method is widely used in healthcare research and is proven to be a rigorous and feasible way to obtain consensus. In the absence of randomized controlled trials, expert opinion can be helpful in exploring patient characteristics and other factors that might be associated with successful TPO‐RA discontinuation [Citation14].
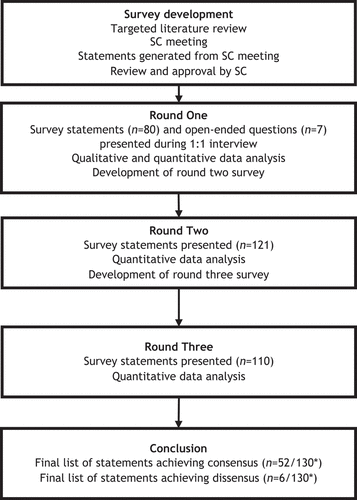
The present Delphi panel methodology included a one-to-one interview round and two survey rounds (). Three HCPs (NC, FZ, and TL) with expertise in the tapering and discontinuation of TPO-RAs in patients with ITP formed the Delphi Panel Steering Committee (SC), providing input into study design, panel selection, survey development, and interpretation of the results.
Panel selection
The SC and the study sponsor identified 13 HCPs from 10 European countries and invited them to participate in the Delphi panel, with the objective to reach the target sample size of 12 KOLs (the recommended sample size for a Delphi panel is typically 5–20 panelists [Citation17]). A sample size of 12 was deemed appropriate provided the limited pool of HCPs with expertise in tapering and discontinuing TPO-RAs. Invited panelists were required to be practicing hematologists with relevant experience in autoimmune hematologic diseases, which was assessed using the following inclusion criteria for screening: (1) have at least two years of experience working with patients with ITP; or (2) have at least one publication on this disease area. HCPs also had to have a good understanding of written English. Participating KOLs signed a written contract with the study sponsor and received an honorarium for their participation. Oral consent to participate in the study was also collected during the first one-to-one interview.
According to the governance arrangements for research ethics committees, review is not required for research involving healthcare professionals recruited as research participants by virtue of their professional role [Citation18]. Therefore, institutional ethics committee approval was deemed unnecessary.
Preparation
The research team used PubMed to conduct a targeted literature review on tapering and discontinuing TPO-RAs in adults and children with ITP. The targeted search aimed to develop a summary of evidence supporting existing guidelines for the cessation of TPO-RA treatment, including evidence on sustained response off treatment (SRoT), between January 2005 and December 2020. The summary included evidence from three case reports and case series reports [Citation19–21], three cohort studies [Citation22–24], four analyses of clinical trial data [Citation8,Citation10,Citation25,Citation26], and five expert consensus studies on administration and tapering practices of TPO-RAs in patients with ITP [Citation7,Citation13,Citation14,Citation27,Citation28]. No standard tool was used to formally appraise the quality of evidence. A SC meeting was held in June 2021 to discuss the findings of the targeted review and outline the research objectives. The Delphi panel statement framework developed by the SC is presented in . The SC discussion was used to develop the first survey draft, which included consensus statements and open-ended questions for each framework’s domain. Noticeably, the developed survey included items regarding tapering and discontinuation regimens for eltrombopag and romiplostim. Other TPO-RAs, such as avatrombopag, were not included, as they were not the focus of the identified literature.
Table I. Consensus statements framework.
Procedure
The Delphi panel was conducted between September and December 2021. The consensus statements addressing the objectives were shared with the panelists across three survey rounds. During each round, they were asked to rate the extent to which they agreed with each statement using either Likert scales or binary responses.
Round one
In Round One, panelists were presented with 80 statements and seven open-ended questions during a one-to-one interview with a member of the research team. All statements were presented with a 5-point Likert response scale (1 = Completely Disagree, 2 = Disagree, 3 = Neutral, 4 = Agree, 5 = Completely Agree). Open-ended questions were asked only during the first round to generate further statements for Round Two. Panelists were also encouraged to provide comments on the statements, which offered additional qualitative insights to refine them. Median ratings were calculated for each item using Microsoft Excel, and results were analyzed as per the analysis rules in .
Table II. Analysis rules.
Rounds two and three
In Round Two, panelists were emailed an electronic survey with a total of 121 statements, customized with panelists’ individual responses and the group mean ratings of statements brought forward from Round One.
Following quantitative analysis of Round Two survey data, 11 statements were removed (one achieving consensus and 10 not receiving the minimum level of response threshold). Round Three included 110 statements, which were presented to panelists in a customized survey with their individual responses and group mean ratings.
Data analysis
After each round, quantitative survey responses were extracted for each statement into a Microsoft Excel database and were assigned a score/code (i.e., 1–5, 1–3, or 1, 2) corresponding to each Likert/binary response scale. The interquartile range (IQR) was calculated and used to summarize the extent of the spread of the data. Central tendencies (mean, median, and mode) were calculated to present the group’s responses back to panelists, and percentage response frequencies for each statement were calculated to determine whether consensus had been achieved. Consensus definition was determined a priori with the SC alongside the following set of analysis rules ().
Results
Panel composition
The panel was composed of 12 HCPs from nine European countries (Belgium, Czech Republic, France, Germany, Norway, Spain, Slovenia, Switzerland, the United Kingdom). All 12 participants completed the three rounds of the survey. All panelists were practicing hematologists and had experience in tapering and discontinuing TPO-RAs in patients with ITP. As per the Delphi methodology, panelists remained anonymous to one another for the entire duration of the panel to control for the influence of dominant individuals [Citation29].
Overview of survey results
Panelists were presented a total of 130 statements over the three survey rounds. At the end of Round Three, 52 statements (40%) achieved consensus (response pattern of ≥80% “Agree”), and six statements (4.6%) achieved dissensus (response pattern of ≥80% “Disagree”). Topics that achieved consensus and dissensus are presented in .
Table III. Summary of survey topics that achieved consensus or dissensus.
Table IV. Tapering regimens that achieved consensus for eltrombopag and romiplostim.
Domain 1: selection of patients for tapering and discontinuation
Within section one, 19 out of 59 statements reached consensus (32.2%).
General considerations for patients’ selection
Panelists agreed on the appropriateness of considering tapering and discontinuation of TPO-RAs for patients with ITP who are clinically stable (platelet count >50 × 109, no bleeding events or large fluctuations in platelet count) and have a low-risk lifestyle (low risk of physical injuries and associated bleeding manifestations). No consensus or dissensus was reached on whether tapering is appropriate in patients who have an at-risk lifestyle (high risk of physical injuries and associated bleeding manifestations), experience high levels of anxiety related to their condition, have a history of resistance to other treatments, or are on anticoagulants or antiplatelets. Furthermore, the panel did not reach consensus or dissensus on whether the patient’s access to healthcare (e.g., distance from healthcare facilities) should influence the choice of attempting discontinuation nor the patient’s history of thrombotic episodes in the six months prior to tapering. Panelists did also not reach consensus agreement or disagreement on whether it is appropriate to consider discontinuation in patients who have been or will soon be vaccinated against SARS-CoV-2.
Patient’s age
No consensus was achieved on the role of the patient’s age in the decision to taper TPO-RAs. However, panelists disagreed on considering tapering only for young patients (below 40 years of age).
Response to TPO-RAs
According to the panel, the decision to attempt discontinuation does not depend on the dose of TPO-RAs required by the patient to maintain a stable response. If the patient presents a favorable risk profile (platelet count ≥100 × 109/L, no bleeding events, and no large fluctuations in platelet counts) there is no dosage threshold of TPO-RAs above which tapering cannot be considered.
Patient’s time on TPO-RA treatment
The panel determined that it is appropriate to consider tapering and discontinuation in patients who have been on TPO-RA treatment for a minimum of six months prior to attempting discontinuation.
Patient’s platelet count
Ideal candidates for tapering and discontinuation should have achieved and maintained a stable mean platelet count ≥100 × 109/L for a minimum of two to three months. For patients with platelet count <100 × 109/L, a safe platelet count (platelet count >50 × 109 and <100 × 109, however the panel did not achieve consensus on the definition of safe platelet count) should be maintained for at least six months prior tapering. Whereas there was no consensus on whether discontinuation can be attempted for patients who present a stable mean platelet count ≥50 × 109/L, the panel determined that it is not appropriate to consider discontinuation for patients who present a stable mean platelet count ≥30 × 109/L.
Bleeding history
No statement achieved consensus in this subdomain. Panelists reached dissensus on considering patients ineligible for tapering and discontinuation if they have a history of bleedings (minor and/or major) should they present normal or above normal platelet count regardless of if they are using anticoagulants.
The patient’s perspective and motivation to attempt treatment discontinuation
The panel agreed on the importance of patients feeling motivated to attempt discontinuation. However, prior to tapering, patients should be well informed of the possibility that they may not succeed in discontinuing the treatment. It is the clinician’s role to explain to the patient that they might experience a worsening of their platelet count and require a re-initiation of treatment. During Round One, panelists were asked the reasons to attempt discontinuation. Among their answers, the following reasons reached consensus: (1) the patient wants a child; (2) the patient fears the long-term toxicity of TPO-RAs; and (3) the patient suffers side effects while on TPO-RAs. Reasons for discontinuation that achieved neither consensus or dissensus were: (1) the patient’s quality of life may be increased if they are treatment-free as this may make them feel healed; (2) the patient wants to reduce their visits to a healthcare facility; (3) the patient wants to reduce the financial burden of their treatment; and (4) the patient suffers the burden of the treatment’s dietary restrictions.
Domain 2: tapering and discontinuation strategies for TPO-RAs
Twenty-nine out of 65 statements reached consensus in domain two (44.6%), and none reached dissensus.
Discontinuation regimens for eltrombopag and romiplostim
The tapering regimens that achieved consensus for the two TPO-RAs analyzed are reported in .
Duration of tapering
Consensus was achieved on the appropriateness of tapering the dose of the TPO-RA for two to three months prior to attempting discontinuation. The panel also reached consensus on considering tapering in a slower fashion (six to 12 months) for patients showing suboptimal response to TPO-Ras (patients who show a response to the drug below the therapeutic goal - i.e. who do not achieve platelet count ≥ 50×109 - after 7–28 days of treatment at the maximum recommended dose).
Patient management during tapering
Although panelists achieved consensus on the appropriateness of monitoring patients while tapering at intervals no longer than four weeks, they could not agree on the optimal duration of monitoring intervals. There was no consensus or dissensus on the appropriateness of monitoring patients at intervals of one or two weeks. Consensus was reached on monitoring a patient’s clinical status at any time during tapering and discontinuation should they experience minor or major mucocutaneous bleeding, bruises, extreme physical weakness/fatigue, anxiety related to their ITP, or any sign of infection (fever, fatigue, etc.).
Management of platelet count drop during tapering
Consensus was achieved on the need to reintroduce the TPO-RA at a minimum dose needed to trigger a response should the patient experience a sudden drop in platelet count (e.g., the count drops below 30 × 109/L) or should they experience a bleeding event associated with a mild platelet count reduction. Panelists also agreed on reintroducing the same TPO-RA should the patient need to restart treatment. No consensus or dissensus was achieved on whether a patient in relapse could be rescued with a different TPO-RA if the response to the previous TPO-RA treatment was suboptimal.
Relapse and successful discontinuation
The panel achieved consensus on defining patient relapse as a platelet count drop below 20–30 × 109/L. Additionally, two possible causes of relapse reached consensus: (1) viral infections; and (2) vaccinations. Panelists did not demonstrate consensus or dissensus on the rate of successful discontinuation in their clinical practice. All four statements on the percentage of patients who successfully discontinue TPO-RA treatment (10% of patients, 30% of patients, 40% of patients, and 50% of patients) reached a low level of agreement (agreement <60%).
Predictors of success/failure
The only predictor for successful discontinuation that reached consensus was the patient’s mean platelet count prior to tapering. Panelists agreed that patients with a stable mean platelet count above 100 × 109/L may be more likely to achieve a sustained response after discontinuation compared to patients with a lower mean platelet count. The panel did not reach consensus on whether the duration of a patient’s ITP, a quick response to the TPO-RA, younger age (<40 years), and secondary ITP could predict a higher chance of successful discontinuation.
Domain 3: post discontinuation and sustained response off treatment
Four statements out of six reached consensus in domain three (66%), and none achieved dissensus. The panel agreed on defining a patient in SRoT if, after discontinuing TPO-RAs, they maintain a platelet count above 50 × 109/L after treatment discontinuation. The panel did not reach consensus or dissensus on considering discontinuation successful if a patient who is not receiving TPO-RAs maintains a platelet count of 20 × 109/L to 30 × 109/L.
Discussion
A validated Delphi panel methodology was used to develop a set of consensus and dissensus statements for clinical experts in nine European countries on tapering and discontinuing TPO-RAs in patients with ITP. To our knowledge, this is the first study that presents the aggregated consensus of experts across multiple European countries on tapering and discontinuing TPO-RAs.
The results of the survey regarding the selection of patients for tapering and discontinuation are largely aligned with guidelines found in the literature in that the tapering and discontinuation of TPO-RAs can be considered in clinically stable patients, with stable platelet count, whose lifestyle does not expose them to excessive physical risks, and who have been on TPO-RA treatment for a minimum of six months [Citation14,Citation27].
The panel agreed on the appropriateness of tapering for patients with a platelet count above 100 × 109/L that has been maintained for a minimum of six months and the inappropriateness of doing so when the patient’s platelet count drops below 30 × 109/L. Our study was unable to determine whether tapering should be considered for patients with a stable mean platelet count of 50 × 109/L. By contrast, Zaja et al. [Citation27]. conducted a survey of 11 international experts and concluded that TPO‐RAs can be tapered in patients with a stable platelet count above 50 × 109/L that is maintained for at least six months without concomitant therapy. Although this is aligned with our panel guidance of considering tapering in patients who have maintained a safe platelet count for a minimum of six months, elsewhere the literature lacks agreement on the definition of a safe platelet count [Citation11,Citation18,Citation19]. The question remains as to whether it is appropriate to taper and discontinue in patients with a platelet count between 30 × 109/L and 100 × 109/L.
The panel recognized the importance of assessing the patient’s motivation to discontinue treatment, as well as the clinician’s role in motivating the patient to attempt treatment discontinuation. Experts agreed on the appropriateness of attempting discontinuation only in motivated patients. The role of the patient’s perspective is also reported in a retrospective study using the Spanish Eltrombopag Registry [Citation30], which identified patient request among the factors influencing the decision to attempt discontinuation. The importance of engaging patients in their healthcare through shared decision-making is well recognized by several national and international groups [Citation31,Citation32]. The reasons motivating patients to attempt discontinuation should be further researched to develop recommendations on how to best involve patients and maximize shared decision-making in ITP.
The tapering regimens for eltrombopag and romiplostim recommended by the experts of this Delphi panel are comparable to the ones advised by the expert panel in Zaja et al. [Citation27]. However, the regimens differ in the recommended duration of the final dose administration interval prior to discontinuation. The differences may be due to the lack of studies on tapering regimens and on patient responses to tapering. The multiple tapering regimens of romiplostim that reached consensus may also indicate that different tapering approaches may be required for specific patients. There have been no formal guidelines regarding the tapering and discontinuation of TPO-RAs [Citation33]. Previous expert consensus suggests that tapering and discontinuation can be attempted also with avatrombopag by increasing the intervals between doses [Citation14]. Future real-world evidence studies or trial data should support the identification of best tapering regimens for different patient scenarios and different TPO-RAs. Our panel’s recommendation to taper over the course of two to three months is aligned overall with the available literature, which recommends slow tapering to improve the overall outcome [Citation7,Citation8,Citation27].
Similarly to the results in Cuker et al. [Citation14], this study panel did not reach consensus on a specific schedule for patient monitoring. The clinicians agreed only that intervals should be no longer than four weeks during the tapering process. Our panel and Cuker et al. [Citation13] are aligned on recommending monitoring the patient’s clinical situation (including platelet count) should the patient experience bleeding events, signs of infections, or fatigue during tapering and after discontinuation. Both panels also recommend reinitiating treatment if the patient’s platelet count drops below 30 × 109/L. This differs from the threshold platelet count of 50 × 109/L that would trigger treatment initiation indicated in a previous review of practice among UK hematologists [Citation34].
According to the expert panel convened for this study, the patient’s platelet count prior to tapering is the only predictive factor of successful discontinuation. In alignment with available evidence, the panel could not determine whether disease duration, treatment history, or patient’s age can predict SRoT after TPO-RA discontinuation [Citation8,Citation11,Citation24,Citation35].
Panelists were not aligned on the percentage of patients who successfully discontinue a TPO-RA and achieve a SRoT (defined as patients who maintain a platelet count above 50 × 109/L after treatment discontinuation). This reflects the variability in the reported response rates in the literature, which may be attributed to studies conducted with small sample sizes, different patient populations and follow-up periods, or different definitions of sustained response. For instance, in the Spanish registry study [Citation30] and the French study [Citation36], 40% to 70% of patients with complete response on a TPO-RA were able to achieve treatment discontinuation successfully. Preliminary results of the ESTIT study, a phase 2, prospective study to explore the role of eltrombopag given as second-line treatment in patients with ITP [Citation37], found that 30% of respondents achieved SRoT. Lucchini et al. [Citation37] showed that SRoT may be achievable in 38% and 50% of patients who meet the International Working Group criteria of response and complete response [Citation38], respectively, at the end of treatment (week 24) with eltrombopag. This highlights the need to conduct prospective, real-world evidence studies with large data sets to robustly define SRoT with TPO-RAs and provide clinical evidence on the rate of successful discontinuation.
Conclusion
This study identified consensus for tapering and discontinuing TPO-RAs in ITP patients that could be deemed applicable in nine European countries. This Delphi panel’s findings echo those of previous consensus studies on the appropriateness of attempting to taper and discontinue TPO-RAs in stable patients with ITP who have maintained a platelet count above 100 × 109/L for a minimum of six months. In addition, eligible patients should have a stable lifestyle that doesn’t expose them to an excessive risk of physical trauma. The patient’s platelet count prior to discontinuation remains the only predictive factor for successful discontinuation agreed upon by experts. Also largely aligned with recommendations from previous studies is the panel’s recognition that it is important to understand the patient’s perspective on tapering and discontinuing TPA-RAs and motivation for doing so.
More than half the survey’s statements did not achieve consensus or dissensus, which indicates the existence of knowledge gaps as well as differences in tapering and discontinuation practices across KOLs from nine European countries. Key knowledge gaps include: (1) additional patient characteristics to consider before deciding to taper and discontinue (e.g., age, vaccination status, TPO-RA dose required to maintain a response); (2) schedule for monitoring patients during tapering and after discontinuation; (3) predictors of successful discontinuation; and (4) overall rates of successful discontinuation. The persistence of knowledge gaps and discrepancies in clinical practices identified by this Delphi panel highlights the need to conduct prospective, real-world evidence studies to identify best practices and develop guidelines that can provide the basis for a pan-European, evidence-based approach to tapering and discontinuing TPO-RAs.
Limitations
We recognize that these consensus statements reflect the opinion of a small group of individuals. Panelists were recruited from the SC members’ networks; it is thus possible they come from a similar school of thought. Additionally, the response option “I don’t know,” as suggested by Vogel et al. [Citation39], was not available in this study, meaning panelists were unable to indicate when they did not know the answer to a statement. The lack of a pilot study was mitigated by the one-to-one interviews in the first round to determine comprehensibility and clarify any statements. Lastly, the 80 statements added as a result of panelists’ comments during Round One were presented to the panelists in two rounds only, as opposed to all three rounds.
Acknowledgments
The authors would like to thank the panelists who contributed to this study with their knowledge and expertise: Dr. A. Janssens, Dr. L. Cervinek, Dr. G. Moulis, Dr. S. Cheze, Dr. H. Ostermann, Prof. M. Rummel, Prof. W. Ghanima, Prof. B. Skopec, Prof. I. Jarque, Assoc. Prof. M. L. Lozano, Prof. T. Kuehne, and Dr. V. McDonald.
Disclosure statement
This study was sponsored by Novartis Pharmaceuticals. GLTJ has received research grants from Novartis Pharmaceuticals, Amgen, Sobi, and Grifols, and speaker honoraria from Novartis Pharmaceuticals, Amgen, Sobi, Grifols, Momenta, UCB, and Argenx. CN has received honoraria from Novartis Pharmaceuticals, Rigel, Amgen, Grifols, UCB, Principia, Sanofi, and Sobi and provided research support to Novartis Pharmaceuticals and Rigel. ZF has reported personal fees from Novartis Pharmaceuticals, Amgen, Argenx, Grifols, Janssen, AbbVie, Takeda, and Sobi. AB is an employee of OPEN Health, which received support from Novartis Pharmaceuticals for this study.
Additional information
Funding
References
- Kistangari G, McCrae KR. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013;27(3):495–8. doi:10.1016/j.hoc.2013.03.001.
- Kohli R, Chaturvedi S. Epidemiology and clinical manifestations of immune thrombocytopenia. Hämostaseologie. 2019;39(3):238–249. doi:10.1055/s-0039-1683416.
- Trotter P, Hill QA. Immune thrombocytopenia: improving quality of life and patient outcomes. Pat Relat Outcome Meas. 2018;9:369. doi:10.2147/PROM.S140932.
- European Medicines Agency. Guideline on the clinical development of medicinal products intended for the treatment of chronic primary immune thrombocytopenia. Oncology Working Party, editor. London, UK; 2014.
- Moulis G, Palmaro A, Montastruc J-L, Godeau B, Lapeyre-Mestre M, Sailler L. Epidemiology of incident immune thrombocytopenia: a nationwide population-based study in France. Blood. 2014;124(22):3308–3315. doi:10.1182/blood-2014-05-578336.
- Lambert MP, Gernsheimer TB. Clinical updates in adult immune thrombocytopenia. Blood. 2017;129(21):2829–2835. doi:10.1182/blood-2017-03-754119.
- Cooper N, Hill QA, Grainger J, Westwood J-P, Bradbury C, Provan D, Thachil J, Ramscar N, Roy A. Tapering and discontinuation of thrombopoietin receptor agonist therapy in patients with immune thrombocytopenia: results from a modified Delphi panel. Acta Haematol. 2021;144(4):418–426. doi:10.1159/000510676.
- Bussel JB, Mahmud SN, Brigstocke SL, Torneten SM. Tapering eltrombopag in patients with chronic ITP: how successful is this and in whom does it work? Blood. 2015;126(23):1054. doi:10.1182/blood.V126.23.1054.1054.
- Khellaf M, Michel M, Quittet P, Viallard J-F, Alexis M, Roudot-Thoraval F, Cheze S, Durand J-M, Lefrère F, Galicier L, et al. Romiplostim safety and efficacy for immune thrombocytopenia in clinical practice: 2-year results of 72 adults in a romiplostim compassionate-use program. Blood. 2011;118(16):4338–4345. doi:10.1182/blood-2011-03-340166.
- Saleh MN, Bussel JB, Cheng G, Meyer O, Bailey CK, Arning M, Brainsky A. Safety and efficacy of eltrombopag for treatment of chronic immune thrombocytopenia: results of the long-term, open-label EXTEND study. Blood. 2013;121(3):537–545. doi:10.1182/blood-2012-04-425512.
- Leven E, Miller A, Boulad N, Haider A, Bussel JB. Successful discontinuation of eltrombopag treatment in patients with chronic ITP. Blood. 2012;120(21):1085. doi:10.1182/blood.V120.21.1085.1085.
- Rodeghiero F, Carli G. Beyond immune thrombocytopenia: the evolving role of thrombopoietin receptor agonists. Ann Hematol. 2017;96(9):1421–1434. doi:10.1007/s00277-017-2953-6.
- Carpenedo M, Baldacci E, Baratè C, Borchiellini A, Buccisano F, Calvaruso G, Chiurazzi F, Fattizzo B, Giuffrida G, Rossi E, et al. Second-line administration of thrombopoietin receptor agonists in immune thrombocytopenia: Italian Delphi-based consensus recommendations. Ther Adv Hematol. 2021;12:20406207211048361. doi:10.1177/20406207211048361.
- Cuker A, Despotovic JM, Grace RF, Kruse C, Lambert MP, Liebman HA, Lyons RM, McCrae KR, Pullarkat V, Wasser JS, et al. Tapering thrombopoietin receptor agonists in primary immune thrombocytopenia: expert consensus based on the RAND/UCLA modified Delphi panel method. Res Pract Thromb Haemost. 2021;5(1):69–80. doi:10.1002/rth2.12457.
- Dalkey N, Helmer O. An experimental application of the Delphi method to the use of experts. Manage Sci. 1963;9(3):458–467. doi:10.1287/mnsc.9.3.458.
- Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: characteristics and guidelines for use. Am J Public Health. 1984;74(9):979–983. doi:10.2105/AJPH.74.9.979.
- Rowe G, Wright G. Expert opinions in forecasting: the role of the Delphi technique. In: Scott Armstrong J, editor. Principles of forecasting. New York: Springer New York; 2001. p. 125–144.
- Furniss D, Randell R, O’Kane AA, Taneva S, Mentis H, Blandford A. Fieldwork for healthcare: guidance for investigating human factors in computing systems. Synth Lect Assistive Rehabil Health Preserving Technol. 2014;3(2):1–146.
- González-López TJ, Sánchez-González B, Pascual C, Arefi M, de Cabo E, Alonso A, Martín-Salces M, Jiménez-Bárcenas R, Calbacho M, Galan P, et al. Sustained response after discontinuation of short- and medium-term treatment with eltrombopag in patients with immune thrombocytopenia. Platelets. 2015;26(1):83–86. doi:10.3109/09537104.2013.870987.
- Rashidi A, Blinder M. Combination therapy in relapsed or refractory chronic immune thrombocytopenia: a case report and literature review. J Clin Pharm Therap. 2016;41(5):453–458. doi:10.1111/jcpt.12421.
- Ghadaki B, Nazi I, Kelton JG, Arnold DM. Sustained remissions of immune thrombocytopenia associated with the use of thrombopoietin receptor agonists. Transfusion. 2013;53(11):2807–2812. doi:10.1111/trf.12139.
- Iino M, Sakamoto Y, Sato T. Treatment-free remission after thrombopoietin receptor agonist discontinuation in patients with newly diagnosed immune thrombocytopenia: an observational retrospective analysis in real-world clinical practice. Int J Hematol. 2020;112(2):159–168. doi:10.1007/s12185-020-02893-y.
- González‐López TJ, Pascual C, Álvarez‐román MT, Fernández-Fuertes F, Sánchez-González B, Caparrós I, Jarque I, Mingot-Castellano ME, Hernández-Rivas JA, Martín-Salces M, et al. Successful discontinuation of eltrombopag after complete remission in patients with primary immune thrombocytopenia. Am J Hematol. 2015;90(3):E40–43. doi:10.1002/ajh.23900.
- Červinek L, Mayer J, Doubek M. Sustained remission of chronic immune thrombocytopenia after discontinuation of treatment with thrombopoietin-receptor agonists in adults. Int J Hematol. 2015;102(1):7–11. doi:10.1007/s12185-015-1793-1.
- Zhang L, Zhang M, Du X, Cheng Y, Cheng G. Safety and efficacy of eltrombopag plus pulsed dexamethasone as first‐line therapy for immune thrombocytopenia. Brit J Haematol. 2020;189(2):369–378. doi:10.1111/bjh.16327.
- Wong RS, Saleh MN, Khelif A, Salama A, Portella MSO, Burgess P, Bussel JB. Safety and efficacy of long-term treatment of chronic/persistent ITP with eltrombopag: final results of the EXTEND study. Blood. 2017;130(23):2527–2536. doi:10.1182/blood-2017-04-748707.
- Zaja F, Carpenedo M, Baratè C, Borchiellini A, Chiurazzi F, Finazzi G, Lucchesi A, Palandri F, Ricco A, Santoro C, et al. Tapering and discontinuation of thrombopoietin receptor agonists in immune thrombocytopenia: real-world recommendations. Blood Rev. 2020;41:100647. doi:10.1016/j.blre.2019.100647.
- Provan D, Arnold DM, Bussel JB, Chong BH, Cooper N, Gernsheimer T, Ghanima W, Godeau B, González-López TJ, Grainger J, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780–3817. doi:10.1182/bloodadvances.2019000812.
- Taylor E. We agree, don’t we? The Delphi method for health environments research. HERD. 2020;13(1):11–23. doi:10.1177/1937586719887709.
- Gonzalez-Lopez TJ, Fernandez-Fuertes F, Izquierdo MCP, Caparros I, Bernat S, Pastoriza C, Martínez-Badas MP, Olivera PE, Caballero G, Hernandez-Rivas JA, et al. Long term discontinuation of eltrombopag after remission in primary immune thrombocytopenia: 8-year follow-up data from 15 Spanish centers. Blood. 2019;134(Supplement_1):2352. doi:10.1182/blood-2019-129274.
- Fowler FJ Jr, Levin CA, Sepucha KR. Informing and involving patients to improve the quality of medical decisions. Health Aff. 2011;30(4):699–706. doi:10.1377/hlthaff.2011.0003.
- Hawley ST, Morris AM. Cultural challenges to engaging patients in shared decision making. Patient Educ Counsel. 2017;100(1):18–24. doi:10.1016/j.pec.2016.07.008.
- Song F, Al-Samkari H. Management of adult patients with immune thrombocytopenia (ITP): a review on current guidance and experience from clinical practice. J Blood Med. 2021;12:653. doi:10.2147/JBM.S259101.
- Thachil J, Bagot C, Bradbury C, Cooper N, Lester W, Grainger JD, Lowe G, Evans G, Talks K, Sibson K, et al. A United Kingdom Immune Thrombocytopenia (ITP) forum review of practice: thrombopoietin receptor agonists. Brit J Haematol. 2018;180(4):591–594. doi:10.1111/bjh.14395.
- Mahevas M, Guillet S, Viallard J-F, Gobert D, Malphettes M, Cheze S, Lefrere F, Audia S, Bonnotte B, Lambotte O, et al. Rate of prolonged response after stopping thrombopoietin-receptor agonists treatment in primary immune thrombocytopenia (ITP): results from a nationwide prospective multicenter interventional study (STOPAGO). Blood. 2021;138(Supplement 1):583. doi:10.1182/blood-2021-152767.
- Mahévas M, Fain O, Ebbo M, Roudot-Thoraval F, Limal N, Khellaf M, Schleinitz N, Bierling P, Languille L, Godeau B, et al. The temporary use of thrombopoietin‐receptor agonists may induce a prolonged remission in adult chronic immune thrombocytopenia. Results of a French observational study. Brit J Haematol. 2014;165(6):865–869. doi:10.1111/bjh.12888.
- Lucchini E, Palandri F, Volpetti S, Vianelli N, Auteri G, Rossi E, Patriarca A, Carli G, Barcellini W, Tosi P, et al. Eltrombopag as second line therapy in adult patients with primary immune thrombocytopenia (ITP) in attempt to achieve long-term remission. preliminary analysis of a phase ii, multicenter, prospective study by gimema group (the ESTIT study). Blood. 2018;132(Supplement 1):1135. doi:10.1182/blood-2018-99-113314.
- Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, Bussel JB, Cines DB, Chong BH, Cooper N, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–2393. doi:10.1182/blood-2008-07-162503.
- Vogel C, Zwolinsky S, Griffiths C, Hobbs M, Henderson E, Wilkins E. A Delphi study to build consensus on the definition and use of big data in obesity research. Int J Obesity. 2019 Dec 1;43(12):2573–2586. doi:10.1038/s41366-018-0313-9.