Abstract
Celiac (gluten intolerant) sprue is a life-long threatening disease. The only effective treatment thus far is to maintain a gluten-free diet. Sensitive and reliable double antibody sandwich enzyme-linked immunosorbent assay (ELISA) and Immunoswab assay were developed by using chicken egg yolk anti-gliadin immunoglobulin Y as capturing antibody and monoclonal anti-gliadin IgG (HYB-314) antibody as detecting antibody for the detection of gliadin. The detection limits of the double antibody sandwich ELISA and Immunoswab assay were 5 ng/ml in phosphate buffered saline and 1.25 µg/ml in 60% ethanol, respectively. The Immunoswab was a more convenient and rapid detection system, but was less sensitive as compared to the double antibody sandwich ELISA developed.
Introduction
Celiac disease (CD) or gluten intolerance, one of the most permanent food intolerances, is induced by ingestion of gluten proteins from wheat, barley, rye and possibly oats. Gluten consists of prolamin and glutenin proteins. The principal toxic components of wheat prolamin include proline and glutamine rich proteins called gliadins. CD is triggered by gliadin peptides that are not digested by human protease. When CD individuals consume foods containing gluten, it causes a loss of villous structure in their proximal small intestine mucosa. Destruction of the villi hinders the absorption of nutrients from food into the bloodstream, leading to malabsorption syndrome. The most common symptoms include nausea, diarrhoea, weight loss and malnutrition problems. The only practical treatment so far is to maintain a life-long gluten-free diet, which means avoiding all foods that contain wheat, rye, barley and possibly oats (Catassi et al., Citation1993).
As every CD individual has different tolerance levels to gluten ingestion, a safe daily intake of gluten cannot be set. Thus, a testing system for detecting wheat gliadin (as well as the other cereal prolamins) in a gluten-free diet is needed to ensure a safe diet for CD patients that present a broad range of sensitivity to gluten intake, with clinical manifestations to minimal amounts of gliadin reported (Faulkner-Hogg, Selby, & Loblay, Citation1999).
The European Union, World Health Organization and Codex Alimentarius (Revised Standards for gluten-free foods In Report of 25th Session of the Codex Committee on Nutrition and Foods for Special Dietary Uses, 2003) require reliable measurement of gliadin, rather than all wheat-derived proteins, which include albumins, globulins and starch granule proteins. The official limits described in the Codex Draft Revised Standard (2000) are 20 ppm for foodstuffs naturally gluten-free and 200 ppm for foodstuffs rendered gluten-free. As part of the Food Allergen Labeling and Consumer Protection Act of 2004, the US Food and Drug Administration issued a final rule in 2009, defining a gluten-free food for the food containing <20 ppm gluten.
Immunochemical tests are reliable for the detection of gliadin in food since they provide specific, sensitive recognition of gliadin, based on mammalian polyclonal and monoclonal antibodies. Accordingly, many immunoassays were developed for gliadin detection based on antibody and antigen interaction such as enzyme linked immunosorbent assay (ELISA) (Ellis, Rosen-Bronson, O'Reilly, & Ciclitira, Citation1998; Mills, Spinks, & Morgan, Citation1989; Skerritt & Hill, Citation1990; Sorell et al., Citation1998; Troncone et al., Citation1986; Valdes, Garcia, Llorente, & Mendez, 2003) and competitive ELISA methods (Bermudo et al., Citation2005; Chirdo, Ann, & Fossati, Citation1998; Chirdo, Anon, & Fossati, Citation1995; van den Broeck et al., Citation2009).
While mammalian antibodies against gliadin are extensively used in ELISA, chicken egg yolk antibodies immunoglobulin Y (IgY) are very attractive and economical for the development of immunological test systems. Immunised hens transfer high amount of IgY (200 mg) into their eggs, which can then be non-invasively obtained by simple collection methods (Sunwoo, Lee, Menninen, Suresh, & Sim, 2002). However, there is no report on the immunisation of chickens with gliadin peptides, although chickens can produce more specific antibodies against gliadin than mammalian species.
This study was therefore undertaken to examine the use of antibodies raised in the chicken as a reagent to detect gliadin in ELISA and Immunoswab assay. In this study, the chicken IgY antibody was used for the development of double antibody sandwich ELISA (DAS-ELISA) and Immunoswab assay using IgY as a capture antibody and monoclonal antibody (MAb) as a detection antibody. The new detection systems were then evaluated for their reliability and sensitivity for a gluten intolerance health purpose.
Materials and methods
Reagents and chemicals
Crude Sigma gliadin (G-3375), Freund's incomplete adjuvant, purified chicken IgG, rabbit anti-chicken IgG and rabbit anti-chicken IgG conjugated with horseradish peroxidase (HRPO) were purchased from Sigma (St. Louis, MO, USA). HYB-314 MAb and chicken anti-mouse IgG conjugated with HRPO were purchased from Thermo Fisher Scientific Canada (Ontario, Canada). Blue dextran was purchased from Pharmacia Biotech Inc., (Baie-d'Urfe, QC, Canada). Tetramethylbenzidine (TMB) and 2-2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) substrates were purchased from KPL (Frederick, MD, USA). Bio-Rad protein assay kit was purchased from Bio-Rad Laboratory (Ontario Canada).
Instrumentation
Sephacryl S-300 gel filtration column was purchased from GE Healthcare (Quebec, Canada). Microtitre 96-wells plates were purchased from Costar Inc. (Cambridge, MA, USA). The ELISA V max kinetic microplate reader was obtained from Molecular Devices Corp (Sunnyvale, CA, USA).
Preparation of antigen
The isolation of gliadin was performed according to Wieser, Seilmeier, and Beiltz (Citation1994). Briefly, 10 g of crude Sigma gliadin was defatted with butanol under stirring condition for 1 h. After centrifugation (5000 rpm for 15 min), the residual solvent was removed by evaporation in a vacuum drier at room temperature. The step was repeated once. Albumins and globulins were extracted three times with 40 ml of 0.4 M NaCl from the defatted crude Sigma gliadin. The suspension was stirred for 20 min, centrifuged (4500 rpm) for 30 min at room temperature, and subjected to gliadin extraction twice with 40 ml 60% (v/v) aqueous ethanol from the sediment. One milligram of purified gliadin was diluted to 10 ml with deionised water. The solutions containing purified gliadin were then analysed for protein content by the method of BIORAD protein assay with bovine serum albumin (BSA) as a standard at the absorbance of 595 nm. This extracted gliadin was used for the immunisation of chickens and further studies.
Immunisation of chickens
Laying hens were handled in accordance with the guidelines of animal welfare of the Canadian Council on Animal Care approved by the Animal Care and use Committee of University of Alberta (Protocol Number 098/10/10). Sigma gliadin (200 µg/ml protein) was suspended in sterilise phosphate buffered saline (PBS, pH 7.2) and emulsified with an equal volume of Freund's incomplete adjuvant. Eight 23-weeks-old Single Comb White Leghorn chickens were intramuscularly injected with the emulsified saline, with or without gliadin at four different sites (0.25 ml per site) in the breast muscles (two sites per left and right breast muscle). Booster immunisations were given after two weeks and four weeks of the initial immunisation. Eggs were collected daily and stored at 4°C until the extraction of the antibodies.
Purification of IgY antibody
Polyclonal egg yolk IgY against the purified gliadin was produced in laying hens during the immunisation period. The egg yolk was physically separated from the egg white and first mixed gently with eight volumes of cold distilled water (acidified with 0.1 M HCl to give pH 4.0) to avoid possible disruptions of egg yolk granules due to the presence of high concentrations of acid. Cold acidified distilled water (pH 2.0) was then added to make the final dilution of 1:10. After mixing well, the mixture was adjusted to a pH 5.0–5.2 and incubated at 4°C for 12 h. The water soluble fraction (WSF) was obtained by centrifugation at 3125×g at 4°C for 20 min. The supernatant was collected as the IgY rich WSF and titrated by indirect ELISA (mentioned below) using gliadin as a coating antigen.
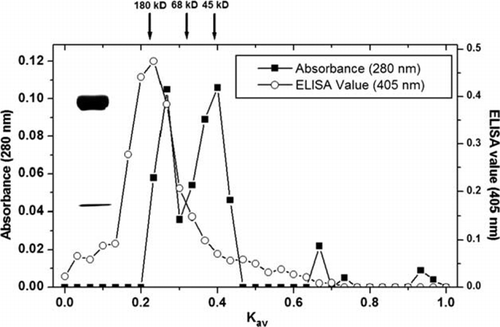
The IgY of high titre was further purified by ammonium sulphate precipitation (60%) followed by Sephacryl S-300 gel chromatography. A portion (1 ml containing 10 mg protein) of WSF was fractionated by using a 1.0×110 cm column of Sephacryl S-300 which was equilibrated and eluted with PBS (0.15 M NaCl, 0.0027 M KCl, 0.0081 M disodium phosphate and 0.0015 M monopotassium phosphate, pH 7.2) at a flow rate of 3 ml/h. Blue dextran and titrated water were used to determine void volume (V o) and total volume (V t) of the column, respectively. The partition coefficient was calculated from the formula: K av=(V e − V o)/(V t − V o), in which V e represents the volume of the peak fraction. The eluates (1 ml) were analysed for protein at 280 nm absorbance and IgY activity by ELISA. The eluates of IgY were pooled, freeze-dried and analysed for protein content, total IgY and specific IgY. All chromatography data presented in this report represent the average of three experiments.
The fraction containing 180 kDa was verified by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS–PAGE) using 10% polyacrylamide gels under non-reducing conditions (50 mM Tris–HCl, pH 6.8, 2% SDS, 0.1% bromophenol blue, 10% glycerol) and stained with Coomassie Brillant Blue.
Indirect ELISA for anti-gliadin IgY quantification
Microtitre plates were coated with 100 µl of gliadin (10 µg/ml of 60% ethanol) and incubated at 37°C for 1 h. The plates were then washed four times with PBS containing 0.05% Tween 20 (PBS-T), filled with 120 µl of 3% BSA solution (w/v) in PBS-T for each well, and incubated at 37°C for 45 min. The BSA solution was then discarded and the wells were washed four times with PBS-T. To each well, 100 µl of WSF (diluted 1:1000 in PBS-T) or column fraction (diluted 1:1000 in PBS-T) was added as a specific IgY, and non-specific IgY as a control prior to incubating at 37°C for 1 h. After washing the plates with PBS-T for four times, 100 µl of rabbit anti-chicken IgY conjugated with HRPO (diluted 1:5000 in PBS-T) was added to incubate at 37°C for 90 min. The plates were then washed again four times with PBS-T to receive 100 µl of freshly prepared substrate solution, ABTS in 0.05 M phosphate citrate buffer (pH 5.0) containing 30% hydrogen peroxide. Optical density reading at 405 nm (OD 405) was taken after 30 min using an ELISA Vmax kinetic microplate reader. The ELISA value of antibody activity was determined by subtracting the value of the control antibody from that of specific antibody.
Preparation of bread sample stock solution
Wheat bread (1 g) was blended in a food blender with 500 ml of 60% ethanol for 5 min at room temperature. The mixtures were centrifuged for 10 min at 2500×g at room temperature. One millilitre of the supernatant was serially diluted with PBS and 60% ethanol into eppendorf tubes for DAS-ELISA and Immunoswab assay, respectively.
Double antibody sandwich ELISA for gliadin detection
Unless indicated otherwise, all incubations were performed at 37°C with four times washing by PBS-T in each step. The assay was carried out with the purified gliadin-specific IgY as a capture antibody and gliadin-specific MAb HYB-314 as a detection antibody. The MAb was raised against a synthetic peptide corresponding to residues KLQPFPQPELPYPQPQ of alpha gliadin peptide (58–73). Wells were coated with 100 µl of gliadin-specific IgY (100 µg/well) in PBS at 4°C overnight. Non-specific binding sites were blocked with 120 µl of 5% BSA for 45 min. The 100 µg of purified gliadin was dissolved in 1 ml of 60% ethanol. One hundred microlitre aliquot of each serially diluted sample (1.28 µg – 0.625 ng) in PBS was added to triplicate wells and incubated at room temperature for 1 h. Then, 100 µl of gliadin-specific MAb (diluted 1:2000 in PBS-T) was added to each well and incubated at 37°C for 1 h. After washing, 100 µl of chicken anti-mouse IgG conjugated with HRPO (diluted 1:5000 in PBS-T) was added to each well and incubated at 37°C for 1 h. Finally, each well was incubated with 100 µl of freshly prepared TMB peroxidase substrate for 5 min at room temperature. Optical density reading at 650 nm was taken by an ELISA V max kinetic microplate reader. The ELISA value of antibody activity was determined by subtracting the value of the control antibody from that of specific antibody.
Assay quality control
Within-plate and between-plate precision profiles were constructed using, respectively, six replicate standard curves on one plate, and six replicate plates, each with triplicate standard curves. The performance of assay has been evaluated by precision studies determining the coefficient of variation.
Immunoswab assay for gliadin detection
Different concentrations of gliadin antigens were spiked in 60% ethanol and aliquoted in eppendorf tubes (50 µl). The tagged calgiswab was mixed with 50 µl of gliadin-specific IgY capture antibody (100 µg/ml) in PBS pH 7.4 to incubate and dry for 10 and 5 min, respectively, at room temperature. The mixture was then fixed with 50 µl of 95% ethanol for 1 min and dried for 5 min at room temperature. Swabs were then blocked with 5% BSA (50 µl) for 10 min at room temperature and washed with PBS (0.05% BSA) at pH 7.4 for five times by a simple fill and aspiration step in a test tube. The washed swabs were then incubated for 5 min with different concentrations of gliadin antigen spiked in ethanol solution (60%). The swabs were then incubated with gliadin-specific MAb (10 µg/ml) for 5 min in a separate eppendorf tube (50 µl) at room temperature. The detection of the chicken IgY bound to antigen was conducted by incubating the swabs in 50 µl of chicken anti-mouse IgG–HRPO (1:5000 diluted in 1% BSA) for 5 min at room temperature. The swabs were then carefully washed as described above and incubated with 50 µl of TMB substrate for colour development. Control swabs were also incubated at the same time in PBS solution. Between steps, swabs were washed with PBS for 5 min. A digital camera with high pixel size and optical zoom was used to capture images of the blue colour with and without eppendorf tubes.
Statistical analyses
The student t-test (one-tailed t-test) was used to analyse for significant differences (P<0.05) between the control (zero antigen) and samples.
Results
IgY antibody production
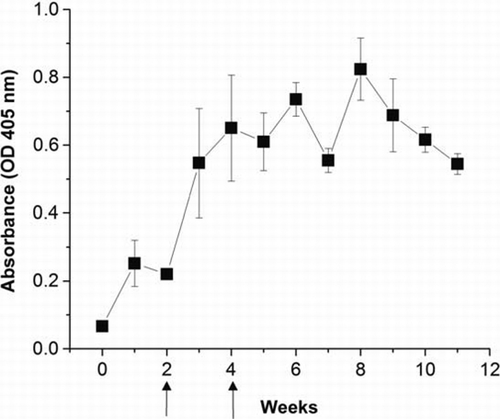
The changes of anti-gliadin antibody activity in the egg yolk from chickens hyperimmunised with gliadin peptides were determined by indirect ELISA (). The activity of anti-gliadin IgY was detected on day 0, rapidly increased (P<0.05) at week 2, and reached to the highest level at week 8 with a gradual reduction, thereafter.
Figure 1. Specific IgY antibody ELISA values in the egg yolk from chickens immunised with Sigma gliadin (200 µg/ml protein) in PBS, emulsified with Freund's incomplete adjuvant. Booster immunisations were given at 2 and 4 weeks after the initial immunisation. Values are the mean of quadruple samples, with vertical bars indicating the standard deviation.
The total IgY concentration in the egg yolk was fairly constant (9.2±1.5 mg/ml) in the chickens hyperimmunised with gliadin during the whole experimental period. Both protein and total IgY concentrations in the eggs of 4–10 weeks of immunisation were similar regardless of the immunisation. The titre of gliadin-specific IgY pooled from egg yolks (4–10 weeks of immunisation) was 0.982±0.045 OD. The titre is higher in the gliadin immunised chickens than in the non-immunised chickens (P<0.05) shown at 0.078±0.005 OD. In comparison, immunised chickens produced specific anti-gluten protein IgY that was approximately 13.6 times higher than that obtained from non-immunised chickens. The presence of specific IgY against gliadin in non-immunised chicken is expected from the commercial feed containing wheat grain as one of major feed ingredients in Canada.
Purification of IgY antibody
For the development of the sensitive DAS-ELISA and Immunoswab assay in this study, crude IgY fractions from immunised hens were further purified by ammonium sulphate (60%) and Sephacryl S-300 gel chromatography. Both elution profile and ELISA values of the IgY were determined (). The peak fractions with molecular weight of 180 kDa at Kav 0.16–0.24 were pooled and then lyophilised for further uses. SDS–PAGE showed that the purity of IgY (180 kDa) at the peak of fractions was >90% by electrophoresis ().
Double antibody sandwich ELISA for gliadin detection
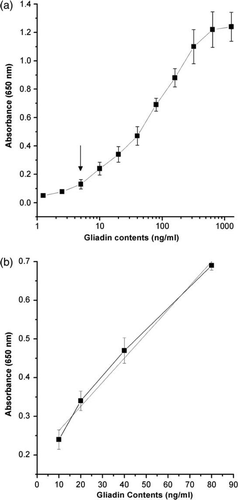
The sandwich ELISA is a common enzyme immunoassay for the detection of certain proteins. In this study, the specificity and detection limit of DAS-ELISA were tested using anti-gliadin IgY and MAb (HYB-314) as capture and detection antibodies, respectively. For the test, different amount (200–20 µg/well) of the IgY was coated on the plate and various dilution (1:100–1:2000) of MAb (HYB-314) was added and incubated at different times and temperatures. The pre-condition results aid to the development of the DAS-ELISA. The DAS-ELISA showed that the gliadin was detected as low as 5 ng/ml (a) and the detectable range in the standard curve was between 10 and 80 ng/ml (b). The low detection limit (10 ng/ml) could identify food samples containing as low as 1 mg gliadin per 100 g dry food, which is equivalent to 10 ppm of gliadin when diluted to 1:1000 ratio.
Figure 3. Standard curve of DAS-ELISA for the detection limit of 5 ng/ml of gliadin (a) and the working linear range at 10–80 ng/ml of gliadin (b). Vertical bars indicate standard deviation. Straight line indicates the linear fit ‘y=0.006x+0.201, R 2=0.99’.
To determine the reproducibility of the DAS-ELISA, the validity was examined further by precision testing. The intra- and inter-assay coefficient variations were 7.2% and 9.8%, respectively when they were evaluated by replicate measurements (n=6) of serially diluted gliadin fractions on a microtitre plate.
Three bread samples were quantified with the DAS-ELISA for gliadin contents at dilutions of 1:512 to 1:8192 from the stock sample preparation. Gliadin contents calculated based on reference to the standard curve, ranged from 23.65±8.29 to 31.95±5.96 mg/g of bread samples (), demonstrating 2.4–3.2% gliadin contents in these bread samples. Three gluten-free labelled samples were also quantified with the DAS-ELISA for gliadin contents at dilutions of 1:32–1:512 from the stock sample preparation. The gliadin content in the gluten-free products was determined to be lower than the limit of detection (5 ppm) of the DAS-ELISA developed.
Table 1. Gliadin contents in bread samples at dilutions of 1:512 to 1:8192 in PBS from the stock sample preparation by DAS-ELISA (ng/ml; mean±SD).
Immunoswab assay
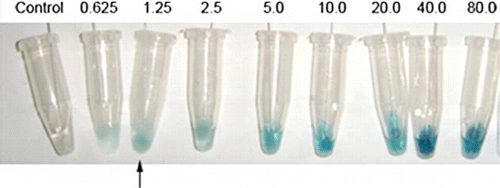
The development of antibody-based Immunoswab assay () involved three main steps: (1) sample application to the capture antibody (chicken egg yolk IgY) in coating swabs, (2) recognition of the captured gliadin by the detection MAb antibody (HYB-314) and (3) initiation of colour reaction to the HRPO-labelled MAb (chicken anti-mouse IgG). The time required to perform the assay with both monoclonal and polyclonal antibodies was approximately 30 min excluding the time of coating IgY and blocking with BSA. The detection limit of Immunoswab was 1.25 µg/ml, while the limit of detection for the DAS-ELISA was 5 ng/ml, which is 4000 times more sensitive. The control swab showed no colour in all the assays.
Figure 4. Immunoswab assay for detection of gliadin. Increased intensity of swab colour observed with increasing gliadin concentration (from left to right) with the control swab (left side) tested in the absence of antigen. Arrow indicates the detection limit of gliadin.
Three bread samples were tested with the developed Immunoswab assay for detecting gliadin at dilutions of 1:2–1:32 from the stock sample preparation. Gliadin detected in the assay was expressed by positive gliadin (+) ().
Table 2. Gliadin detection in bread samples at dilutions of 1:2 to 1:128 from the stock sample preparation in 60% ethanol by Immunoswab assay.
Discussions
Four laying hens immunised with purified Sigma gliadin produced high level of anti-gliadin IgY during the immunisation period. It seems that gliadin (consisting of 689 and 691 amino acids) is a highly immunogenic antigen to produce greatly specific egg yolk antibodies, IgY. The use of laying hens as potent antibody producers has numerous advantages over mammalian because of the high quantity of antibody in egg, the convenience of handling egg over the serum, low maintenance fee for chicken and numerous productions of the eggs. Hens usually lay about 250 eggs (approximately 4000 g of egg yolk) in a year while the serum from a rabbit is about 40 ml. One gram of egg yolk from the immunised hen contains about 10 mg of IgY, whereas 1 ml of rabbit serum yields about 35 mg of IgG. For example, an immunised hen yields 40 g of IgY in a year compared to 1.4 g of IgG from an immunised rabbit, which indicates that a hen can produce antibody nearly 30 times as much as than that of a rabbit in the same time (Hatta et al., Citation1993; Sim, Sunwoo, & Lee, Citation2000). The use of polyclonal IgY may improve the gliadin detection efficacy due to the multiple epitopes on gliadin and also has an economical advantage over the MAb.
Among gliadin peptides, a 33-mer peptide from α-gliadin was responsible for initiation of the inflammatory response for CD patients (Shan et al., Citation2002). The development of DAS-ELISA with chicken IgY could be a useful tool to detect gliadin in foods. The antibody can be partially purified by a simple dilution method (Akita & Nakai, Citation1993). The water soluble IgY fraction showed only 30% purity, indicating that the rest of the fraction was contaminated with α and β-livetins, lipoproteins and fatty acid molecules, which could reduce the sensitivity of ELISA. In this study, highly purified egg yolk IgY was obtained by elimination of fat and other proteins and used as a reagent for both DAS-ELISA and Immunoswab assay.
There are several reports regarding the proportion of specific antibody in total IgY when chickens were immunised with various antigens. The proportion of bovine proteoglyan-specific IgY in the total egg yolk IgY was 9.0% (Li, Nakano, Chae, Sunwoo, & Sim, Citation1998). The percentage of specific IgY in total IgY from egg yolks ranged from approximately 7 to 16% against whole bacteria including Salmonella enteritidis, Salmonella typhimurium (Lee, Sunwoo, Menninen, & Sim, Citation2002), E. coli O157:H7 (Sunwoo et al., Citation2002), E. coli 987P (Sunwoo, Lee, Gujral, & Suresh, Citation2010) and Clostridium perfringens (Song, Kim, Cho, & Sunwoo, Citation2009). The percentage of specific antibody in total IgY ranged from 5 (anti-insulin antibody) to 28% (anti-mouse IgG antibody) (Hatta et al., Citation1997). Our results showed 7.9% of the gliadin-specific IgY concentration in total IgY determined by the quantitative ELISA as previously reported (Sunwoo et al., Citation2002).
In this study, Sigma gliadin immunised as an antigen was used as a standard for the DAS-ELISA. The DAS-ELISA is comprised of capture and detection antibodies with high specificity due to the sandwich reaction of antibodies with an antigen. In some studies using MAb as both capturing and detecting antibodies, the detection of the gliadin is often poor due to single epitope targeting. For example, a homo sandwich ELISA using a MAb to the α-gliadin 33-mer has a detection limit of 100 ng/ml gliadin (Skerritt & Hill, Citation1990), while the limit of hetero sandwich ELISA, with polyclonal antibody, was 15 ng/ml (Ellis et al., Citation1994). In the recent reports, using PN3 MAb antibody as a competitive ELISA showed the detection limit of 1.2 ppm for Sigma gliadin (Bermudo Redondoa et al., 2005). The single antibody sandwich ELISA based on R5 MAb as both coating and detecting Ab showed the detection limit of 1.56 ppm gliadin (Valdes et al., Citation2003).
Since IgY-based DAS-ELISA showed the similar detection limit of gliadin ranged from 5 ng in the present study, the specificity of the capture IgY described here makes it well suited for simple and rapid quantification of gliadin in foods. As a result, it appeared to be sensitive enough to measure gliadin contents since the DAS-ELISA showed a good standard curve at gliadin contents of 10–80 ng/ml.
There is a growing desire for a new gliadin assay that is easy to use and accessible on site compared to the cumbersome ELISA. Immunoswab is faster and more cost-effective in screening than the traditional ELISA, requiring specific instruments such as microplate reader and washer.
The Immunoswab assay, developed with the same antibody for coating and detecting in DAS-ELISA, showed the detection limit of 1.25 ppm. When tested with breads after diluting with 60% ethanol, the gliadin contents were ranged from 21.76 to 0.34 µg/ml. In , the presence of gliadin is expressed as positive gliadin (+) by Immunoswabs for dilutions of 1:2–1:32 (equivalent to 21.76–1.36 µg/ml by DAS-ELISA), which complies with the detection limit of the Immunoswab mentioned earlier.
Celiac patients can use this detection system to test gliadin contamination in food in order to prevent immunogenic reaction to susceptible persons. Food industries can also employ the use of this rapid test for quality assurance of raw material as well as for monitoring gluten contamination throughout the food processing. Although the detection limit of the Immunoswab assay greatly influenced by the types of foods, the method generally detects gliadin as low as 1 ppm or less in foods.
Conclusions
Two detection methods (DAS-ELISA and Immunoswab assay) were developed with highly specific IgY antibody against gliadin. Since IgY can be simply produced in large quantity with a high titre, it was used to replace other sources of polyclonal antibodies or monoclonal antibodies which were conventionally used in ELISA and Immunoswab detection systems. IgY egg yolk antibody can be an alternative antibody source of DAS-ELISA and Immunoswab by using as capturing reagent along with other MAbs (HYB, R5, PN3 or others) as detection antibody to effectively reduce the cost and limit of detection. Both DAS-ELISA and Immunoswab detection systems have low detection limits of 5 ng/ml and 1.25 µg/ml, respectively, enabling gliadin detection in foods as well as foods rendered gluten-free (1–200 ppm).
Acknowledgements
This work was supported by Alberta Livestock and Meat Agency Ltd. (#2007L009R).
References
- Akita , E.M. and Nakai , S. 1993 . Comparison of four purification methods for the production of immunoglobulins from eggs laid by hens immunized with an enterotoxigenic E. coli strain . Journal of Immunological Methods , 160 : 207 – 214 .
- Bermudo Redondoa , M.C. , Griffina , P.B. , Garzon Ransanza , M. , Ellisc , H.J. , Ciclitirac , P.J. and O'Sullivan , C.K. 2005 . Monoclonal antibody-based competitive assay for the sensitive detection of coeliac disease toxic prolamins . Analytical Chimica Acta , 551 : 105 – 114 .
- Catassi , C. , Rossini , M. , Ratsch , I.M. , Bearzi , I. , Santinelli , A. Castagnani , R. 1993 . Dose dependent effects of protracted ingestion of small amounts of gliadin in coeliac disease children: A clinical and jejunal morphometric study . Gut , 34 : 1515 – 1519 .
- Chirdo , F.G. , Ann , M.C. and Fossati , C.A. 1998 . Development of high-sensitive enzyme immunoassays for gliadin quantification using the streptavidin-biotin amplification system . Food and Agricultural Immunology , 10 : 143 – 155 .
- Chirdo , F.G. , Anon , M.C. and Fossati , C.A. 1995 . Optimization of a competitive ELISA with polyclonal anti-bodies for quantification of prolamins in foods . Food and Agricultural Immunology , 7 : 333 – 343 .
- Ellis , H.J. , Doyle , A.P. , Weiser , H. , Sturgess , R.P. , Day , P. and Ciclitira , P.J. 1994 . Measurement of gluten using a monoclonal antibody to a sequenced peptide of alpha-gliadin from the coeliac-activating domain I . Journal of Biochemical and Biophysical Methods , 28 : 77 – 82 .
- Ellis , H.J. , Rosen-Bronson , S. , O'Reilly , N. and Ciclitira , P.J. 1998 . Measurement of gluten using a monoclonal antibody to a coeliac toxic peptide of A gliadin . Gut , 43 : 190 – 195 .
- Faulkner-Hogg , K.B. , Selby , W.S. and Loblay , R.H. 1999 . Draft revised standards for gluten-free foods, report of 25th session of the codex committee on nutrition and food . Scandinavian Journal of Gastroenterology , 34 : 784 – 799 .
- Hatta , H. , Tsuda , K. , Akachi , S. , Kim , M. , Yamamoto , T. and Ebina , T. 1993 . Oral passive immunization effect of anti-human rotavirus IgY and its behavior against proteolytic enzymes. Bioscience, Biotechnology . and Biochemistry , 57 : 1077 – 1081 .
- Hatta , H. , Tsuda , K. , Ozeki , M. , Kim , M. , Yamamoto , T. Otake , S. 1997 . Passive immunization against dental plaque formation in humans: Effect of a mouth rinse containing egg yolk antibodies (IgY) specific to Streptococcus mutans . Caries Research , 31 : 268 – 274 .
- Lee , E.N. , Sunwoo , H.H. , Menninen , K. and Sim , J.S. 2002 . In vitro studies of chicken egg yolk antibody (IgY) against Salmonella enteritidis and Salmonella typhimurium . Poultry Science , 81 : 632 – 641 .
- Li , X. , Nakano , T. , Chae , H.S. , Sunwoo , H.H. and Sim , J.S. 1998 . Production of chicken egg yolk antibody (IgY) against bovine proteoglycan . Canadian Journal of Animal Science , 78 : 287 – 291 .
- Mills , E.N.C. , Spinks , C.A. and Morgan , M.R.A. 1989 . A two-site enzyme-linked immunosorbent assay for wheat gliadins . Food and Agricultural Immunology , 1 : 19 – 27 .
- Shan , L. , Molberg , Ø. , Parrot , I. , Hausch , F. , Filiz , F. Gray , G.M. 2002 . Structural basis for gluten intolerance in celiac sprue . Science , 297 : 2275 – 2279 .
- Sim , J.S. , Sunwoo , H.H. and Lee , E.N. 2000 . “ Ovoglobulin Y ” . In Natural food antimicrobial systems , Edited by: Naidu , A.S. 227 – 252 . New York , NY : CRC Press .
- Skerritt , J.H. and Hill , A.S. 1990 . Monoclonal antibody sandwich enzyme immunoassays for determination of gluten in foods . Journal of Agricultural and Food Chemistry , 38 : 1771 – 1778 .
- Song , M.S. , Kim , C.J. , Cho , W.I. and Sunwoo , H.H. 2009 . Growth inhibition of Clostridium perfringens vegetative cells and spores using chicken immunoglobulin y . Food Safety , 29 : 511 – 520 .
- Sorell , L. , Lopez , J.A. , Valdes , I. , Alfonso , P. , Camafeita , E. Acevedo , B. 1998 . An innovative sandwich ELISA system based on an antibody cocktail for gluten analysis . FEBS Letters , 439 : 46 – 50 .
- Sunwoo , H.H. , Lee , E.N. , Gujral , N. and Suresh , M.R. 2010 . Growth inhibition of Escherichia coli 987P by neutralizing IgY antibodies . Open Immunology Journal , 3 : 1 – 8 .
- Sunwoo , H.H. , Lee , E.N. , Menninen , K. , Suresh , M.R. and Sim , J.S. 2002 . Growth inhibitory effect of chicken egg yolk antibody (IgY) on Escherichia coli O157:H7 . Journal of Food Science , 67 : 1486 – 1494 .
- Troncone , R. , Vitale , M. , Donatiello , A. , Farris , E. , Roosi , G. and Auricchio , S. 1986 . A sandwich enzyme immunoassay for wheat gliadin . Journal of Immunological Methods , 92 : 21 – 23 .
- Valdes , I. , Garcia , E. , Llorente , M. and Mendez , E. 2003 . Innovative approach to low-level of gluten determination in food using a novel sandwich enzyme-linked immunosorbent assay protocol . European Journal of Gastroenterology Hepatology , 15 : 465 – 474 .
- van den Broeck , H.C. , America , A.H.P. , Smulders , M.J.M. , Bosch , D. , Hamer , R.J. Gilissen , L.J.W.J. 2009 . A modified extraction protocol enables detection and quantification of celiac disease-related gluten proteins from wheat . Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences , 877 : 975 – 982 .
- Wieser , H. , Seilmeier , W. and Beiltz , H.D. 1994 . Quantitative determination of gliadin subgroups from different wheat cultivars . Journal of Cereal Science , 19 : 149 – 153 .