ABSTRACT
A total of 600 samples of milk from different species [buffalo (150), cow (150), goat (150), and sheep (150)] were analyzed for aflatoxin M1 (AFM1) contamination using high-performance liquid chromatography and enzyme-linked immunosorbent assay (ELISA) methods. AFM1 contamination was found in buffalo (38.6%), cow (45.3%), goat (33.3%), and sheep (36.6%) milk. The mean value of AFM1 was 0.026 µg L−1 in buffalo, 0.018 µg L−1 in cow, 0.014 µg L−1 in goat, and 0.017 µg L−1 in sheep milk. In all types of milks, the level of AFM1 concentration was higher in milk obtained from urban and semi-urban areas, whereas it was found minimal in milk from rural areas. The results of the analysis of AFM1 level by the ELISA analysis (ng L−1) was observed in 46.5% of all samples. The amount of AFM1 in 16% buffalo, 44% cow, 10% goat, and 12% sheep milk samples was above the maximum tolerance limit accepted by the European Union.
1. Introduction
Aflatoxins are potent toxic, carcinogenic, mutagenic, and immunosuppressive agents produced as secondary metabolites by the fungi of the genus Aspergillus, particularly Aspergillus flavus, Aspergillus parasiticus, and Aspergillus nomius in/on variety of foods, dairy products, and feeds (Galvano et al., Citation2001; Lanyasunya, Wamae, Musa, Olowofeso, & Lokwaleput, Citation2005). Aflatoxins are of economic and health importance because of their ability to contaminate agricultural and dairy commodities worldwide, in particular, cereals, oilseeds, and milk resulting in contaminated human food and ruminants feeds (Peraica, Marija, Domijan, & Cvjetkovic, Citation2002; Yiannikouris & Jouany, Citation2002). The occurrence of aflatoxins is influenced by different environmental factors; hence, the extent of contamination will vary with geographic location, agricultural and agronomic practices, and the susceptibility of commodities to fungal invasion during pre-harvest storage and/or processing periods (Kim et al., Citation2000; Fung & Clark, Citation2004). Aflatoxin contamination in milk and milk products occurs in two ways; either toxins pass to milk with ingestion of feeds contaminated with aflatoxin or as subsequent contamination of milk and milk products with fungi producing aflatoxin (Celik, Sarimehmetoglu, & Kuplulu, Citation2005; Sarimehmetoglu, Kuplulu, & Celik, Citation2003). Aflatoxin M1 (AFM1) is a hydroxylated metabolite of aflatoxin B1 (AFB1) that can be found in the milk of animals that are fed with AFB1-contaminated feed. Cytochrome P450-associated enzymes covert the animals consuming aflatoxin B1 hydroxylate during feeding it into AFM1 in liver and thus contaminates milk and milk products (Prandini et al., Citation2009). There is a linear relationship between the concentration of AFM1 in milk and of AFB1 in feed for dairy cattle. It has been estimated that about 0.3–6.2% of AFB1 consumed by dairy cattle is metabolized to AFM1 and excreted in milk (Fallah, Citation2010). Although AFM1 is less mutagenic and carcinogenic (as a proved carcinogen in Group 1) than AFB1 and it exhibits a high level of genotoxic activity and certainly represents a health hazard because of its possible accumulation and linkage to DNA (Shundo & Sabino, Citation2006). Since AFM1 has been evaluated as a possible human carcinogen, the cancer risk arising from AFM1 contamination in milk is a serious problem in food safety (Sugiyama, Hiraoka, & Sugita-Konishi, Citation2008). Many countries have regulations to control the levels of AFB1 in foodstuffs and have introduced maximum permissible levels of AFM1 in milk in order to reduce the health risk from this source, but regulatory limits throughout the world are influenced by economic considerations and may vary from one country to another, such as Australia follows the Codex regulation and Switzerland the European Union (EU) regulation. In order to establish a limit for AFM1 in milk and other dairy products in India, preliminary studies are needed to examine its occurrence and level in different areas of the country. Most of the developed countries have set or proposed legal regulations for AFM1 levels in milk and dairy products to reduce this hazard (Celik et al., Citation2005; Creppy, Citation2002). Analytical methods that combine simplicity, a high detection sensitivity and a high analytical throughput are required for the effective screening and monitoring of AFM1 in foodstuffs at ppm levels. High-performance liquid chromatography (HPLC) and enzyme-linked immunosorbent assay (ELISA) are generally used in routine analysis for AFM1 in milk (Shephard et al., Citation2012). Notably, HPLC is a complex and time-consuming method to implement and it requires costly and bulky instrumentation. ELISA has none of these shortcomings. ELISA is a widely accepted standard screening method (Ediage, Di Mavungu, Goryacheva, van Peteghem, & De Saeger, Citation2012). Therefore, the presence of AFM1 in milk and dairy products may pose a threat, mainly to children who are considered to be the major consumers of milk and dairy products in many countries globally where milk production and consumption is high. Therefore, this study was carried out with the objective of investigating the presence of AFM1 in raw milk from cow, buffalo, goat, and sheep from India using HPLC and enzyme-linked immunosorbent assay (ELISA) analyses.
2. Materials and methods
2.1. Milk samples
Samples (1 L) of buffalo (n = 150), cow (n = 150), goat (n = 150), and sheep milk (n = 150) were collected from different dairy farms and vendors from June to December 2013 from different districts of Maharashtra, India. The area for milk collection was divided into three categories, namely, urban, semi-urban, and rural, as the milk is collected at these different areas depending on the type of feed and fodder supplement and grazing conditions. The milk samples were either analyzed immediately or stored in a freezer (−20°C) till tested or in the case of delayed analysis. At the time of analysis, samples were brought up to room temperature.
2.2. Chemicals
Acetonitrile and standard of AFM1 (10 µg mL−1 in acetonitrile) was purchased from Sigma-Aldrich (Mumbai, India). The immunoaffinity columns AFM1 TM for HPLC were obtained from VICAM (Watertown, MA, USA). Doubled distilled water (Millipore water purification system Merck, Mumbai, India) was used for the analysis. All other chemicals used were of analytical grade. A working solution of 0.2 µg mL−1 was prepared from the stock solution of standard and was stored in a tightly stoppered bottle at 5°C.
2.3. Sample preparation
AFM1 was determined as per the previous AOAC International (Citation2005; method 2000.08). In brief, the milk samples (100 mL) of each species were warmed at 40°C in a water bath and then centrifuged at 5000 × g for 10 min at 5°C. The fat layer was removed completely and then milk was passed through a filter paper (Whatmann No. 4). AFM1 was extracted using AOAC Official Method Citation2000.Citation08 (Citation2005). In brief, 10 mL chloroform and 1 µL salt solution (10 g NaCl in 50 mL H2O) were added into a 25 mL falcon tube containing 5 mL of milk (previously centrifuged) securely stoppered and mixed gently. The volume of chloroform extract was recorded, transferred into a screw-capped borosilicate vial, and then evaporated to dryness. The extract was dissolved in 5 mL acetonitrile defatted twice with 5 mL petroleum ether and evaporated to dryness (Elzupir & Elhussein, Citation2010). The dry film was re-dissolved in 500 µL mobile phase methanol:water:acetic acid (60:35:5) by the HPLC analysis (Dragacci, Grosso, & Gilbert, Citation2001).
2.4. AFM1 analysis by HPLC
The HPLC chromatograph was used for AFM1 determination adopting fluorescence detection (Agilent, Palo alto, CA, USA) with excitation and emission wavelengths of 365 and 435 nm, respectively. The C18 Column 250 × 4.6 mm ID with a particle size of 5 µm (Phenomenex, USA) was used at ambient temperature of 25°C. Methanol, water, and acetic acid (60:35:5) combination was used as the mobile phase. The flow rate was set at 1 mL min−1 with an injection volume of 25 µL. The calibration curve was determined using a series of dilutions containing 600, 1200, and 2400 pg mL−1 of AFM1 standard. The average of correlation factors was 0.959. The calibration curve was determined using a series of calibration solutions of AFM1 in acetonitrile with concentrations of 0.05, 0.1, 0.5, 1.0, 5.0, and 10.0 µg L−1. The retention time for AFM1 was 6.5 min (Hussain & Anwar, Citation2008; Hussain, Anwar, Asi, Munawar, & Kashif, Citation2010).
2.5. Analysis of AFM1 by competitive ELISA
The quantitative analysis of AFM1 in samples was performed by competitive ELISA using a ELISA plate reader (ELX800, Bio-Tek Instruments, USA). One hundred microliters of the AFM1 standard solutions and test samples in duplicate were added to the wells of micro-titer plate precoated with antibodies for AFM1 and incubated for 60 min at room temperature (20–25°C) in the dark. Then, the liquid was poured out of the wells and the wells were filled with 250 μL washing buffer and poured out the liquid again. This washing step repeated twice. In the next stage, 100 μL of enzyme conjugate was added to occupy the remaining free binding sites and in the washing step 250 μL of washing buffer washed any unbound enzyme conjugates. Then, 50 μL of enzyme substrate and 50 μL of chromogen were added to the wells and incubated for 30 min at room temperature in the dark. The reaction was stopped by adding of 100 μL stop solution to each well and absorbance was measured at 450 nm in the spectrophotometer ELISA plate reader. The results expressed by the means of the three analyses. The absorbance values obtained for the standards and samples were divided by the absorbance of the first standard (zero standards) and multiplied by 100. Therefore, the zero standards are considered 100% and the absorbance values are expressed in percentage. The considered limit for positive samples was 5 ng L−1 AFM1 (Rahimi, Bonyadian, Rafei, & Kazemeini, Citation2010).
3. Statistical analysis
The results of the present study are presented as mean ± SD (n = 3). The AFM1 concentration results were statistically analyzed by applying one-way analysis of variance and considered statistically difference at 95% confidence levels.
4. Results and discussion
The composition of milk from different animal species might be affected by a number of factors, such as age, area, breed, feed, and seasonal changes. A number of studies have been undertaken to determine AFM1 contamination in different milk produced by species such as buffalo, cow, sheep, goat, and camel (Bognanno et al., Citation2006; Gallo, Moschini, & Masoero, Citation2008; Garg, Murthy, Bhanderi, & Sherasia, Citation2004; Virdis, Corgiolu, Scarano, Pilo, & De-santi, Citation2008). Here, we presented the AFM1 contamination in different milk produced by species such as buffalo, cow, sheep, and goat from Indian remnants. gives the calibration curve of standard solutions of AFM1 with concentrations of 0.05, 0.1, 0.5, 1.0, 5.0, and 10.0 µg L−1 by HPLC. The standard solutions of concentration ranges from 0.05 to 10 µg L−1 AFM1 were used to find a calibration/standard curve as described by the following regression equation: Y = −1.6246 + 19.821X, where Y is the area and X is the amount of AFM1. The result showed the linearity of the standard curve over the range studied. The coefficient of determination (R2) was 0.9997. The limit of detection (LOD) was defined as the minimum level at which the analyte could be detected. The LOD was determined as 0.005 µg AFM1 L−1 of milk. The study revealed that the percentage of AFM1 contamination found in buffalo's milk was 38.6%, cow's milk 45.3%, goat's 33.3%, and sheep's 36.6%. The results of the analyses of AFM1 level by the ELISA analysis (ng L−1) in the milk of the buffalo, cow, goat, and sheep are given in . The presence of AFM1 was observed in 46.5% of all samples. The overall mean level of AFM1 in the samples was 34.15 ± 2.2 ng L−1. However, none of the above studied samples was higher than the maximum tolerance level (50 ng L−1) but, 39 (12.5%) of samples were higher than maximum tolerance limit accepted by EU and Codex Alimentarious Commission (50 ng L−1) (European Commission (EC), Citation2006). The amount of AFM1 in 16% of contaminated buffalo milk samples, 44% of contaminated cow milk samples, 10% of contaminated goat milk samples, and 12% of contaminated sheep milk samples was above the maximum tolerance limit accepted by the EU. However, none of the contaminated milk samples of camel milk exceeded the EU action level for AFM1. Statistical analysis of the data showed that the percentage of AFM1 contamination in cow milk was more than other milks (62%), whereas in buffalo milk, goat milk, and sheep milk it was 50%, 34%, and 40%, respectively. The concentration of AFM1 in all of samples was lower than the standard limit (50 ng L−1) as accepted by the EU and Codex Alimentarious Commission (50 ng L−1) (EC, Citation2006) but 16% of buffalo milk, 44% of cow milk, 10% of goat milk, and 12% of sheep milk samples had higher than the maximum tolerance limit (50 ng kg−1) accepted by the EU/Codex Alimentarius Commission for AFM1 (Codex Alimentarius Commission [CAC] action, Citation2001; EC, Citation2006). The low presence of AFM1 in goat and sheep as compared to buffalo and cow milk is probably related to the fact that these species in India are mainly fed by grazing and they are fed on stored grains for three to four months only. The European Community and Codex Alimentarius Commission (CAC) prescribed that the maximum level of AFM1 in liquid milk and dried or processed milk products should not exceed 50 ng L−1 or 50 ng kg−1 (CAC, Citation2001; Creppy, Citation2002). This limit has been established following the ALARA (as low as reasonably achievable) principle. gives the detail of contamination of AFM1 in buffalo, cow, sheep, and goat milk samples. The higher concentration of AFM1 is found in urban and semi-urban area milk samples as compared to rural area milk samples the reason being that the feeding practices varied in these localities. In urban and semi-urban areas, there is less availability of green fodder and there is excessive use of concentrated feeds such as cottonseed cake, corn, soybean, wheat straw, paddy straw, and wheat bran as compared to rural areas (Hussain, Anwar, Munawar, & Asi, Citation2008). The percentage of contamination in cow and buffalo milk samples is relatively high as compared to sheep and goat milk samples (). AFM1 levels in milk and dairy products are important since many people use milk and dairy products in their diets frequently especially babies and children need milk and dairy products. For this reason, AFM1 in milk and dairy products should be controlled systematically (Sarimehmetoglu et al., Citation2003; Celik et al., Citation2005). The results of this and some previous studies about the factual contamination of milk and dairy products with AFM1 imply that more emphasis should be given to the routine AFM1 inspection of milk and dairy products in India. In addition, governmental agencies need to inform both farmers and dairy companies about the importance of AFB1 and AFM1 and the consequences of AFM1 presence in their products. However, milk could also be a source of toxic substances such as AFM1. Since AFM1 has been evaluated as a possible human carcinogen, the cancer risk arising from AFM1 contamination in milk is a serious problem in food safety (Sugiyama et al., Citation2008). The occurrence of AFM1 in milk makes it a particular risk factor for humans because of its importance as a foodstuff for adults and especially for children (Atasever, Atasever, & Ozturan, Citation2011). There are many reports on AFM1 contamination in dairy products, including reports from Slovenia (Torkar & Vengus, Citation2008), North Africa (Elgerbi, Aidoo, Candlish, & Tester, Citation2004), Turkey (Tekinsen & Eken, Citation2008; Unusam, Citation2006), Brazil (Garrido, Iha, Santos-Ortolani, & Duarte-Fávaro, Citation2003; Oliveira, Franco, Rosim, & Fernandes, Citation2011), Portugal (Martins & Martins, Citation2004), Pakistan (Hussain & Anwar, Citation2008), and Iran (Rahimi et al., Citation2010). In the EU, the maximum residue level (EU-MRL) of AFM1 is 50 ng kg−1 for milk and dairy products and 25 ng kg−1 for milk-based baby food (EC, Citation2006). These levels are based on the ALARA principle and are among the lowest levels in the world (European Food Safety Agency, Citation2004). Several studies showed that AFM1 is relatively stable to heat and high temperature with pasteurization, sterilization (UHT techniques), autoclaving, freezing, fermenting preparation, and cold storage of various dairy products and also the level of AFM1 does not change in contaminated milk (Bakirci, Citation2001; Tavakoli, Kamkar, Riazipour, Mozaffari-Nejad, & Rafati, Citation2013). Most of the developed countries have set or proposed legal regulations for AFM1 levels in milk and dairy products to reduce this hazard. These regulations vary from one country to another and are dependent on economic considerations (Rahimi et al., Citation2010). Therefore, the presence of AFM1 in milk and dairy products may pose a threat, mainly to children who are considered to be the major consumers of milk and dairy products in those countries ().
Figure 1. Calibration curve for standard solutions of AFM1 with concentrations of 0.05, 0.1, 0.5, 1.0, 5.0, and 10.0 µg L−1 by the HPLC analysis. The standard solutions of concentration ranges from 0.05 to 10 µg L−1 AFM1 were used to find the calibration/standard curve as described by the following regression equation: Y = −1.6246 + 19.821X, where Y is the area and X is the concentration of AFM1. The result showed the linearity of the standard curve over the range studied. The coefficient of determination (R2) was 0.9997.
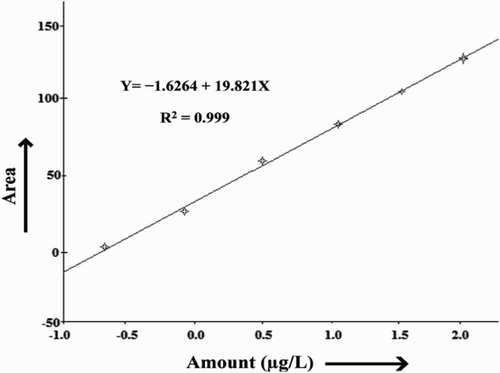
Figure 2. Area-wise variation of AFM1 in raw milk of different dairy species. This study also conducted with respect to different areas and the data revealed that there is a variation in the concentration of AFM1 in different localities studied.
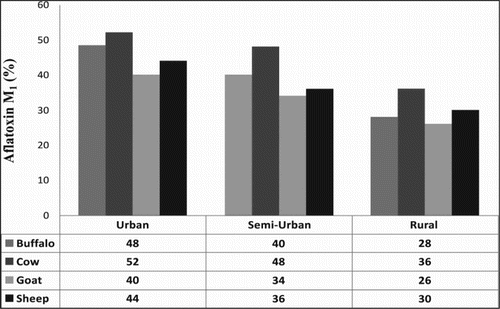
Table 1. AFM1 level (µg L−1) in milk of different species in urban semi-urban and rural areas (n = 3) by the HPLC method.
Table 2. Occurrence of AFM1 level in milk of different species (n = 3) by the ELISA method.
5. Conclusion
In conclusion, this study has shown the serious risk for public health since all age groups including infants and children's consume milk worldwide. It is also extremely important to maintain low levels of AFM1 in the feeds of dairy animals. Therefore, this study was carried out with the objective to investigate the presence of AFM1 in raw milk of cow, buffalo, goat, and sheep from India. Despite the lack of regulatory limit for AFM1 in milk, the survey data of this study may offer information useful in the determination of whether the occurrence of AFM1 in milk samples may be considered as a possible risk for consumer health and whether food regulatory guidelines for AFM1 in dairy products are needed. This study further can be utilized or referred to study global contamination of AFM1 milk from different locations in the world.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This research work was supported by KU-Research Professor Program-2015, Konkuk University, Seoul, South Korea.
References
- AOAC Official Method 2000.08. (2005). Aflatoxin M1 in liquid milk, immunoaffinity column by liquid chromatography. Natural toxins (chap. 49, pp. 45–47). Official Methods of Analysis of AOAC International, 18th edition, AOAC International. Gaithersburg, Maryland 20877-2417, USA.
- Atasever, M. A., Atasever, M., & Ozturan, K. (2011). Aflatoxin M1 levels in retail yoghurt & ayran in Erzurum in Turkey. Turkish Journal of Veterinary & Animal Sciences, 35, 59–62.
- Bakirci, I. (2001). A study on the occurrence of aflatoxin M1 in milk & milk products produced in Van province of Turkey. Food Control, 12, 47–51. doi: 10.1016/S0956-7135(00)00020-7
- Bognanno, M., Fauci, L. L., Ritieni, A., Tafuri, A., De-Lorenzo, A., Micari, P., … Galvano, F. (2006). Survey of the occurrence of aflatoxin M1 in ovine milk by HPLC & its confirmation by MS. Molecular Nutrition & Food Research, 50, 300–305. doi: 10.1002/mnfr.200500224
- Celik, T. H., Sarimehmetoglu, B., & Kuplulu, O. (2005). Aflatoxin M1 contamination in pasteurised milk. Veterinarski Arhiv, 75, 57–65.
- Codex Alimentarius Commission. (2001). Comments submitted on the draft maximum level for Aflatoxin M1 in milk. Codex committee on food additives & contaminants 33rd session, Hauge.
- Creppy, E. E. (2002). Update of survey regulation & toxic effects of mycotoxins in Europe. Toxicology Letters, 127, 19–28. doi: 10.1016/S0378-4274(01)00479-9
- Dragacci, S., Grosso, F., & Gilbert, J. (2001). Immunoaffinity column cleanup with liquid chromatography for determination of aflatoxin M1 in liquid milk: Collaborative study. Journal of AOAC International, 842, 437–443.
- Ediage, E. N., Di Mavungu, J. D., Goryacheva, I. Yu., van Peteghem, C., & De Saeger, S. (2012). Multiplex flow-through immunoassay formats for screening of mycotoxins in a variety of food matrices. Analytical and Bioanalytical Chemistry, 403, 265–278. doi: 10.1007/s00216-012-5803-3
- European Food Safety Agency. (2004). Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to aflatoxin B1 as undesirable substance in animal feed. EFSA Journal, 39, 1–27.
- Elgerbi, A. M., Aidoo, K. E., Candlish, A. G., & Tester, R. F. (2004). Occurrence of aflatoxin M1 in randomly selected in North African milk & cheese samples. Food Additives & Contaminants, 21, 592–597. doi: 10.1080/02652030410001687690
- Elzupir, A. O., & Elhussein, A. M. (2010). Determination of aflatoxin M1 in dairy cattle milk in Khartoum State Sudan. Food Control, 21, 945–946. doi: 10.1016/j.foodcont.2009.11.013
- European Commission. (2006). European Commission regulation EC No. 321 1881 2006 of 19 December, 2006, setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union, 364, 5–24.
- Fallah, A. A. (2010). Assessment of aflatoxin M1 contamination in pasteurized and UHT milk marketed in central part of Iran. Food and Chemical Toxicology, 48, 988–991. doi: 10.1016/j.fct.2010.01.014
- Fung, F., & Clark, R. F. (2004). Health effects of mycotoxins: A toxicological overview. Journal of Toxicology, 42, 217–234.
- Gallo, A., Moschini, M., & Masoero, F. (2008). Aflatoxins absorption in the gastrointestinal tract & in vaginal mucosa in lactating dairy cows. Italian Journal of Animal Science, 71, 53–63.
- Galvano, F., Galofaro, V., Ritieni, A., Bognanno, M., De-Angelis, A., & Galvano, G. (2001). Survey of the occurrence of aflatoxin M1 in dairy products marketed in Italy: Second year of observation. Food Additives & Contaminants, 18, 644–646. doi: 10.1080/02652030118086
- Garg, M. R., Murthy, T. N., Bhanderi, B. M., & Sherasia, P. L. (2004). Excretion of aflatoxin B1 into milk as M1 in cows & buffaloes. Indian Veterinary Journal, 813, 334–335.
- Garrido, N. S., Iha, M. H., Santos-Ortolani, M. R., & Duarte-Fávaro, R. M. (2003). Occurrence of aflatoxins M1 & M2 in milk commercialized in Ribeirão Preto-SP Brazil. Food Additives & Contaminants, 20, 70–73. doi: 10.1080/0265203021000035371
- Hussain, I., & Anwar, J. (2008). A studies on contamination of aflatoxin M1 in raw milk in the Punjab province of Pakistan. Food Control, 19, 393–395. doi: 10.1016/j.foodcont.2007.04.019
- Hussain, I., Anwar, J., Asi, M. R., Munawar, M. A., & Kashif, M. (2010). Aflatoxin M1 contamination in milk from five dairy species in Pakistan. Food Control, 21, 122–124. doi: 10.1016/j.foodcont.2008.12.004
- Hussain, I., Anwar, J., Munawar, M. A., & Asi, M. R. (2008). Variation of levels of aflatoxin M1 in raw milk from different localities in the central areas of Punjab Pakistan. Food Control, 19, 1126–1129. doi: 10.1016/j.foodcont.2007.12.002
- Kim, E. K., Shon, D. H., Ryu, D., Park, J. W., Hwang, H. J., & Kim, Y. B. (2000). Occurrence of aflatoxin M1 in Korean dairy products determined by ELISA & HPLC. Food Additives & Contaminants, 17, 59–64. doi: 10.1080/026520300283595
- Lanyasunya, T. P., Wamae, L. W., Musa, H. H., Olowofeso, O., & Lokwaleput, I. K. (2005). The risk of mycotoxins contamination of dairy feed & milk on smallholder dairy farms in Kenya. Pakistan Journal of Nutrition, 43, 162–169.
- Martins, M. L., & Martins, H. M. (2004). Aflatoxin M1 in yoghurts in Portugal. International Journal of Food Microbiology, 91, 315–317. doi: 10.1016/S0168-1605(02)00363-X
- Oliveira, C. A. F., Franco, R. C., Rosim, R. E., & Fernandes, A. M. (2011). Survey of aflatoxin M1 in Minas cheese from the North-east region of São Paulo Brazil. Food Additives & Contaminants B, 4, 57–60. doi: 10.1080/19393210.2010.538934
- Peraica, M., Marija, A., Domijan, J. Z., & Cvjetkovic, B. (2002). Prevention of exposure to mycotoxins from food & feed. Archives of Industrial Hygiene & Toxicology, 53, 229–237.
- Prandini, A., Tansini, G., Sigolo, S., Filippi, L., Laporta, M., & Piva, G. (2009). On the occurrence of aflatoxin M1 in milk & dairy products. Food & Chemical Toxicology, 47, 984–991. doi: 10.1016/j.fct.2007.10.005
- Rahimi, E., Bonyadian, M., Rafei, M., & Kazemeini, H. R. (2010). Occurrence of aflatoxin M1 in raw milk of five dairy species in Ahvaz Iran. Food & Chemical Toxicology, 48, 129–131. doi: 10.1016/j.fct.2009.09.028
- Sarimehmetoglu, B., Kuplulu, O., & Celik, T. H. (2003). Detection of aflatoxin M1 in cheese samples by ELISA. Food Control, 15, 45–49. doi: 10.1016/S0956-7135(03)00006-9
- Shephard, G. S., Berthiller, F., Burdaspal, P. A., Crews, C., Jonker, M. A., Krska, R., … Whitaker, T. B. (2012). Developments in mycotoxin analysis: An update for 2010–2011. World Mycotoxin Journal, 5(1), 3–30. doi: 10.3920/WMJ2011.1338
- Shundo, L., & Sabino, M. (2006). Aflatoxin M1 in milk by immunoaffinity column clean up with TLC/HPLC determination. Brazilian Journal of Microbiology, 37, 164–167. doi: 10.1590/S1517-83822006000200013
- Sugiyama, K., Hiraoka, H., & Sugita-Konishi, Y. (2008). Aflatoxin M1 contamination in raw bulk milk & the presence of aflatoxin B1 in corn supplied to dairy cattle in Japan. Shokuhin Eiseigaku Zasshi, 49, 352–355. doi: 10.3358/shokueishi.49.352
- Tavakoli, H. R., Kamkar, A., Riazipour, M., Mozaffari-Nejad, A. S., & Rafati, H. (2013). Assessment of aflatoxin M1 levels by enzyme-linked immunosorbent assay in yoghurt consumed in Tehran Iran. Asian Journal of Chemistry, 25, 2836–2838.
- Tekinsen, K. K., & Eken, H. S. (2008). Aflatoxin M1 levels in UHT milk & kashar cheese consumed in Turkey. Food & Chemical Toxicology, 46, 3287–3289. doi: 10.1016/j.fct.2008.07.014
- Torkar, K. G., & Vengus, T. (2008). The presence of yeasts moulds & aflatoxin M1 in raw Milk & cheese in Slovenia. Food Control, 19, 570–577. doi: 10.1016/j.foodcont.2007.06.008
- Unusam, N. (2006). Occurrence of aflatoxin M1 in UHT milk in Turkey. Food & Chemical Toxicology, 44, 1897–1900. doi: 10.1016/j.fct.2006.06.010
- Virdis, S., Corgiolu, G., Scarano, C., Pilo, A. L., & De-santi, E. P. L. (2008). Occurrence of aflatoxin M1 in tank bulk goat milk & ripened goat cheese. Food Control, 19, 44–49. doi: 10.1016/j.foodcont.2007.02.001
- Yiannikouris, A., & Jouany, J. P. (2002). Mycotoxins in feeds & their fate in animals: A review. Animal Research, 51, 81–99. doi: 10.1051/animres:2002012

