ABSTRACT
Morinda citrifolia L. (noni) has been indicated to treat a variety of disorders; however, important aspects surrounding the consumption of products derived from noni still require clarification. We investigated the immune-related effects produced by the consumption of noni fruit juice by C57BL/6 mice. In the intestine, IL-4 and IL-10 levels were reduced following consumption of the fruit juice at a 1:100 dilution. However, when the highest concentrations were administered, IFN-γ, TNF-α and IL-12 levels increased in this organ. Similarly, IFN-γ, TNF-α, IL-12, IL-4, IL-23 and IL-10 levels increased in the liver. In the kidney, IL-12 production decreased following consumption of the juice at a 1:100 dilution. In the intestine, only a mild edema was observed followed by the consumption of noni fruit juice at the highest concentrations. Overall, noni fruit juice consumption did not cause any significant disturbances in liver or kidney outside of immune modulation.
GRAPHICAL ABSTRACT
Introduction
The Morinda citrifolia L. (Rubiaceae) plant, also known as “noni,” has been used for over 2000 years as food and medicine either alone or in combination with other plants. Noni appears to have originated in Asia and is also cultivated in Oceania, the Caribbean, Central America and northern South America (Wang et al., Citation2002; Whistler, Citation1985).
Noni plant leaves have been used as a topical medicine to treat skin wounds (Pawlus & Kinghorn, Citation2007). Commercial consumption of noni juice has also increased in recent decades (Dixon, McMillen, & Etkin, Citation1999). Different preparations of the plant, including extracts of the leaves, stem bark, and roots, serve as reservoirs of molecules that, both alone and in combination, have been used in vivo and in vitro to constrain cancer cell proliferation (Gupta, Banerjee, Pathak, Sharma, & Singh, Citation2013; Huang et al., Citation2016), infections (Huang, Ko, Yan, & Wang, Citation2014; Suzuki et al., Citation2015), inflammation (Basar, Uhlenhut, Hogger, Schone, & Westendorf, Citation2010) and diabetes (Lee et al., Citation2012), among other diseases. Great effort has been made by researchers around the world to better understand the mechanisms related to the effects observed with noni use. Extracts of the noni plant have shown both anti-inflammatory and pro-inflammatory effects (Dussossoy et al., Citation2011; Palu et al., Citation2008), and what often distinguishes these findings is the experimental model used. Approximately 200 bioactive molecules can be derived from noni, as reviewed by Singh (Citation2012), but the aspects governing the biological behavior of these molecules, whether alone or in combination, are not fully defined.
Different studies have demonstrated the beneficial effects of noni and its derivative products (Gooda Sahib Jambocus et al., Citation2016; Lee et al., Citation2012; Lin, Chang, Yang, Tzang, & Chen, Citation2013; Wang et al., Citation2002); however, the safety and value of these products remain controversial. For example, a few cases of hepatitis were reported after noni juice consumption (Millonig, Stadlmann, & Vogel, Citation2005; Stadlbauer, Weiss, Payer, & Stauber, Citation2008). However, these results have been challenged, for example, one study showed that noni fruit juice exposure had no impact on liver cell toxicity either in vitro or in vivo, even when over 90 ml of noni fruit juice/kg/day was administered for 90 days to Sprague-Dawley rats (West, Su, & Jensen, Citation2009).
Although the immunomodulatory effects of noni and its derivatives have already been described in vivo, how these substances affect cytokine production in key metabolic and excretory organs, including the intestine, liver and kidney, has not yet been explored. Due to the physiological and metabolic importance of these organs, this study aimed to evaluate the impact of short-term consumption of fresh noni fruit juice in mice on organ architecture, cell toxicity and organ functionality as well as on cytokine production.
Materials and methods
Collection and botanic identification of the plant
The fruits used in this study were obtained from the monoculture of 150 noni plants located 18°43′47.23″S and 48°6′49.50″O (data from Google Earth, 2013) on the farm “Boa Vontade” (Municipality of “Araguari – Triângulo Mineiro/MG,” Brazil). All the material was prepared in accordance with common herborization methodology (Oliveira & Akisue, Citation2000) and was deposited in the Herbarium of the Universidade Federal de Uberlândia (HUFU Herbarium) under the registration number HUFU-67210 as Morinda citrifolia L. (Rubiaceae).
Juice extraction process
Morinda citrifolia (noni) juice was prepared in the Laboratory of Pharmacognosy of University of Uberaba, Uberaba, Minas Gerais Brazil. M. citrifolia fruit was manually and randomly collected from 150 plants, washed in ozonized water and kept at room temperature for 3–5 days. The fruits were mechanically pulped in a fruit depulper; after seed removal, the resulting pulp was centrifuged under refrigeration at 4000 rpm until a supernatant was obtained, which was considered 100% (v/v) juice and stored at −70°C until further use.
Animal studies
Male C57BL/6 mice aged 6–8 weeks and weighing 20–25 g were maintained under a controlled temperature (25°C) in specific pathogen-free and standard controlled environmental conditions with a 12-h light/dark cycle and food and water ad libitum in the animal housing facility at the Federal University of Triângulo Mineiro. All animal studies were performed in accordance with the Institutional Animal Care and Use Committee of the Federal University of Triângulo Mineiro (Brazil) under protocol 275. The experiments were performed with eight mice/group as follows: saline, healthy control mice treated with saline; pure noni, animals treated with the pure M. citrifolia fruit juice; noni 1:10, mice treated with a 1:10 dilution of the fruit juice; and noni 1:100, mice treated with a 1:100 dilution of the fruit juice. Treatments were administered orally for nine consecutive days in a volume of 100 µl per mouse. Each animal was placed in an individual metabolic cage 24 h prior to euthanasia.
Peripheral blood collection, leukocyte counts and biochemical parameters
Blood smears were stained with panoptic stain (Laborclin Products for Laboratory Ltd, Pinhais, PR, Brazil). Total white blood cells were counted using a Neubauer chamber. Plasma was used to measure total protein (g/dl), plasma albumin (g/dl), plasma globulin (g/dl), aspartate transaminase (U/l), alanine transaminase (U/l), alkaline phosphatase (U/l), urinary excretion (ml/24 h), urinary protein (mg/dl), urinary urea (mg/dl), creatinine clearance (ml/min), plasma sodium (mmol/L), plasma potassium (mmol/L) and plasma chloride (mmol/L). All analyses were performed using an automated system (Cobas® Integra 400) following the manufacturer’s instructions (Roche Diagnostics Ltd, Rotkreuz, Switzerland). Glomerular filtration rate was estimated by determining creatinine clearance as previously described (Rodrigues, Miguel, Napimoga, Oliveira, & Lazo-Chica, Citation2014).
Cytokine quantification by ELISA
The cytokines IL-10, IL-17, IFN-γ, TNF-α, IL-12, IL-4 and IL-23 were quantified by subjecting tissue homogenates to enzyme-linked immunosorbent assays (ELISA) according to the manufacturer’s instructions (BD Biosciences, San Jose, CA, USA).
Histology and histopathological analysis
To assess macroscopic damage, each tissue was exposed longitudinally, flushed with Phosphate buffered saline, placed in 10% buffered formalin for 24 h and then processed for paraffin embedding followed by microtomy. Tissue sections (5 μm) were obtained and stained with hematoxylin and eosin. For histopathological analysis, kidney evaluation was performed. This consisted of analyses of two regions (cortex and medulla) and the following four compartments: glomeruli, tubules, interstitium and vessels. In the liver, the following regions in the parenchyma were evaluated: port space, periportal, intermediate zone and lobular center. Within these regions, the presence of degenerative changes and necrotic and inflammatory infiltrate were assessed. In the intestine, the mucosa, sub mucosa, muscle and serosa were evaluated. These intestinal sections were also assessed for the presence of edema, inflammatory infiltrate and epithelial abnormalities.
Images were captured using a 10× objective through a digital video camera (Evolution MP 5.0 color Media Cybernetics, Silver Spring, MD, USA) coupled to a light microscope (Nikon – Eclipse 50i, Melville, NY, USA). Morphometry was performed using Image-Pro Insight (Media Cybernetics). Inflammatory infiltrate was measured based on the damaged area containing inflammatory infiltrate divided by the total area of tissue visualized in the acquired image and was expressed as a percentage (%). Histopathological analysis was performed by a trained pathologist who was blinded to treatment information.
Data analysis and statistics
For all of the variables, the normal distribution and homogeneous variance were tested. When the distribution was considered normal and the variance was homogeneous, parametric tests were used (unpaired Student’s t-test or one-way ANOVA with Tukey’s post hoc test). In cases of non-Gaussian data distribution, the Mann–Whitney or Kruskal–Wallis (Dunn’s) nonparametric tests were used. The results were expressed as the mean ± SD. The observed differences were considered significant when p < .05 (5%). Statistical analysis was performed using GraphPad Prism, version 5.0 (La Jolla, CA, USA).
Results
Noni juice consumption increases leukocyte numbers in a dose-dependent manner
Because treatment with drugs and/or natural compounds may cause variations in body weight, weight loss was measured in mice treated with or without noni juice. Regardless of the concentration used, the mice that were administered the fruit juice did not show any differences in weight loss ((a)) compared to their vehicle-treated counterparts.
Figure 1. Noni fruit juice increases leukocyte counts in a dose-dependent manner. Experiments were performed with the following treatment groups (eight mice/group): saline, healthy control mice administered saline; pure noni, animals administered pure juice from M. citrifolia fruit; noni 1:10, mice administered a 1:10 dilution of juice; noni 1:100, mice administered a 1:100 dilution of juice. Noni juice was administered orally in a volume of 100 µl per mouse for nine consecutive days. Body weight variations (a) were recorded during the noni juice regimen, and total leukocytes (b), lymphocytes (c) neutrophils (d) and monocytes (e) were recorded on the day of euthanasia. *p < .05.
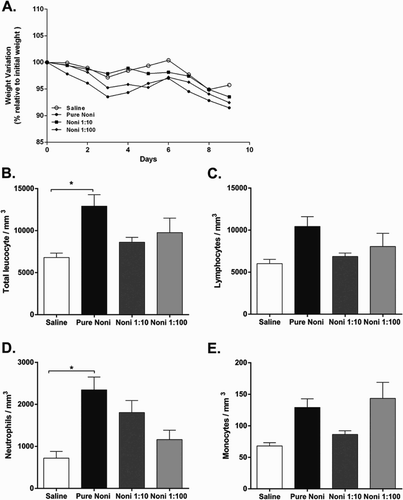
Owing to the importance of modulating circulating leukocyte populations in different physiological and pathological conditions, we assessed the effects of noni juice consumption on leukocyte populations in the blood. Notably, only the pure juice was capable of increasing the number of total leukocytes ((b)). This increase appeared to result from changes in the polymorph nuclear cell population ((d)) rather than the mononuclear cell population, specifically to changes in lymphocyte ((c)) and monocyte populations ((e)).
Noni juice consumption does not affect liver or kidney function
The liver and kidney have key roles in metabolism and excretion; therefore, we assessed the impact of a 9-day period of noni juice consumption on the functionality of these organs. Blood tests were performed in mice administered vehicle or noni juice, and the results are shown in and . Remarkably, noni juice consumption did not alter liver or kidney function, regardless of the concentration used ( and ). These results suggest that oral administration of noni juice for 9 days does not affect body weight and does not impact biochemical markers of liver and kidney functionality. However, noni juice consumption did increase leukocyte populations in a dose-dependent manner, as shown in (b).
Table 1. Liver function – biochemical plasma profiles of mice treated with saline or noni juice.
Table 2. Kidney function – biochemical plasma profiles of mice treated with saline or noni juice.
Noni juice modulates cytokine levels in the intestine
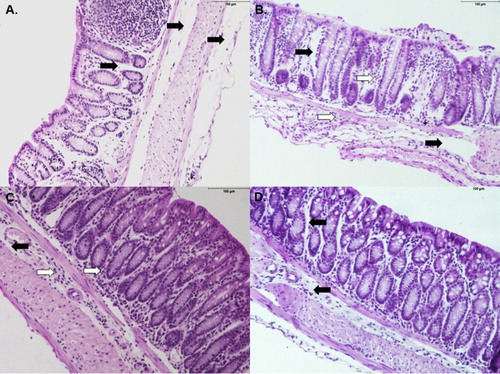
Three steps are required for the metabolism of ingested elements: absorption, metabolism and excretion. To investigate the impact of noni juice consumption on these functions, the architecture and immune response of the intestine were assessed. The intestine was the first organ assessed because it has a key role in drug metabolism and absorption. The vehicle-treated group ((a)) showed mild edema in all of the epithelial layers, which was similar to that observed in the 1:100 noni group ((d)). Notably, mice that received noni fruit juice at higher concentrations (pure and 1:10) displayed greater edema and increased inflammatory infiltrate in the mucosal and sub mucosal layers. The edema and inflammatory infiltrate were more pronounced in the mice administered pure juice ((b,c)).
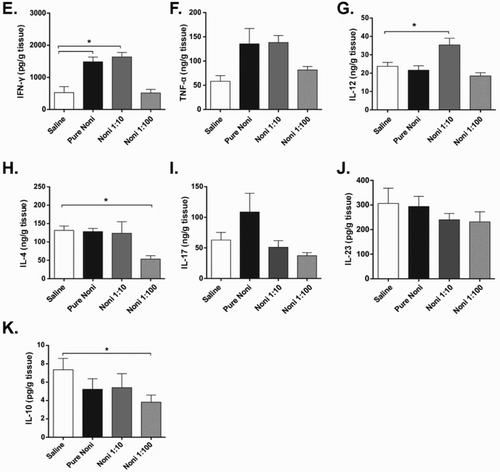
Figure 2. Noni juice modulates cytokine levels in the intestine. Experiments were performed with the following treatment groups (eight mice/group): saline, healthy control mice administered saline; pure noni, animals administered pure juice from M. citrifolia fruit; noni 1:10, mice administered a 1:10 dilution of juice; noni 1:100, mice administered a 1:100 dilution of juice. Noni fruit juice was given orally in a volume of 100 µl per mouse for nine consecutive days. Colon segments were collected, processed and analyzed at the conclusion of the 9-day treatment. (a) Histopathological analysis of colon tissue from saline-treated mice. In the lamina propria, there was slight edema (black arrows), normal infiltrate of mononuclear cells and lymphoid aggregates (primary lymphoid follicle). There was also slight edema and serous fluid in the submucosal layer (black arrows). The muscular layer was normal. (b): Upper right panel: in the mice administered pure juice, edema (black arrows) and cellular infiltrate were observed in the mucosal and submucosal layers (white arrows). (c) Lower left panel: mice in the noni 1:10 group exhibited changes similar to those observed in B, namely, edema (black arrows) and cellular infiltrate, but with lower intensity (white arrows). (d) Lower right panel: mice in the noni 1:100 group showed slight swelling of the mucosa and submucosa (black arrows). Enzyme-linked immunosorbent assay (ELISA) was used to assess gut homogenates for the presence of (e) IFN-γ, (f) TNF-α, (g) IL-12, (h) IL-4, (i) IL-17, (j) IL-23 and (k) IL-10. The results are expressed as nanograms of cytokine per milliliter of homogenate supernatant and normalized by tissue weight. *p < .05.
Next, we investigated the impact of noni juice on cytokine production in the intestine. Treatment with either pure or 1:10 diluted noni juice increased the levels of the inflammatory cytokines IFN-γ ((e)) and TNF-α ((f)). Similarly, augmented levels of IL-12 ((g)) were detected but only when the juice was diluted 1:10. Treatment using the 1:100 dilution reduced the production of the Th2 cytokine IL-4 ((h)) and the putative regulatory cytokine IL-10 ((k)). However, no effects on the intestinal production of IL-17 and IL-23 were observed ((i,j), respectively). Taken together, these results suggest that noni juice has different effects on cytokine production within the intestine. Furthermore, noni juice appeared to induce edema and increase the presence of inflammatory infiltrate within intestinal tissues when administered at the highest concentration.
Noni juice differentially modulates cytokine levels in the liver
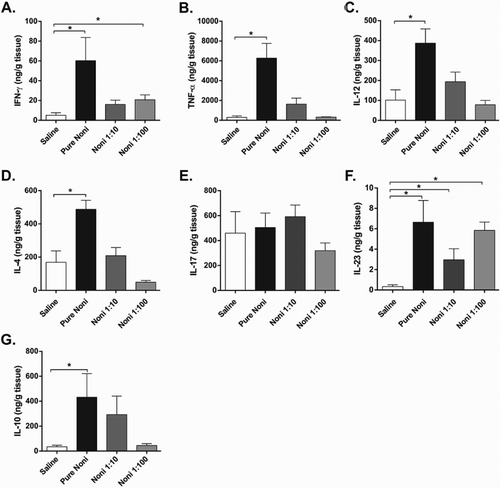
Next, we investigated the effects of noni juice on the liver because of the importance of this organ in metabolism. Interestingly, regardless of the concentration used, no alterations in liver architecture were observed in the mice receiving noni juice compared to those receiving vehicle (data not shown). Remarkably, similarly to what was observed in the intestine, noni juice modulated the levels of different cytokines in the liver. When pure juice was administered, the levels of IFN-γ ((a)), TNF-α ((b)), IL-12 ((c)) and IL-4 ((d)) increased. Furthermore, all juice concentrations tested increased the levels of IL-23 ((f)) compared to those measured in mice receiving vehicle. Pure noni juice also increased the production of the putative regulatory cytokine IL-10 ((g)), although not to the extent observed with IL-23. However, noni juice did not increase IL-17 production in the liver ((e)). Altogether, these results suggest that noni juice has a dose-dependent effect on cytokine production in the liver.
Figure 3. Noni juice modulates cytokine levels in the liver. Experiments were performed with the following treatment groups (eight mice/group): saline, healthy control mice administered saline; pure noni, animals administered pure juice from M. citrifolia fruit; noni 1:10, mice administered a 1:10 dilution of juice; noni 1:100, mice administered a 1:100 dilution of juice. Noni fruit juice was administered orally in a volume of 100 µl per mouse for nine consecutive days. Liver segments were collected, processed and analyzed at the conclusion of the 9-day treatment. Enzyme-linked immunosorbent assay (ELISA) was used to measure the levels of the following cytokines in the tissue homogenates: (a) IFN-γ, (b) TNF-α, (c) IL-12, (d) IL-4, (e) IL-17, (f) IL-23 and (g) IL-10. The results are expressed as nanograms of cytokine per milliliter of homogenate supernatant and normalized by tissue weight. *p < .05.
Cytokine modulation in the kidney is not as pronounced as in other organs
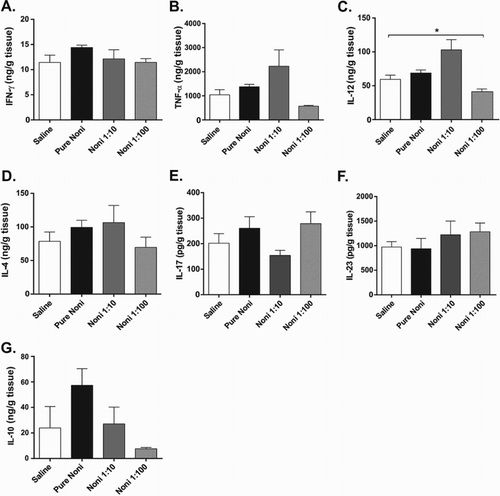
Although no alterations in kidney function were detected in serum samples from the mice receiving noni juice, we assessed the impact of juice intake on kidney architecture and cytokine production. Similar to what was observed in the liver, no histopathological alterations were detected in the kidneys of the mice that received noni juice, regardless of the concentration used (data not shown). Interestingly, only mice receiving noni juice diluted 1:10 showed increased levels of the inflammatory cytokine IL-12 ((c)). However, no alterations were observed in the levels of IFN-γ ((a)), TNF-α ((b)), IL-4 ((d)), IL-17 ((e)), IL-23 ((f)) or IL-4 ((g)). Compared to the other organs examined, noni juice exerted only minor effects on cytokine production in the kidney. These differences in cytokine levels in different organs suggest that the active compounds in noni were almost completely metabolized before reaching the kidney, which explains why only minor changes in cytokine levels were observed in this organ.
Figure 4. Cytokine modulation in kidney was not as prominent as in other organs. Experiments were performed with the following treatment groups (eight mice/group): saline, healthy control mice administered saline; pure noni, animals administered pure juice from M. citrifolia fruit; noni 1:10, mice administered a 1:10 dilution of juice; noni 1:100, mice administered a 1:100 dilution of juice. Noni fruit juice was administered orally in a volume of 100 µl per mouse for nine consecutive days. Kidney samples were collected, processed and analyzed at the conclusion of the 9-day treatment. Enzyme-linked immunosorbent assay (ELISA) was used to measure the levels of the following cytokines in the tissue homogenates: (a) IFN-γ, (b) TNF-α, (c) IL-12, (d) IL-4, (e) IL-17, (f) IL-23 and (g) IL-10. The results are expressed as nanograms of cytokine per milliliter of homogenate supernatant and normalized by tissue weight. *p < .05.
Discussion
The results presented here demonstrate that short-term (9 days) consumption of noni fruit juice can modulate cytokine levels in key metabolic and excretory organs. Furthermore, pure fruit juice intake produced only minor effects on intestinal architecture and inflammatory infiltrate. Although moderate edema was observed following the consumption of either pure noni fruit juice or juice diluted 1:10, no other significant deleterious effects on the functionality and architecture of the liver and kidney were detected.
One of the primary concerns regarding the use of natural products is their potential impact on physiology and organ functionality with regard to absorption, metabolism and excretion. Because the intestine is a key organ involved in absorption and metabolism, the beneficial effects of noni consumption on this organ were already demonstrated (Huang, Liu, Chou, Ko, & Wang, Citation2015). Noni fruit juice extract promoted the growth of probiotic bacteria, primarily Lactobacillus and Bifidobacterium species, in human fecal samples (Huang et al., Citation2015). Furthermore, the extract downregulated intracellular oxidation and inflammation by suppressing cyclooxygenase-2 (COX-2), IL-8, and prostaglandin E2 (PGE2) production in Caco-2 cells (Huang et al., Citation2015). Despite the differences in experimental models and the fact that we did not investigate the impact of noni fruit juice consumption on the modulation of intestinal microbiota, our results showed that the juice produced no deleterious effects with regard to weight loss or intestinal architecture, outside of the presence of edema. In this work, we only evaluated a 9-day course of noni consumption, and therefore we cannot estimate the longer term effects of the juice. Thus, further studies exploring the effects of long-term juice consumption are required.
Liver toxicity is another concern associated with the use of natural products. An aqueous extract of noni fruit protected mice from developing chemically induced hepatic injury and also suppressed elevations in liver enzyme activity (Wang, Nowicki, Anderson, Jensen, & West, Citation2008). The protective effect of noni on liver function was further elucidated in an experimental model of alcohol-induced liver injury in mice (Chang et al., Citation2013). The beneficial role of noni in this case was primarily attributed to the regulation of lipid homeostasis, antioxidant activity and alcohol metabolism (Chang et al., Citation2013). Furthermore, supplementation with noni fruit juice at both 3.6 and 9 ml/kg was able to protect hamsters fed a high-fat diet from developing liver injuries by regulating antioxidant activity and modulating immune responses (Lin et al., Citation2013). Thus far, the majority of published studies have not associated deleterious effects on liver functionality with noni consumption (Chang et al., Citation2013; Gooda Sahib Jambocus et al., Citation2016; Wang et al., Citation2008); however, a few studies have shown that noni consumption might lead to liver injury (Millonig et al., Citation2005; Stadlbauer et al., Citation2008). In the present study, noni fruit juice consumption did not induce histological disturbances in the liver or affect liver enzyme activity, which suggests that short-term consumption of noni fruit juice does not deleteriously affect liver functionality.
Excretion is the final component of consideration for pharmacokinetic analysis; thus, it was necessary to assess kidney function when evaluating the safety of noni juice consumption. However, data regarding the nephrotoxicity associated with the intake of different noni products are scarce. Though not directly toxic, it was shown that patients with chronic kidney disease should avoid 100% noni juice consumption due to its high potassium content (Mueller, Scott, Sowinski, & Prag, Citation2000). The potassium content in noni fruit juice is similar to that in orange and tomato juice, two juices that are also limited in kidney patients (Mueller et al., Citation2000). Additionally, although it did not impair kidney function, noni fruit juice consumption was unable to prevent doxorubicin-induced kidney damage in rats (Buranakarl, Kalandakanond-Thongsong, & Pondeenana, Citation2008). Another study suggested that consumption of noni juice at 5 or 10 mg/kg produces a diuretic effect in normal rats (Shenoy et al., Citation2011). In contrast, the present study showed no differences in potassium urinary levels in mice following noni fruit juice consumption, and no diuretic properties were observed in relation to the juice. Furthermore, kidney function was not impaired regardless of the concentration of juice administered, which reinforces the observation that short-term consumption of noni fruit juice has no deleterious effects on kidney function. We believe that the differences between the results in our present study and previously published studies may result from differences in the experimental model, experimental duration, and type of noni product used.
Among the different effects that result from consumption of noni fruit juice and its derivatives, those related to cancer and immunomodulation are probably the best known. In accordance with our data, previous studies have shown that noni fruit juice consumption modulates immune responses in vivo and in vitro through the suppression of IL-4 production (Hirazumi & Furusawa, Citation1999; Hirazumi, Furusawa, Chou, & Hokama, Citation1994; Palu et al., Citation2008; Sunder, Sujatha, & Kundu, Citation2016) and the induction of IFN-γ, TNF, IL-1ß, IL-10 and IL-12 production (Hirazumi & Furusawa, Citation1999; Palu et al., Citation2008). Additionally, our data indicated that noni fruit juice consumption produces varying effects on the modulation of IL-23, IL-10, IL-4 and IL-12 in different organs, with opposite trends observed in the intestine and liver for the modulation of IL-4 and IL-10. The role of noni fruit juice consumption in the development of a Th1-dominant immune status was shown in a previous study, and this effect helped restrain tumor growth in mice (Furusawa, Hirazumi, Story, & Jensen, Citation2003). Furthermore, the ability of noni fruit powder to stimulate the proliferation of T and B cells was demonstrated in a previous study (Nayak & Mengi, Citation2010). These results reinforce the importance of noni fruit juice as an immune system enhancer.
Finally, as the juice of noni fruit serves as a reservoir of immune modulatory molecules, we cannot underestimate the participation of these molecules in the cytokine modulation observed in the present study. Fatty acid glycosides, which are produced from reactions between fatty acids and glucose, have been isolated from noni fruit. These molecules increase the production of IFN-γ in addition to reducing IL-4 (Smith, Tran, Richards, & Luo, Citation2015), which is a similar pattern to that observed in the intestine (for both cytokines) and liver (only for IFN-γ) in our study. Similarly, a polysaccharide-rich substance known as Noni-ppt that can be isolated from the juice of Noni fruit also showed the ability to induce a Th1 immune status in DBA/2, C57BL/6 and BALB/c mice inoculated with ascites sarcoma 180 (S180) tumor cells (Furusawa et al., Citation2003). When Noni-ppt was partially characterized via chromatography, glucuronic acid, galactose, arabinose and rhamnose were identified as key compounds (Hirazumi & Furusawa, Citation1999). Hirazumi and Furusawa (Citation1999) also showed that peritoneal exudate cells, thymocytes and splenocytes harvested from mice treated with Noni-ppt display increased levels of TNF-α, IL-1β, IL-10, IL-12, IFN- γ and nitric oxide (NO) and reduced levels of IL-4. Although the roles of these molecules were not investigated in the present work, their importance in immune modulation cannot be excluded based on our results.
Altogether, our findings indicate that short-term consumption of noni fruit juice does not trigger any significant disturbances in the liver or kidneys of mice. However, the observation that short-term consumption of noni fruit juice induced edema in the intestine should be further explored in longer term models of consumption. Furthermore, the observed immune effects of noni juice in different organs reinforce the need for additional studies examining the immune modulatory molecules present in this juice and their roles in health and disease.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Beatriz Coutinho de Sousa is a PhD student in Tropical and Infectious Disease in the Federal University of Triângulo Mineiro and has two publications in international journals.
Camila Botelho Miguel, PhD, has eight publications in international journals.
Wellington Francisco Rodrigues, PhD, is a postdoctoral fellow and has eight publications in international journals.
Juliana Reis Machado, PhD, is a professor at the Federal University of Goias and has 38 publications in international journals.
Marcos Vinicius da Silva, PhD, is a postdoctoral fellow and has 36 publications in international journals.
Thiago Alvares da Costa is a PhD student in Tropical and Infectious Disease in the Federal University of Triangulo Mineiro and has five publications in international journals.
Javier Emilio Lazo-Chica is a MD, PhD, Professor at Federal Univeristy of Triangulo Mineiro with 30 published papers in international journals.
Thatiane do Prado Degasperi is an undergraduate student and has one paper published in international journals.
Helioswilton Sales-Campos, PhD, is a postdoctoral fellow and has 14 publications in international journals and experience as a reviewer.
Elizabeth Uber Bucek, PhD, is a professor at the University of Uberaba and has seven publications in international journals.
Carlo José Freire de Oliveira, DVM, PhD, is a professor at the Federal University of Triangulo Mineiro and has 39 publications and experience as a reviewer.
ORCID
Beatriz Coutinho de Sousa http://orcid.org/0000-0001-9300-5502
Camila Botelho Miguel http://orcid.org/0000-0002-1834-1394
Wellington Francisco Rodrigues http://orcid.org/0000-0002-3426-2186
Juliana Reis Machado http://orcid.org/0000-0002-8673-7788
Marcos Vinicius da Silva http://orcid.org/0000-0002-2966-7621
Thiago Alvares da Costa http://orcid.org/0000-0003-2696-5321
Javier Emilio Lazo-Chica http://orcid.org/0000-0003-1950-1895
Thatiane do Prado Degasperi http://orcid.org/0000-0001-5933-6949
Helioswilton Sales-Campos http://orcid.org/0000-0003-3252-2834
Elizabeth Uber Bucek http://orcid.org/0000-0001-5163-4116
Carlo José Freire Oliveira http://orcid.org/0000-0003-2211-7333
Additional information
Funding
References
- Basar, S., Uhlenhut, K., Hogger, P., Schone, F., & Westendorf, J. (2010). Analgesic and antiinflammatory activity of Morinda citrifolia L. (Noni) fruit. Phytotherapy Research, 24(1), 38–42. doi:10.1002/ptr.2863
- Buranakarl, C., Kalandakanond-Thongsong, S., & Pondeenana, S. (2008). Renal catecholamine contents in doxorubicin-treated rats receiving Morinda citrifolia (Noni) juice. Thai J Physiol Sci, 20, 89–96.
- Chang, Y. Y., Lin, Y. L., Yang, D. J., Liu, C. W., Hsu, C. L., Tzang, B. S., & Chen, Y. C. (2013). Hepatoprotection of noni juice against chronic alcohol consumption: Lipid homeostasis, antioxidation, alcohol clearance, and anti-inflammation. Journal of Agricultural and Food Chemistry, 61(46), 11016–11024. doi:10.1021/jf4038419
- Dixon, A. R., McMillen, H., & Etkin, N. L. (1999). Ferment this: The transformation of Noni, a traditional Polynesian medicine (Morinda citrifolia, Rubiaceae). Economic Botany, 53(1), 51–68. doi: 10.1007/BF02860792
- Dussossoy, E., Brat, P., Bony, E., Boudard, F., Poucheret, P., Mertz, C., … Michel, A. (2011). Characterization, anti-oxidative and anti-inflammatory effects of Costa Rican noni juice (Morinda citrifolia L.). Journal of Ethnopharmacology, 133(1), 108–115. doi:10.1016/j.jep.2010.08.063
- Furusawa, E., Hirazumi, A., Story, S., & Jensen, J. (2003). Antitumour potential of a polysaccharide-rich substance from the fruit juice of Morinda citrifolia (noni) on sarcoma 180 ascites tumour in mice. Phytotherapy Research, 17(10), 1158–1164. doi:10.1002/ptr.1307
- Gooda Sahib Jambocus, N., Saari, N., Ismail, A., Khatib, A., Mahomoodally, M. F., & Abdul Hamid, A. (2016). An investigation into the antiobesity effects of Morinda citrifolia L. Leaf extract in high fat diet induced obese rats using a (1)H NMR metabolomics approach. Journal of Diabetes Research, 2016, 2391592, 1–14. doi:10.1155/2016/2391592
- Gupta, R. K., Banerjee, A., Pathak, S., Sharma, C., & Singh, N. (2013). Induction of mitochondrial-mediated apoptosis by Morinda citrifolia (Noni) in human cervical cancer cells. Asian Pacific Journal of Cancer Prevention, 14(1), 237–242. doi: 10.7314/APJCP.2013.14.1.237
- Hirazumi, A., & Furusawa, E. (1999). An immunomodulatory polysaccharide-rich substance from the fruit juice of Morinda citrifolia (noni) with antitumour activity. Phytotherapy Research, 13(5), 380–387. doi: 10.1002/(SICI)1099-1573(199908/09)13:5<380::AID-PTR463>3.0.CO;2-M
- Hirazumi, A., Furusawa, E., Chou, S. C., & Hokama, Y. (1994). Anticancer activity of Morinda citrifolia (noni) on intraperitoneally implanted Lewis lung carcinoma in syngeneic mice. Proc West Pharmacol Soc, 37, 145–146.
- Huang, H. L., Ko, C. H., Yan, Y. Y., & Wang, C. K. (2014). Antiadhesion and anti-inflammation effects of noni (Morinda citrifolia) fruit extracts on AGS cells during Helicobacter pylori infection. Journal of Agricultural and Food Chemistry, 62(11), 2374–2383. doi:10.1021/jf405199w
- Huang, H. L., Liu, C. T., Chou, M. C., Ko, C. H., & Wang, C. K. (2015). Noni (Morinda citrifolia L.) fruit extracts improve colon Microflora and exert anti-inflammatory activities in Caco-2 cells. Journal of Medicinal Food, 18(6), 663–676. doi:10.1089/jmf.2014.3213
- Huang, C., Wei, Y. X., Shen, M. C., Tu, Y. H., Wang, C. C., & Huang, H. C. (2016). Chrysin, abundant in Morinda citrifolia fruit water-EtOAc extracts, combined with Apigenin synergistically induced apoptosis and inhibited migration in human breast and liver cancer cells. Journal of Agricultural and Food Chemistry, 64(21), 4235–4245. doi:10.1021/acs.jafc.6b00766
- Lee, S. Y., Park, S. L., Hwang, J. T., Yi, S. H., Nam, Y. D., & Lim, S. I. (2012). Antidiabetic effect of Morinda citrifolia (Noni) fermented by Cheonggukjang in KK-A(y) diabetic mice. Evid Based Complement Alternat Med, 2012, 163280. doi:10.1155/2012/163280
- Lin, Y. L., Chang, Y. Y., Yang, D. J., Tzang, B. S., & Chen, Y. C. (2013). Beneficial effects of noni (Morinda citrifolia L.) juice on livers of high-fat dietary hamsters. Food Chemistry, 140(1–2), 31–38. doi:10.1016/j.foodchem.2013.02.035
- Millonig, G., Stadlmann, S., & Vogel, W. (2005). Herbal hepatotoxicity: Acute hepatitis caused by a Noni preparation (Morinda citrifolia). European Journal of Gastroenterology & Hepatology, 17(4), 445–447. doi: 10.1097/00042737-200504000-00009
- Mueller, B. A., Scott, M. K., Sowinski, K. M., & Prag, K. A. (2000). Noni juice (Morinda citrifolia): hidden potential for hyperkalemia? American Journal of Kidney Diseases, 35(2), 310–312. doi: 10.1016/S0272-6386(00)70342-8
- Nayak, S., & Mengi, S. (2010). Immunostimulant activity of noni (Morinda citrifolia) on T and B lymphocytes. Pharmaceutical Biology, 48(7), 724–731. doi: 10.3109/13880200903264434
- Oliveira, F., & Akisue, G. (2000). Coleta de Plantas Fanerógamas Fundamentos de Farmacobotânica (Vol. 1, pp. 9–12). São Paulo: Ed Atheneu.
- Palu, A. K., Kim, A. H., West, B. J., Deng, S., Jensen, J., & White, L. (2008). The effects of Morinda citrifolia L. (noni) on the immune system: its molecular mechanisms of action. Journal of Ethnopharmacology, 115(3), 502–506. doi:10.1016/j.jep.2007.10.023
- Pawlus, A. D., & Kinghorn, D. A. (2007). Review of the ethnobotany, chemistry, biological activity and safety of the botanical dietary supplement Morinda citrifolia (noni). Journal of Pharmacy and Pharmacology, 59(12), 1587–1609. doi:10.1211/jpp.59.12.0001
- Rodrigues, W. F., Miguel, C. B., Napimoga, M. H., Oliveira, C. J., & Lazo-Chica, J. E. (2014). Establishing standards for studying renal function in mice through measurements of body size-adjusted creatinine and urea levels. BioMed Research International, 2014, 872827. doi:10.1155/2014/872827
- Shenoy, J. P., Pai, P. G., Shoeb, A., Gokul, P., Kulkarni, A., & Kotian, M. (2011). An evaluation of diuretic activity of Morinda Citrifolia (LINN) (NONI) fruit juice in normal rats. International Journal of Pharmacy and Pharmaceutical Sciences, 3(2), 119–121.
- Singh, D. (2012). Morinda citrifolia L.(Noni): A review of the scientific validation for its nutritional and therapeutic properties. Journal of Diabetes and Endocrinology, 3(6), 77–91. doi: 10.5897/JDE10.006
- Smith, R. E., Tran, K., Richards, K. M., & Luo, R. (2015). Dietary carbohydrates that modulate the immune system. Clinical Immunology, Endocrine & Metabolic Drugs, 2(1), 35–42. doi: 10.2174/221270700201151216151927
- Stadlbauer, V., Weiss, S., Payer, F., & Stauber, R. E. (2008). Herbal does not at all mean innocuous: The sixth case of hepatotoxicity associated with Morinda citrifolia (noni). The American Journal of Gastroenterology, 103(9), 2406–2407. doi:10.1111/j.1572-0241.2008.02010_8.x
- Sunder, J., Sujatha, T., & Kundu, A. (2016). Effect of Morinda citrifolia in growth, production and immunomodulatory properties in livestock and poultry: A review. Journal of Experimental Biology and Agricultural Sciences, 4(3, Suppl.), 249–265. doi: 10.18006/2016.4(3S).249.265
- Suzuki, M., Tung, N. H., Kwofie, K. D., Adegle, R., Amoa-Bosompem, M., Sakyiamah, M., … Shoyama, Y. (2015). New anti-trypanosomal active tetracyclic iridoid isolated from Morinda lucida Benth. Bioorganic & Medicinal Chemistry Letters, 25(15), 3030–3033. doi:10.1016/j.bmcl.2015.05.003
- Wang, M. Y., Nowicki, D., Anderson, G., Jensen, J., & West, B. (2008). Liver protective effects of Morinda citrifolia (Noni). Plant Foods for Human Nutrition, 63(2), 59–63. doi:10.1007/s11130-008-0070-3
- Wang, M. Y., West, B. J., Jensen, C. J., Nowicki, D., Su, C., Palu, A. K., & Anderson, G. (2002). Morinda citrifolia (Noni): a literature review and recent advances in Noni research. Acta Pharmacologica Sinica, 23(12), 1127–1141.
- West, B. J., Su, C. X., & Jensen, C. J. (2009). Hepatotoxicity and subchronic toxicity tests of Morinda citrifolia (noni) fruit. The Journal of Toxicological Sciences, 34(5), 581–585. doi: 10.2131/jts.34.581
- Whistler, W. A. (1985). Traditional and herbal medicine in the Cook Islands. Journal of Ethnopharmacology, 13(3), 239–280. doi: 10.1016/0378-8741(85)90072-8