Abstract
F11 receptor (F11R)/Junctional Adhesion Molecule –A (JAM-A) is a transmembrane protein which belongs to the immunoglobulin superfamily of cell adhesion molecules. F11R/JAM-A is present in epithelial cells, endothelial cells, leukocytes, and blood platelets. In epithelial and endothelial cells, it takes part in the formation of tight junctions. In these structures, molecules of F11R/JAM-A located on adjacent cells form homodimers and thus take part in stabilization of cellular layer integrity. In leukocytes, F11R/JAM-A was shown to play role in their transmigration through the vascular wall. Paradoxically, the function of F11R/JAM-A in blood platelets, where it was primarily discovered, is much less understood. It has been proven to regulate downstream signaling of αIIbβ3 integrin and to mediate platelet adhesion under static conditions. It was also shown to contribute to transient interactions of platelets with inflamed vascular wall. The review is aimed at summarizing the current state of knowledge of the platelet pool of F11R/JAM-A. The article also presents perspectives of the future research to better understand the role of this protein in hemostasis, thrombosis, and other processes where blood platelets are involved.
Plain Language Summary
The molecule of a complex name F11R/JAM-A is a protein which was primarily discovered on blood platelets. Later, the presence of the same molecule was confirmed on endothelial cells and epithelial cells. From the moment of the discovery, most of the research was focused on the role of this protein in the latter types of cells. It was found to be an important element of so-called tight junctions. These structures are crucial for maintaining of integrity and selective permeability of cellular layers composed of these types of cells. In the following years, the presence of F11R/JAM-A has also been reported on leukocytes. An important role of specific type of leukocytes is their penetration to the sites of inflammation. Interplay of F11R/JAM-A present on endothelium and that on leukocyte is involved in this process. But what about the role of this protein in blood platelets where it was originally discovered? There is limited knowledge regarding this issue. It was found to play a role in the ability of platelets to adhere to a surface under static conditions, but it is not known if the same is true under flow. Is the protein necessary for platelets to aggregate and form thrombus? Genetically engineered mice were created which lack this protein in blood platelets to answer this question. These platelets were abnormally reactive, as it transpired that the protein plays a role of a negative regulator to one of the most important mechanisms, which triggers platelet aggregation. But is this inhibitory function the only task F11R/JAM-A has to fulfil in platelets? Presented review collects all the knowledge regarding this protein in blood platelets and tries to show interesting routes which need exploration.
Introduction
The purpose of this review is to aggregate the current status of knowledge of F11R/JAM-A (F11 receptor/junctional adhesion molecule A) in blood platelets. Historically, the protein was first discovered in platelets as a target of an activating F11 antibody.Citation1 Shortly after it was also confirmed as a functional part of tight junctions in endothelium and epitheliumCitation2 and its expression was also found in immune cellsCitation3 and smooth muscle cells.Citation4 In the following years, a number of studies addressed the function of this protein in a range of physiological processes and an overwhelming number of studies addressed F11R/JAM-A function in these phenomena. At the same time, only a minority of studies were aimed at understanding of F11R/JAM-A function in platelets and in the processes where platelets are involved. As of writing this article, there were 645 original publications mentioning JAM-A. For only 39 of those, platelets are within the main focus of the study. Therefore, only about 6% of papers mention the platelet population of F11R/JAM-A in anything more than a passing acknowledgment of its existence. The presented work is aimed at a review of this relatively limited amount of experimental data.
The search was conducted using keywords and controlled vocabulary within three databases: PMC, Embase, and Cochrane library. The primary keyword and grouping variable for search results were different names of the protein in question (JAM-A/F11R/JAM-1/CD321/Junctional molecule A/Junctional adhesion molecule 1). The search parameters were further filtered by use of additional terms and phrases for related topics such as a junctional molecule, tight junction, platelet function, platelet proteome, platelet transcriptome, platelet omics, atherosclerosis, arteriosclerosis, and atherogenesis. Papers with fewer than five iterations of primary keywords were screened for secondary terms. Aggregated result of less than 10 keywords/terms was a basis for article exclusion unless the article provided further sources in its bibliographical references. Given the relative scarcity of valid sources, no articles were excluded based on species or the date of publication.
Historical background of F11R/JAM-A discovery
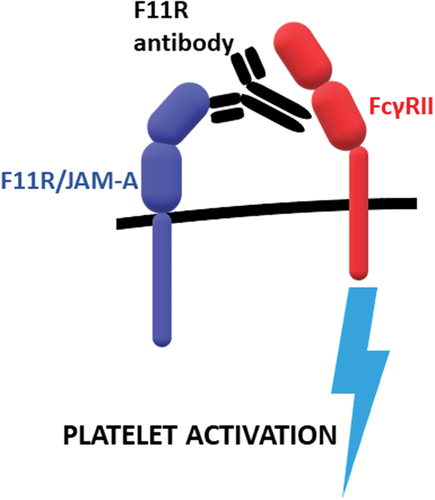
In 1990, Kornecki et al. showed that certain antibodies which had the capability of activating human blood platelets were binding to a protein target which was not known at the time. The protein was named F11 receptor (F11R) after the name of the clone of these antibodies (F11).Citation1 Platelet activation induced by binding of the antibodies to F11R was primarily shown to be dependent on the interaction of Fc fragment of F11 antibodies with FcγRII receptor present on blood plateletsCitation5 (). In 1998, a similar protein was isolated during studies on tight junctions in epithelial and endothelial cell monolayers.Citation2 The protein was recognized as an immunoglobulin and named after its function as Junctional Adhesion Molecule (JAM). In the wake of full sequencing of the protein, the homology of JAM-A and F11R was established.Citation6,Citation7 As an effect of this convergent discovery, the two names of the protein exist simultaneously in scientific publications. This status quo gained legitimacy by the decision of the Human Genome Nomenclature committee which has approved F11R as a symbol of the gene number AF207907, and the number BC021876 for the murine molecule, while JAM-A was mentioned as one of the alias symbols among others such as PAM-1, JCAM, JAM-1, JAMA, and CD321.
Basics of structure and function of F11R/JAM-A
Structure of F11R/JAM-A
JAM-A is a transmembrane protein belonging to the immunoglobulin superfamily (IgSF).Citation2,Citation8 Several JAMs have been recognized based on their structure,Citation9,Citation10 however, other members of the JAM family remain outside the scope of this paper. The other proteins from this family are JAM-B which populates mainly tight junctions and was not detected in blood platelets and JAM-C which is expressed on blood platelets and plays a role of a counterreceptor for the leukocyte integrin Mac-1.Citation11 JAM-A has a molecular weight of 29 kDaCitation1,Citation5 (core protein) which can reach between 32 kDaCitation12 and 43 kDaCitation13 as a result of post-translational modifications (N-glycosylation) or due to differences in mRNA splicing.Citation14 The protein consists of an extracellular part and transmembrane domain which ends in a short cytoplasmic tail. The extracellular part contains two Ig-like domains, a membrane distal D1 domain, and membrane proximal D2 domain (). In human F11R/JAM-A, the distal domain is of V-type and the proximal is of I-type.Citation15 In turn, murine protein consists of membrane distal V-type and a proximal C-type domain.Citation16,Citation17 The extracellular chain contains in its structure a motif which takes part in the homophilic-dimerization of two adjacent F11R/JAM-A molecules known as cis-dimerization.Citation18,Citation19 A distal Ig domain also contains a motif responsible for the trans-homo-dimerization i.e. dimerization of two F11R/JAM-A molecules located on adjacent cells. The trans-dimerization motif lies on the opposite side to the cis dimerization interface, ensuring non-exclusive access of the binding sites.Citation20,Citation21 A short linker chain between Ig domains forces a 125°Citation19,Citation22 bend in its conformation.
Figure 2. Structure and molecular interactions of the extracellular component F11R/JAM-A a) structure of F11R/JAM-A, b) cis-homophilic dimerization – two molecules of F11R/JAM-A located in the same cell interact with each other via D1 domain, c) trans-homophilic interactions – two, or more F11R/JAM-A dimers located on adjacent cells interact with each other via D1 domain, the site of interaction is different from the one responsible for cis-homophilic dimerization, d) heterophilic interaction with lymphocyte function-associated antigen (LFA-1) - LFA-1 is the only identified to date heterophilic ligand for F11R/JAM-A, the interaction occurs via D2 domain.
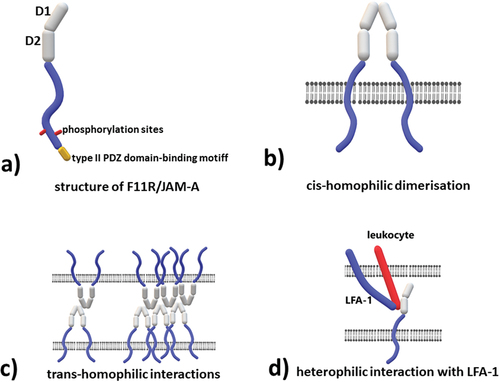
JAM-A has a single-pass transmembrane domain which ends in a short cytoplasmic tail with a PDZ binding motifCitation23,Citation24 which is characteristic for transmembrane receptors and signal transduction and is usually associated with internal proteins like ZO-1 or the actin cytoskeleton.Citation25
Interactions of F11R/JAM-A with other proteins
Homo- and hetero-dimerization
As mentioned above, F11R/JAM-A molecules are capable of forming homodimers. This homodimerization occurs in cis-configuration when the dimer is formed by the molecules located in the same cell (). The molecules can also interact in the trans-configuration when they are located on the membrane of two adjacent cellsCitation20 (). It is not clear whether cis-homodimerization is required for trans-homophilic interactions. Some data suggest that monomeric F11R/JAM-A can also interact with its counterpart on an adjacent cell.Citation20 The sites responsible for cis- and trans-homophilic interactions lay in distinct parts of D1 domain of the molecule.Citation20 Both types of interactions are believed to be associated with the function of the protein which will be described further.
The only reported ligand capable of forming a heterodimer with F11R/JAM-A is the lymphocyte function-associated antigen (LFA-1)Citation26 – leukocyte integrin involved in a plethora of processes such as adhesion or extravasation of lymphocytes. The site of interaction with LFA-1 is located in the proximal D2 domain of JAM-A/F11R ().
Interaction between F11R/JAM-A and other representatives of the JAM family such as JAM-B and JAM-C was reported in Danio rerio.Citation27
It is important to mention that both dimeric and monomeric form of F11R/JAM-A undergoes shedding from the cell membrane and thus occurs also in a soluble form.Citation1,Citation28 Two sheddases were identified to be responsible for this process: ADAM17 and to a lesser extent ADAM10.Citation13,Citation29,Citation30 The soluble form consists of both extracellular D1 and D2 domains. Such a soluble form of the protein is able to interact with intact, membrane-bound F11R/JAM-A.Citation31
Interactions with integrins and tetraspanins
F11R/JAM-A interacts laterally with integrins and tetraspanins. At least two tetraspanins, CD9 and CD151Citation21,Citation32,Citation33, act as intermediaries in the formation of ternary complexes of F11R/JAM-A with integrins such as α3β1 or αvβ3 in endothelial or epithelial cellsCitation32–35 or with platelet-specific αIIbβ3.Citation12 Importantly, in such complexes, F11R/JAM-A is present in a monomeric formCitation12,Citation32 ().
Figure 3. Interaction of F11R/JAM-A with αIIbβ3. Monomeric F11R/JAM-A interacts with αIIbβ3 via tetraspanin CD9.
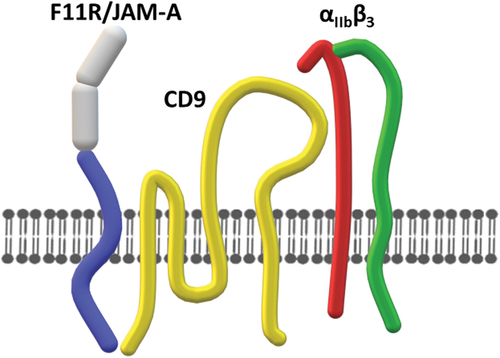
The architecture of these interactions seems to be complex and depends on the type of cells in question. In endothelial cells, interaction of CD9 with JAM-A requires the PDZ domain-binding motif of JAM-A and therefore is mediated by the intracellular portion of JAM-A.Citation32 In contrast, in MDCKII cells, JAM-A interacts with α3β1 via the extracellular JAM-A domain.Citation33
Proteins associated with intracellular part of F11R/JAM-A
The intracellular COOH terminus of F11R/JAM-A contains a type II PDZ domain-binding motif. It has been shown to interact with several PDZ domain-containing proteins: AF-6 (afadin) and ZO-1Citation25 in endothelial cells, afadin and PDZ-GEF2 but not with ZO-1,Citation36 with ZO-2, afadin and PDZ-GEF1,Citation37 CASK,Citation38 MUPP1,Citation39 and PAR3Citation40 in epithelial cells.
Function of JAM-A/F11R in endothelial cells, epithelial cells, and leukocytes
To date, JAM-A/F11R has been shown to be an important player in a large number of processes such as inflammation, tumor progression, and metastasis, differentiation of T-helper lymphocytes, or entry of viruses to host cells. The presented work is intended to review the current state of knowledge on JAM-A/F11R in blood platelets. This cannot be done, however, without outlining the information on this protein which, as stated above, was to a large extent gathered in other cellular models. To avoid an extensive review of JAM-A/F11R function in other types of cells which a reader can familiarize with in other excellent papers, such as,Citation16,Citation41 we briefly introduce arbitrarily selected aspects of JAM-A/F11R function in endothelial cells, epithelial cells, and leukocytes, which we considered most relevant for further discussion of JAM-A/F11R function in blood platelets.
Component of tight junctions
F11R/JAM-A is a component of tight junctions. In these structures, extracellular parts of F11R/JAM-A molecules are assembled as cis-homodimers interacting via trans-homodimerization with cis-homodimers on adjacent cells.Citation2 The intracellular part of the protein is associated with a complex of proteins which integrates tight junctions with the actin filaments. These trans-homophilic interactions are crucial for maintaining the integrity and regulation of permeability of an endothelial or epithelial layer. There are, however, discrepancies as to whether F11R/JAM-A plays a structural or regulatory role in these phenomena. A profound analysis of JAM-A’s role in the physiology of tight junctions and discussion regarding these discrepancies can be found in excellent reviews by Hartmann et al.Citation42 and Steinbacher et al.Citation21
An important aspect of F11R/JAM-A physiology in the endothelium is that upon inflammatory activation of endothelial cell, the protein translocates from tight junctions to the apical side of the cell and thus to the luminal surface of the blood vessel.Citation43,Citation44 This in turn facilitates the adhesion of monocytes and platelets to the inflamed vascular wall and therefore perpetuates the inflammatory process.
Adhesion of leukocytes to vascular wall and their transmigration
Leukocytes interact via their LFA-1 with endothelial F11R/JAM-A. Such interactions facilitate anchoring and migration of T cells in staticCitation26 as well as flow conditions.Citation45 A similar effect was shown for monocytesCitation46 and neutrophils, but the extent was lesser, due to redundancy provided by an alternate pathway employing Mac-1/ICAM-1 binding.
The affinity studies suggest that JAM easily adopts the dimeric state and preferentially forms hetero- rather than homodimers.Citation10,Citation15,Citation20,Citation23,Citation47 This is likely the mechanism used in the diapedesis, facilitating disruption of tight junctions. Once the LFA-1-induced disruption of the barrierCitation48 occurs, the uncoupled JAMs can disrupt deeper JAMs, therefore propagating the opening of the paracellular path, “unzipping” tight junctions in the direction of diapedesis.Citation49
F11R/JAM-A in blood platelets
Expression, dimerization, and phosphorylation
F11R/JAM-A is present in all phases of platelet life, starting from megakaryocytes,Citation50 through to matured platelets where it can be found on the cell surface and inside the alpha granules,Citation51 ending on platelet-derived microparticles.
Evaluation of the number of copies of F11R/JAM-A on the surface of human blood platelets revealed approx. 8,000 copies,Citation1 while the number of total copies based on proteomic analysis of the whole platelet was estimated at approx. 13,000 copies.Citation52,Citation53 The difference between a total number of copies and that on the surface suggests that some amount can be stored internally and presented on the surface upon activation. The latter is in agreement with the presence of the protein in alpha granulesCitation51 and with recent findings that platelet activation results in an increase of F11R/JAM-A expression revealed by flow cytometry.Citation31 Total number of copies of F11R/JAM-A places it among other abundant and crucial proteins such as integrin β3, αIIb, or glycoprotein Ibβ (expressed in tens of thousands of copies) and GPVI which (expressed in approximately 10,000 copies) and hints at significant role of F11R/JAM-A in the functioning of blood platelets.
In non-activated human platelets, F11R/JAM-A is predominantly present as a monomer and is associated with tetraspanin CD9.Citation12 In blood platelets, CD9 is associated with αIIbβ3 integrin and was shown to negatively regulate its functionCitation35 and to be involved in microparticle release from activated platelets.Citation54 When platelets were activated with F11 antibodies, a shift from monomeric to dimeric form of F11R/JAM-A was observed and this process was associated with a decrease in CD9 binding to F11R/JAM-A and an increase in binding of αIIbβ3. The latter observation is in contrast to what was observed in thrombin or fibrinogen-activated platelets where F11R/JAM-A dissociated from the complex with integrin αIIbβ3.Citation55 Similarly, in endothelial and epithelial cells, their activation resulted in dissociation of F11R/JAM-A from the complexes with integrins rather than association.Citation32,Citation34 Assuming that activation with F11 antibodies is to some extent different than that triggered by “classical” agonists, it can be summarized that similarly to epithelial and endothelial cells F11R/JAM-A in platelets dissociates from the complex with integrins upon platelet activation. There is no evidence, however, whether in platelets activated with “classical” agonists dissociated F11R/JAM-A forms dimers as it does in F11-activated platelets. The function of F11R/JAM-A is regulated by its phosphorylation. Upon platelet activation with thrombin, the protein is phosphorylated at Ser284 by PKC, to a lesser extent when activated with collagen, and no phosphorylation on Ser284 was observed in ADP-activated platelets.Citation5,Citation56 In resting blood platelets, F11R/JAM-A is phosphorylated on a tyrosine residue (human Y280 or murine Y281). Platelet activation and dissociation of F11R/JAM-A from integrin αIIbβ3 results in dephosphorylation in this site.Citation55 This dephosphorylation was shown to be dependent on protein phosphatase non-receptor type 1 (PTPN1) constitutively associated with F11R/JAM-A.Citation57 Tyrosine phosphorylation has also been shown to occur as a result of CLEC-2 cascade activation in platelets.Citation58 Analysis of phosphoproteomics of platelets revealed that F11R/JAM-A is in a group of proteins that respond with an early and sustained modification upon activation of platelets with ADP.Citation59
F11R/JAM-A in platelet activation, regulation of αIIbβ3 integrin signaling
From the moment of its discovery, F11R/JAM-A has been associated with the capacity to regulate blood platelet function. The original study has shown that F11 antibodies, which bound to the F11R protein, activated blood platelets by interacting with FcγRII.Citation5 As it turned out, there were also FcγRII -independent effects of F11 antibodies. Adhesion of platelets and spreading on immobilized F11 antibodies was not inhibited by blocking of FcγRII, suggesting that activation elicited by binding of F11 antibodies to F11R/JAM-A was independent of this receptor.Citation7 Furthermore, the presence of F11 caused platelet aggregation in response to normally subthreshold concentrations of ADP, thrombin, or collagen. This effect was sustained even when FcγRII was blocked by specific antibodiesCitation12 which confirmed that there was a component of platelet activation mediated directly by F11 antibodies binding to F11R/JAM-A and independent of crosslinking of the antibodies to FcγRII. The exact nature of this FcγRII-independent activation has not been elucidated.
New light has been shed on F11R/JAM-A’s role in the regulation of platelet activity by experiments performed on F11R/JAM-A knock-out mice. The association of F11R/JAM-A with αIIbβ3 integrin turned out to have a regulatory function and a lack of this protein in the murine model of F11R/JAM-A deficiency resulted in platelet hyperreactivity.Citation60 In vivo thrombosis in these mice was enhanced and their platelets showed higher reactivity toward agonists than that presented in wild-type animals. Further studies provided an explanation of the molecular mechanism of this phenomenon. It has been known before that downstream outside-in signaling of integrins is mediated via c-Src kinases associated with integrins. These kinases are maintained in an inactive state by phosphorylation mediated by Csk kinases.Citation61 F11R/JAM-A turned out to associate in resting platelets with Csk and keep it in the proximity of αIIbβ3 associated c-Src thus limiting αIIbβ3-mediated outside-in signaling.Citation55 Csk binding to F11R/JAM-A was dependent on tyrosine 280 phosphorylation on F11R/JAM-A. Dephosphorylation of this tyrosine upon platelet activation caused dissociation of Csk from F11R/JAM-A; therefore, releasing its inhibitory effect on c-Src. In F11R/JAM-A-deficient platelets, Csk is not present in the proximity of αIIbβ3 associated c-Src and, as an effect, outside-in signaling from αIIbβ3 is enhanced and these platelets are more prone to activation by a lower level of stimulus than their wild-type counterparts (). Importantly, authors have shown that in F11R/JAM-A-deficient platelets Csk is basically not co-immunoprecipitated with αIIbβ3 suggesting that F11R/JAM-A is the main protein responsible for ensuring Csk-dependent inhibitory activity on outside-in signaling from αIIbβ3.
Figure 4. F11R/JAM-A regulates outside-in signaling of αIIbβ3. In resting platelets Csk kinase is associated with F11R/JAM-A and remains in proximity to αIIbβ3-associated c-Src kinase. Phosphorylation of c-Src mediated by Csk limits outside-in signaling of αIIbβ3. Dissociation of F11R/JAM-A from the proximity of αIIbβ3 upon platelet activation, or F11R/JAM-A deficiency results in cessation of Csk inhibitory effect on c-Src. This in turn enhances outside-in signaling from the integrin.
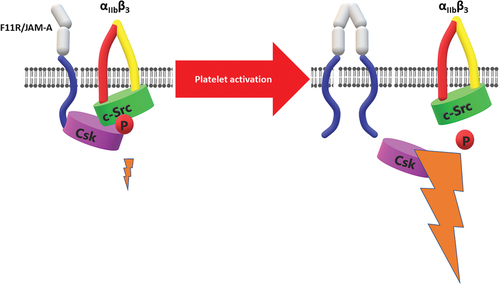
Estimates of F11R/JAM-A number of copies in human blood platelets mentioned above show that F11R/JAM-A molecules are outnumbered by αIIbβ3 by 3 to 4 times and therefore not all copies of αIIbβ3 could be negatively regulated by F11R/JAM-A at the same time in 1:1 manner. Alternatively, it could be speculated that Csk associated with F11R/JAM-A maintains such a pool of c-Src molecule in a phosphorylated, inactive state which is sufficient to effectively limit αIIbβ3-dependent outside-in signaling.
Recently a novel mechanism of platelet activity regulation by F11R/JAM-A was proposed where binding of soluble F11R/JAM-A to the protein located in the platelet membrane triggers a cascade of platelet activation.Citation31 The authors were prompted to study the effects of soluble F11R/JAM-A on blood platelets by an observation that serum levels of the protein were increased in patients with acute coronary syndrome and patients with higher serum concentrations of soluble F11R/JAM-A were at higher risk of recurrent myocardial infarction. Authors reported that the presence of soluble F11R/JAM-A increased the kinetics of platelet spreading on fibrinogen and enhanced platelet response to agonists such as ADP or TRAP, measured both by assessing integrin activation and degranulation, with the use of flow cytometry and by evaluating platelet aggregation. Thrombosis in vitro and in vivo in a murine model was also increased in presence of soluble F11R/JAM-A. These effects were associated with enhanced phosphorylation of p-Src kinase which bears some similarity to the effects of F11R/JAM-A knock-out observed in mice. Authors suggest that soluble F11R/JAM-A by interacting with F11R/JAM-A located in the platelet membrane may emulate trans-homophilic interaction of these two molecules in adjacent cells as it occurs in epithelial or endothelial cells and thus propagate the outside-in signaling. The authors noticed that the physiological relevance of this finding might be disputed as the concentrations of soluble F11R/JAM-A which caused the described effect in experimental conditions were several orders of magnitude higher than that measured in human serum samples. They argued, however, that the concentration of soluble F11R/JAM-A in the thromboinflammatory milieu may be in fact higher than that assessed in the plasma due to intense in situ shedding of the protein from inflamed endothelium.
F11R/JAM-A role in platelet adhesion and consequences for atherosclerosis
Considering that trans-homophilic interactions of F11R/JAM-A molecules contribute to the formation of tight junctions, it could be assumed that such interactions can translate to adhesive properties. Engagement of molecules located on blood platelet and their counterparts presented on activated endothelium or on another platelet could therefore be involved in platelet adhesion to an inflamed vascular wall or platelet recruitment to the growing thrombus.
Such a concept has been positively verified in static conditions as human blood platelets were shown to adhere to immobilized F11R/JAM-A.Citation31,Citation43 Similarly, platelet adhesion to activated endothelial cells in vitro in static conditions was partially blocked by a soluble form of F11R/JAM-A, peptides which constituted fragments of F11R/JAM-ACitation43 or by silencing of F11R/JAM-A gene.Citation62 This indicated that in static conditions, F11R/JAM-A-dependent interactions can contribute to platelet adhesion.
Whether these interactions could ensure platelet adhesion under flow conditions is less evident. Deposition of platelet-derived microparticles on activated endothelium under flow conditions was shown to be partially mediated by F11R/JAM-A as it was reduced in the presence of soluble F11R/JAM-A.Citation63 In line with this, lower deposition of platelet-derived chemokine RANTES was observed when F11R/JAM-A-deficient mouse platelets were perfused over activated endothelial cells expressing F11R/JAM-A when compared to deposition of F11R/JAM-A expressing platelets perfused over the same cells.Citation64 In ApoE−/− mice treated with the peptide which specifically blocked F11R/JAM-A trans-homophilic dimerizationCitation65 and platelet adhesion to inflamed endothelium under static conditions, platelets interacted less efficiently with vascular wall.Citation66
Contrary to these observations, soluble F11R/JAM-A has been shown to increase platelet adhesion to inflamed endothelium in vitro under flow conditions which was attributed by the authors to the activating effect of soluble F11R/JAM-A on platelets.Citation31
Another result which undermines the notion that F11R/JAM-A enhances the ability of platelets to adhere to the vascular wall was obtained in a study using hyperlipidemic mice with platelet-specific F11R/JAM-A deficiency. These mice were characterized by a higher release of platelet-derived microparticles and higher adhesion of platelets to activated endothelium than mice expressing F11R/JAM-A in blood platelets.Citation57 The mice lacking F11R/JAM-A in blood platelets also more rapidly developed atherosclerotic lesions. These findings were further confirmed by wire injury of the vascular wall in the same animal model. The site of vascular injury was more effectively covered with platelets in mice with F11R/JAM-A-deficient platelets than in mice whose platelets expressed F11R/JAM-A.Citation67 Interestingly, interactions observed in vitro under flow conditions of monocytes with adherent platelets were more pronounced in the case of F11R/JAM-A-deficient platelets than with F11R/JAM-A expressing platelets. These effects were explained by higher reactivity of F11R/JAM-A deficient platelets in comparison with WT platelets in a mechanism explained in the previous section.
The higher reactivity of F11R/JAM-A-deficient platelets causes certain difficulties in terms of understanding the role of trans-homophilic interactions of F11R/JAM-A in platelet adhesion with the use of knock-out models. Any potential effect caused by the lack of these interactions can be masked by an enhancement in the activity of other mechanisms in these platelets. The fact that F11R/JAM-A is not indispensable for both aggregation and adhesion does not mean that the protein is devoid of any other function that regulation of αIIbβ3 activity.
As it was discussed above, studies of the role of platelet F11R/JAM-A in the development of atherosclerosis provide conflicting results. One of the aspects that has not been addressed is the level of JAM-A expression on blood platelets among the human population. Taking into account its crucial role in the regulation of outside-in signaling of αIIbβ3 integrin, it would be interesting to assess whether F11R/JAM-A expression in platelets correlates with platelet reactivity, cardiovascular disease, and/or cardiovascular events as it has been shown for the soluble portion of the protein.Citation31,Citation68
Perspectives
It is still not clarified what is the role of the formation of cis-homodimers in platelets upon activation. It is suggested that cis-dimerization of monomers dissociated from αIIbβ3 keeps them at bay from the integrin and therefore allows the outside-in signaling during platelet activation. Are therefore cis-dimers only a storage form of the protein, or have they some other role to play after platelet activation? Would the inhibition of cis-homodimerization impair this mechanism and limit outside-in signaling thus decreasing platelet reactivity? Animal models expressing in blood platelets F11R/JAM-A with site-specific mutations in the D1 domain, which is responsible for cis-dimerization, would greatly help to answer these questions.
Based on F11R/JAM-A’s involvement in tight junctions formation, an obvious question occurs: can this protein take part in the formation of similar connections between blood platelets during thrombus formation or in any other interaction between platelets and other types of cells? The only evidence published to date in favor of such a role of F11R/JAM-A is an electron microscopy image revealing clusters of F11R/JAM-A on contacts of thrombin-activated platelets.Citation56 This raises another question: why do blood platelets contain some of the proteins which play a part in tight junctions formation? According to the platelet proteome identification, approx. 2,400 copies of claudin-5, 1,100 copies of claudin-3, and 3,900 copies of zonula occludens 2 (ZO-2) were detected.Citation52,Citation53,Citation69 In concert with this, a recent conference communication presented on International Society on Thrombosis and Haemostasis congress in 2022 in London reported enriched ZO-2 in platelet-platelet contacts.Citation70 Both proteins are essential components of tight junctions in epithelial and endothelial cells. Claudins are transmembrane proteins directly involved in formation of cell contacts while ZO-2 is a peripheral membrane protein which connects actin cytoskeleton with transmembrane components of tight junctions such as claudins. On the other hand, no occludins, another important transmembrane protein component of tight junctions, were found in platelets’ proteome. These data suggest that blood platelets may form a kind of tight junction analogs. Taking into account the role of tight junctions in regulation of permeability of endothelial and epithelial layers, it is conceivable that similar structures on platelet-platelet contacts may be responsible for the regulation of thrombus porosity.
The involvement of platelets in the regulation of immunological processes is well known and was profoundly reviewed.Citation71,Citation72 Until today, there are no data regarding platelet F11R/JAM-A involvement in such regulation. There are however certain indications suggesting such a possibility. It has been recently shown that F11R/JAM-A present on dendritic cells is involved in regulation of T cells differentiation.Citation73 The site of contact of these two types of cells where the interaction of the molecules crucial for the process occurs is called immunological synapse. This structure is known to contain LFA-1Citation74 which, as mentioned above, is the only known protein interacting with F11R/JAM-A other than F11R/JAM-A itself. Taking this into account, it could be assumed that platelet F11R/JAM-A may be involved in immunomodulatory processes.
It has been shown that F11R/JAM-A in endothelial cells acts as a mechanosensor.Citation75 Tension or shear stress imposed on this protein resulted in a series of intracellular signaling events which led to increased cell stiffness. Since a growing thrombus is subjected to a constant shear stress, it is conceivable that platelet F11R/JAM-A located on the luminal thrombus surface as well as the portion of the protein engaged in trans-homophilic interactions between adjacent platelets in deeper layers of thrombus can affect the mechanistic properties of platelets. This in turn could have an impact on thrombus stability.
There is still much to be learned about the many physiological roles of platelet F11R/JAM-A. The innocuous junctional protein might be so much more than its humble name implies. Only time and concerted effort will tell.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kornecki E, Walkowiak B, Naik UP, Ehrlich YH. Activation of human platelets by a stimulatory monoclonal antibody. J Biol Chem. 1990;265(17):10042–9. doi:10.1016/S0021-9258(19)38776-9. Epub 1990 Jun 15.
- Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142(1):117–27. doi:10.1083/jcb.142.1.117. Epub 1998 Jul 14.
- Williams LA, Martin-Padura I, Dejana E, Hogg N, Simmons DL. Identification and characterisation of human junctional adhesion molecule (JAM). Mol Immunol. 1999;36(17):1175–88. doi:10.1016/S0161-5890(99)00122-4. Epub 2000 Mar 04.
- Azari BM, Marmur JD, Salifu MO, Cavusoglu E, Ehrlich YH, Kornecki E, Babinska A. Silencing of the F11R gene reveals a role for F11R/JAM-A in the migration of inflamed vascular smooth muscle cells and in atherosclerosis. Atherosclerosis. 2010;212(1):197–205. doi:10.1016/j.atherosclerosis.2010.05.014. Epub 2010 Jul 16.
- Naik UP, Ehrlich YH, Kornecki E. Mechanisms of platelet activation by a stimulatory antibody: cross-linking of a novel platelet receptor for monoclonal antibody F11 with the Fc gamma RII receptor. Biochem J. 1995;310(Pt 1):155–62. doi:10.1042/bj3100155. Epub 1995 Aug 15.
- Arrate MP, Rodriguez JM, Tran TM, Brock TA, Cunningham SA. Cloning of human junctional adhesion molecule 3 (JAM3) and its identification as the JAM2 counter-receptor. J Biol Chem. 2001;276(49):45826–32. doi:10.1074/jbc.M105972200. Epub 2001 Oct 09.
- Sobocka MB, Sobocki T, Banerjee P, Weiss C, Rushbrook JI, Norin AJ, Hartwig J, Salifu MO, Markell MS, Babinska A, et al. Cloning of the human platelet F11 receptor: a cell adhesion molecule member of the immunoglobulin superfamily involved in platelet aggregation. Blood. 2000;95(8):2600–9. doi:10.1182/blood.V95.8.2600.
- Mandell KJ, Parkos CA. The JAM family of proteins. Adv Drug Deliv Rev. 2005;57(6):857–67. doi:10.1016/j.addr.2005.01.005. Epub 2005 Apr 12.
- Aurrand-Lions M, Johnson-Leger C, Wong C, Du Pasquier L, Imhof BA. Heterogeneity of endothelial junctions is reflected by differential expression and specific subcellular localization of the three JAM family members. Blood. 2001;98(13):3699–707. doi:10.1182/blood.V98.13.3699. Epub 2001 Dec 12.
- Bradfield PF, Nourshargh S, Aurrand-Lions M, Imhof BA. JAM family and related proteins in leukocyte migration (Vestweber series). Arterioscler Thromb Vasc Biol. 2007;27(10):2104–12. doi:10.1161/ATVBAHA.107.147694. Epub 2007 Jul 7.
- Santoso S, Sachs UJ, Kroll H, Linder M, Ruf A, Preissner KT, Chavakis T. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J Exp Med. 2002;196(5):679–91. doi:10.1084/jem.20020267. Epub 2002 Sep 5.
- Sobocka MB, Sobocki T, Babinska A, Hartwig JH, Li M, Ehrlich YH, Kornecki E. Signaling pathways of the F11 receptor (F11R; a.k.a. JAM-1, JAM-A) in human platelets: F11R dimerization, phosphorylation and complex formation with the integrin GPIIIa. J Recept Signal Transduct Res. 2004;24(1–2):85–105. doi:10.1081/RRS-120034252. Epub 2004 Sep 4.
- Koenen RR, Pruessmeyer J, Soehnlein O, Fraemohs L, Zernecke A, Schwarz N, Reiss K, Sarabi A, Lindbom L, Hackeng TM, et al. Regulated release and functional modulation of junctional adhesion molecule a by disintegrin metalloproteinases. Blood. 2009;113(19):4799–809. doi:10.1182/blood-2008-04-152330. Epub 2009 Mar 5.
- Sobocki T, Sobocka MB, Babinska A, Ehrlich YH, Banerjee P, Kornecki E. Genomic structure, organization and promoter analysis of the human F11R/F11 receptor/junctional adhesion molecule-1/JAM-A. Gene. 2006;366(1):128–44. doi:10.1016/j.gene.2005.08.025. Epub 2005 Dec 13.
- Prota AE, Campbell JA, Schelling P, Forrest JC, Watson MJ, Peters TR, Aurrand-Lions M, Imhof BA, Dermody TS, Stehle T. Crystal structure of human junctional adhesion molecule 1: implications for reovirus binding. Proc Natl Acad Sci U S A. 2003;100(9):5366–71. doi:10.1073/pnas.0937718100. Epub 2003 Apr 17.
- Ebnet K. Junctional adhesion molecules (JAMs): cell adhesion receptors with pleiotropic functions in cell physiology and development. Physiol Rev. 2017;97(4):1529–54. doi:10.1152/physrev.00004.2017. Epub 2017 Sep 22.
- Bonilha CS, Benson RA, Brewer JM, Garside P. Targeting opposing immunological roles of the junctional adhesion molecule-a in autoimmunity and cancer. Front Immunol. 2020;11:602094. doi:10.3389/fimmu.2020.602094. Epub 2020 Dec 17.
- Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): more molecules with dual functions? J Cell Sci. 2004;117(1):19–29. doi:10.1242/jcs.00930. Epub 2003 Dec 06.
- Kostrewa D, Brockhaus M, D’Arcy A, Dale GE, Nelboeck P, Schmid G, Mueller F, Bazzoni G, Dejana E, Bartfai T, et al. X-ray structure of junctional adhesion molecule: structural basis for homophilic adhesion via a novel dimerization motif. Embo J. 2001;20(16):4391–8. doi:10.1093/emboj/20.16.4391. Epub 2001 Aug 14.
- Monteiro AC, Luissint AC, Sumagin R, Lai C, Vielmuth F, Wolf MF, Laur O, Reiss K, Spindler V, Stehle T, et al. Trans-dimerization of JAM-A regulates Rap2 and is mediated by a domain that is distinct from the cis-dimerization interface. Mol Biol Cell. 2014;25(10):1574–85. doi:10.1091/mbc.e14-01-0018. Epub 2014 Mar 29.
- Steinbacher T, Kummer D, Ebnet K. Junctional adhesion molecule-A: functional diversity through molecular promiscuity. Cell Mol Life Sci. 2018;75(8):1393–409. doi:10.1007/s00018-017-2729-0. Epub 2017 Dec 15.
- Weber C, Fraemohs L, Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol. 2007;7(6):467–77. doi:10.1038/nri2096. Epub 2007 May 26.
- Bazzoni G, Martinez-Estrada OM, Mueller F, Nelboeck P, Schmid G, Bartfai T, Dejana E, Brockhaus M. Homophilic interaction of junctional adhesion molecule. J Biol Chem. 2000;275(40):30970–6. doi:10.1074/jbc.M003946200. Epub 2000 Jul 27.
- Songyang Z, Fanning AS, Fu C, Xu J, Marfatia SM, Chishti AH, Crompton A, Chan AC, Anderson JM, Cantley LC. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science. 1997;275(5296):73–7. doi:10.1126/science.275.5296.73. Epub 1997 Jan 03.
- Ebnet K, Schulz CU, Meyer Zu Brickwedde MK, Pendl GG, Vestweber D. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J Biol Chem. 2000;275(36):27979–88. doi:10.1074/jbc.M002363200. Epub 2000 Jun 17.
- Ostermann G, Weber KS, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the β2 integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;3(2):151–8. doi:10.1038/ni755. Epub 2002 Jan 29.
- Kobayashi I, Kobayashi-Sun J, Kim AD, Pouget C, Fujita N, Suda T, Traver D. Jam1a–jam2a interactions regulate haematopoietic stem cell fate through Notch signalling. Nature. 2014;512(7514):319–23. doi:10.1038/nature13623. Epub 2014 Aug 15.
- Salifu MO, Kolff Q, Murty P, Haria DM, Zimpa M, Shakeel M, Lee H, Kornecki E, Babinska A. Relationship between the soluble F11 receptor and markers of inflammation in hemodialysis patients. J Investig Med. 2007;55(3):115–19. doi:10.2310/6650.2007.06041. Epub 2007 May 08.
- Fong KP, Barry C, Tran AN, Traxler EA, Wannemacher KM, Tang HY, Speicher KD, Blair IA, Speicher DW, Grosser T, et al. Deciphering the human platelet sheddome. Blood. 2011;117(1):e15–26. doi:10.1182/blood-2010-05-283838. Epub 2010 Oct 22.
- Garcia BA, Smalley DM, Cho H, Shabanowitz J, Ley K, Hunt DF. The platelet microparticle proteome. J Proteome Res. 2005;4(5):1516–21. doi:10.1021/pr0500760. Epub 2005 Oct 11.
- Rath D, Rapp V, Schwartz J, Winter S, Emschermann F, Arnold D, Rheinlaender J, Buttcher M, Strebl M, Braun MB, et al. Homophilic interaction between transmembrane-JAM-A and soluble jam-a regulates thrombo-inflammation: implications for coronary artery disease. JACC Basic Transl Sci. 2022;7(5):445–61. doi:10.1016/j.jacbts.2022.03.003. Epub 2022 Jun 7.
- Peddibhotla SS, Brinkmann BF, Kummer D, Tuncay H, Nakayama M, Adams RH, Gerke V, Ebnet K. Tetraspanin CD9 links junctional adhesion molecule-A to αvβ3 integrin to mediate basic fibroblast growth factor–specific angiogenic signaling. Mol Biol Cell. 2013;24(7):933–44. doi:10.1091/mbc.e12-06-0481. Epub 2013 Feb 8.
- Tholmann S, Seebach J, Otani T, Florin L, Schnittler H, Gerke V, Furuse M, Ebnet K. JAM-A interacts with α3β1 integrin and tetraspanins CD151 and CD9 to regulate collective cell migration of polarized epithelial cells. Cell Mol Life Sci. 2022;79(2):88. doi:10.1007/s00018-022-04140-5. Epub 2022 Jan 25.
- Naik MU, Mousa SA, Parkos CA, Naik UP. Signaling through JAM-1 and αvβ3 is required for the angiogenic action of bFGF: dissociation of the JAM-1 and αvβ3 complex. Blood. 2003;102(6):2108–14. doi:10.1182/blood-2003-04-1114. Epub 2003 May 17.
- Mangin PH, Kleitz L, Boucheix C, Gachet C, Lanza F. CD9 negatively regulates integrin αIIbβ3 activation and could thus prevent excessive platelet recruitment at sites of vascular injury. J Thromb Haemost. 2009;7(5):900–2. doi:10.1111/j.1538-7836.2009.03322.x. Epub 2009 Feb 21.
- Severson EA, Lee WY, Capaldo CT, Nusrat A, Parkos CA. Junctional adhesion molecule a interacts with Afadin and PDZ-GEF2 to activate Rap1A, regulate β1 integrin levels, and enhance cell migration. Mol Biol Cell. 2009;20(7):1916–25. doi:10.1091/mbc.e08-10-1014. Epub 2009 Jan 30.
- Monteiro AC, Sumagin R, Rankin CR, Leoni G, Mina MJ, Reiter DM, Stehle T, Dermody TS, Schaefer SA, Hall RA, et al. JAM-A associates with ZO-2, afadin, and PDZ-GEF1 to activate Rap2c and regulate epithelial barrier function. Mol Biol Cell. 2013;24(18):2849–60. doi:10.1091/mbc.e13-06-0298. Epub 2013 Jul 26.
- Martinez-Estrada OM, Villa A, Breviario F, Orsenigo F, Dejana E, Bazzoni G. Association of junctional adhesion molecule with calcium/calmodulin-dependent serine protein kinase (CASK/LIN-2) in human epithelial caco-2 cells. J Biol Chem. 2001;276(12):9291–6. doi:10.1074/jbc.M006991200. Epub 2000 Dec 30.
- Hamazaki Y, Itoh M, Sasaki H, Furuse M, Tsukita S. Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J Biol Chem. 2002;277(1):455–61. doi:10.1074/jbc.M109005200. Epub 2001 Nov 2.
- Itoh M, Sasaki H, Furuse M, Ozaki H, Kita T, Tsukita S. Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J Cell Biol. 2001;154(3):491–7. doi:10.1083/jcb.200103047. Epub 2001 Aug 8.
- Czubak-Prowizor K, Babinska A, Swiatkowska M. The F11 receptor (F11R)/junctional adhesion molecule-A (JAM-A) (F11R/JAM-A) in cancer progression. Mol Cell Biochem. 2022;477(1):79–98. doi:10.1007/s11010-021-04259-2. Epub 2021 Sep 18.
- Hartmann C, Schwietzer YA, Otani T, Furuse M, Ebnet K. Physiological functions of junctional adhesion molecules (JAMs) in tight junctions. Biochim Biophys Acta Biomembr. 2020;1862(9):183299. doi:10.1016/j.bbamem.2020.183299. Epub 2020 Apr 06.
- Babinska A, Kedees MH, Athar H, Ahmed T, Batuman O, Ehrlich YH, Hussain MM, Kornecki E. F11-receptor (F11R/JAM) mediates platelet adhesion to endothelial cells: role in inflammatory thrombosis. Thromb Haemost. 2002;88(11):843–50. doi:10.1055/s-0037-1613312. Epub 2002 Nov 13.
- Schmitt MM, Megens RT, Zernecke A, Bidzhekov K, van den Akker NM, Rademakers T, van Zandvoort MA, Hackeng TM, Koenen RR, Weber C. Endothelial junctional adhesion molecule-a guides monocytes into flow-dependent predilection sites of atherosclerosis. Circulation. 2014;129(1):66–76. doi:10.1161/CIRCULATIONAHA.113.004149. Epub 2013 Sep 26.
- Weber C. Novel mechanistic concepts for the control of leukocyte transmigration: specialization of integrins, chemokines, and junctional molecules. J Mol Med (Berl). 2003;81(1):4–19. doi:10.1007/s00109-002-0391-x. Epub 2003 Jan 25.
- Del Maschio A, De Luigi A, Martin-Padura I, Brockhaus M, Bartfai T, Fruscella P, Adorini L, Martino G, Furlan R, De Simoni MG, et al. Leukocyte recruitment in the cerebrospinal fluid of mice with experimental meningitis is inhibited by an antibody to junctional adhesion molecule (JAM). J Exp Med. 1999;190(9):1351–6. doi:10.1084/jem.190.9.1351. Epub 1999 Nov 02.
- Lamagna C, Meda P, Mandicourt G, Brown J, Gilbert RJ, Jones EY, Kiefer F, Ruga P, Imhof BA, Aurrand-Lions M. Dual Interaction of JAM-C with JAM-B and alpha(M)beta2 integrin: function in junctional complexes and leukocyte adhesion. Mol Biol Cell. 2005;16(10):4992–5003. doi:10.1091/mbc.e05-04-0310. Epub 2005 Aug 12.
- Fraemohs L, Koenen RR, Ostermann G, Heinemann B, Weber C. The functional interaction of the β2 integrin lymphocyte function-associated antigen-1 with junctional adhesion molecule-A is mediated by the i domain. J Immunol. 2004;173(10):6259–64. doi:10.4049/jimmunol.173.10.6259. Epub 2004 Nov 6.
- Wojcikiewicz EP, Koenen RR, Fraemohs L, Minkiewicz J, Azad H, Weber C, Moy VT. LFA-1 binding destabilizes the JAM-A homophilic interaction during leukocyte transmigration. Biophys J. 2009;96(1):285–93. doi:10.1529/biophysj.108.135491. Epub 2008 Oct 14.
- Watkins NA, Gusnanto A, de Bono B, De S, Miranda-Saavedra D, Hardie DL, Angenent WG, Attwood AP, Ellis PD, Erber W, et al. A HaemAtlas: characterizing gene expression in differentiated human blood cells. Blood. 2009;113(19):e1–e9. doi:10.1182/blood-2008-06-162958. Epub 2009 Feb 21.
- Maynard DM, Heijnen HF, Horne MK, White JG, Gahl WA. Proteomic analysis of platelet α-granules using mass spectrometry. J Thromb Haemost. 2007;5(9):1945–55. doi:10.1111/j.1538-7836.2007.02690.x. Epub 2007 Aug 29.
- Huang J, Swieringa F, Solari FA, Provenzale I, Grassi L, De Simone I, Baaten C, Cavill R, Sickmann A, Frontini M, et al. Assessment of a complete and classified platelet proteome from genome-wide transcripts of human platelets and megakaryocytes covering platelet functions. Sci Rep. 2021;11(1):12358. doi:10.1038/s41598-021-91661-x. Epub 2021 Jun 13.
- Burkhart JM, Vaudel M, Gambaryan S, Radau S, Walter U, Martens L, Geiger J, Sickmann A, Zahedi RP. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood. 2012;120(15):e73–82. doi:10.1182/blood-2012-04-416594. Epub 2012 Aug 8.
- Dale GL, Remenyi G, Friese P. Tetraspanin CD9 is required for microparticle release from coated-platelets. Platelets. 2009;20(6):361–6. doi:10.1080/09537100903096692. Epub 2009 Aug 07.
- Naik MU, Caplan JL, Naik UP. Junctional adhesion molecule-A suppresses platelet integrin αIIbβ3 signaling by recruiting Csk to the integrin-c–Src complex. Blood. 2014;123(9):1393–402. doi:10.1182/blood-2013-04-496232. Epub 2013 Dec 5.
- Ozaki H, Ishii K, Arai H, Horiuchi H, Kawamoto T, Suzuki H, Kita T. Junctional adhesion molecule (JAM) is phosphorylated by protein kinase C upon platelet activation. Biochem Biophys Res Commun. 2000;276(3):873–8. doi:10.1006/bbrc.2000.3574. Epub 2000 Oct 12.
- Karshovska E, Zhao Z, Blanchet X, Schmitt MM, Bidzhekov K, Soehnlein O, von Hundelshausen P, Mattheij NJ, Cosemans JM, Megens RT, et al. Hyperreactivity of junctional adhesion molecule A-deficient platelets accelerates atherosclerosis in hyperlipidemic mice. Circ Res. 2015;116(4):587–99. doi:10.1161/CIRCRESAHA.116.304035. Epub 2014 Dec 5.
- Izquierdo I, Barrachina MN, Hermida-Nogueira L, Casas V, Moran LA, Lacerenza S, Pinto-Llorente R, Eble JA, de Los Rios V, Dominguez E, et al. A comprehensive tyrosine phosphoproteomic analysis reveals novel components of the platelet CLEC-2 signaling cascade. Thromb Haemost. 2020;120(2):262–76. doi:10.1055/s-0039-3400295. Epub 2020 Jan 5.
- Beck F, Geiger J, Gambaryan S, Solari FA, Dell’Aica M, Loroch S, Mattheij NJ, Mindukshev I, Potz O, Jurk K, et al. Temporal quantitative phosphoproteomics of ADP stimulation reveals novel central nodes in platelet activation and inhibition. Blood. 2017;129(2):e1–e12. doi:10.1182/blood-2016-05-714048. Epub 2017 Jan 7.
- Naik MU, Stalker TJ, Brass LF, Naik UP. JAM-A protects from thrombosis by suppressing integrin αIIbβ3-dependent outside-in signaling in platelets. Blood. 2012;119(14):3352–60. doi:10.1182/blood-2011-12-397398. Epub 2012 Jan 25.
- Obergfell A, Eto K, Mocsai A, Buensuceso C, Moores SL, Brugge JS, Lowell CA, Shattil SJ. Coordinate interactions of Csk, Src, and Syk kinases with αIIbβ3 initiate integrin signaling to the cytoskeleton. J Cell Biol. 2002;157(2):265–75. doi:10.1083/jcb.200112113. Epub 2002 Apr 10.
- Azari BM, Marmur JD, Salifu MO, Ehrlich YH, Kornecki E, Babinska A. Transcription and translation of human F11R gene are required for an initial step of atherogenesis induced by inflammatory cytokines. J Transl Med. 2011;9(1):98. doi:10.1186/1479-5876-9-98. Epub 2011 Jun 28.
- Mause SF, von Hundelshausen P, Zernecke A, Koenen RR, Weber C. Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler Thromb Vasc Biol. 2005;25(7):1512–18. doi:10.1161/01.ATV.0000170133.43608.37. Epub 2005 May 14.
- Zernecke A, Liehn EA, Fraemohs L, von Hundelshausen P, Koenen RR, Corada M, Dejana E, Weber C. Importance of junctional adhesion molecule-A for neointimal lesion formation and infiltration in atherosclerosis-prone mice. Arterioscler Thromb Vasc Biol. 2006;26(2):e10–e13. doi:10.1161/01.ATV.0000197852.24529.4f. Epub 2005 Nov 25.
- Babinska A, Clement CC, Swiatkowska M, Szymanski J, Shon A, Ehrlich YH, Kornecki E, Salifu MO. Development of new antiatherosclerotic and antithrombotic drugs utilizing F11 receptor (F11R/JAM-A) peptides. Biopolymers. 2014;102(4):322–34. doi:10.1002/bip.22503. Epub 2014 May 8.
- Babinska A, Clement CC, Li Y, Wzorek J, Przygodzki T, Talar M, Braun M, Swiatkowska M, Ehrlich YH, Kornecki E, et al. In vivo data: treatment with the F11R/JAM-A peptide 4D decreases mortality and reduces the generation of atherosclerotic plaques in ApoE-deficient mice. Data Brief. 2020;30:105516. doi:10.1016/j.dib.2020.105516. Epub 2020 May 13.
- Zhao Z, Vajen T, Karshovska E, Dickhout A, Schmitt MM, Megens RTA, von Hundelshausen P, Koeppel TA, Hackeng TM, Weber C, et al. Deletion of junctional adhesion molecule a from platelets increases early-stage neointima formation after wire injury in hyperlipidemic mice. J Cell Mol Med. 2017;21(8):1523–31. doi:10.1111/jcmm.13083. Epub 2017 Feb 18.
- Cavusoglu E, Kornecki E, Sobocka MB, Babinska A, Ehrlich YH, Chopra V, Yanamadala S, Ruwende C, Salifu MO, Clark LT, et al. Association of plasma levels of F11 receptor/junctional adhesion molecule-A (F11R/JAM-A) with human atherosclerosis. J Am Coll Cardiol. 2007;50(18):1768–76. doi:10.1016/j.jacc.2007.05.051. Epub 2007 Oct 30.
- Lewandrowski U, Wortelkamp S, Lohrig K, Zahedi RP, Wolters DA, Walter U, Sickmann A. Platelet membrane proteomics: a novel repository for functional research. Blood. 2009;114(1):e10–e19. doi:10.1182/blood-2009-02-203828. Epub 2009 May 14.
- Nagy MB M, Poulter N, Sickmann A, Stephenne X, Brouns S, Koenen R, Ten Cate H, Heemskerk J, Baaten C. ZO-2 enriched tight junction-like structures at sites of platelet-platelet contact. The ISTH 2022 Congress; London. 2022.
- Lam FW, Vijayan KV, Rumbaut RE. Platelets and their interactions with other immune cells. Compr Physiol. 2015;5:1265–80. Epub 2015 Jul 4.
- Ponomarev ED. Fresh evidence for platelets as neuronal and innate immune cells: their role in the activation, differentiation, and deactivation of Th1, Th17, and tregs during tissue inflammation. Front Immunol. 2018;9:406. doi:10.3389/fimmu.2018.00406. Epub 2018 Mar 31.
- Bonilha CS, Benson RA, Scales HE, Brewer JM, Garside P. Junctional adhesion molecule-A on dendritic cells regulates Th1 differentiation. Immunol Lett. 2021;235:32–40. doi:10.1016/j.imlet.2021.05.001. Epub 2021 May 18.
- Dustin ML. The immunological synapse. Cancer Immunol Res. 2014;2(11):1023–33. doi:10.1158/2326-6066.CIR-14-0161. Epub 2014 Nov 5.
- Scott DW, Tolbert CE, Burridge K. Tension on JAM-A activates RhoA via GEF-H1 and p115 RhoGEF. Mol Biol Cell. 2016;27(9):1420–30. doi:10.1091/mbc.E15-12-0833. Epub 2016 Mar 18.