Abstract
Psoriatic arthritis (PsA) affects up to one-third of patients with psoriasis. It is the major comorbidity of psoriasis because of the likelihood that loss of function and permanent disability will develop if initiation of treatment is delayed. Dermatologists are uniquely positioned to recognize early signs of PsA and be the first-line healthcare practitioners to detect PsA in patients with psoriasis. PsA can affect six clinical domains: peripheral arthritis, dactylitis, enthesitis, psoriasis, psoriatic nail disease, and axial disease. However, not every patient will have involvement of all domains and the domains affected can change over time. Complicating the diagnosis is the condition’s similarity with other arthritic diseases and potential heterogeneity. In this article, we provide practical guidance for dermatologists for detecting PsA in patients with psoriasis. We also review the available treatment options by each clinical domain of PsA and give advice on how to interpret the results of PsA clinical trials. Through early recognition of PsA in patients with psoriasis and initiation of proper treatment, dermatologists can help to prevent PsA disease progression, irreversible joint damage, and resultant permanent disability, and improve quality of life.
Introduction
Psoriatic arthritis (PsA) is a potentially progressive, erosive, chronic, heterogeneous, and systemic inflammatory disease that develops in up to 30% of patients with psoriasis and can manifest in up to six different clinical domains, including peripheral arthritis, dactylitis, enthesitis, psoriasis, psoriatic nail disease, and axial disease (Citation1–3). Peripheral arthritis can cause pain in a variety of joints in PsA and commonly involves the knee (41%), finger (26%), hip (19%), ankle (19%), and wrist (16%) (Citation3). Dactylitis is colloquially referred to as ‘sausage digit,’ and is a distinguishing feature of PsA, characterized by uniform swelling of an entire digit that occurs in up to 48% of patients (Citation4). Another distinguishing feature of PsA, enthesitis is present in 35% of patients and is defined as inflammation where the tendon, ligament, or joint capsule inserts into bone (Citation5–7). Psoriatic lesions can develop on the skin and may affect the nails (e.g. pitting). Axial symptoms, which may occur in up to 50% of patients with PsA, result in back stiffness and pain that improves with movement (Citation8,Citation9).
Etiologically, the familial aggregation of psoriasis and PsA is indicative of a genetic basis for psoriatic disease. The major histocompatibility complex is a known susceptibility locus for PsA and psoriasis (Citation10–12), as shown by the observation that nearly 25% of patients with PsA are positive for human leukocyte antigen (HLA)-B*27 (13). Specific HLA alleles are associated with different PsA manifestations, including symmetric sacroiliitis (HLA-B*27:05), asymmetric sacroiliitis (HLA-B*08:01 and HLA-C*07:01), enthesitis (HLA-B*27:05 and HLA-C*01:02), dactylitis (HLA-B*27:05 and HLA-B*08:01), and synovitis (HLA-B*08:01) (Citation14). HLA-Cw*0602 is more common in PsA than the general population and is associated with an earlier age of psoriasis onset (Citation15). Additionally, there is a strong familial aggregation of PsA with first- and second-degree relatives of affected individuals having increased risk of PsA (Citation16,Citation17).
The pathophysiology of PsA is similar to that of psoriasis and involves important cytokines in the interleukin (IL)-23-Th17-IL-17 and tumor necrosis factor (TNF) pathway (e.g. IL-12, IL-17, IL-23, and TNF-α), as well as cytokines such as IL-22 (Citation13,Citation18,Citation19). Thus, it is not surprising that many cytokine-targeting biologics and small-molecule inhibitors that provide effective skin clearance in psoriasis also improve joint symptoms and slow radiographic progression in PsA.
Although PsA is a widely recognized comorbidity in psoriasis, nearly 52% of patients with psoriasis have joint pain without a diagnosis of PsA and the average diagnostic delay for PsA is 5 years (Citation3,Citation20). Dermatologists have an essential role in reducing this diagnostic delay by screening patients with psoriasis for PsA, as 85% of patients develop psoriasis before PsA, and in these cases, PsA frequently develops within 10 years following the appearance of psoriasis (Citation21–23). There are a number of comorbidities that are more common in patients with PsA than in patients with psoriasis without signs of PsA, including inflammatory bowel disease, cardiovascular diseases (including obesity, hypertension, and type 2 diabetes mellitus), uveitis, depression, anxiety, and fatty liver disease (Citation24,Citation25). Thus, this indicates a need for screening all patients with psoriasis.
Early detection and treatment of PsA are critically important for improving long-term patient outcomes, as the disease can progress rapidly and cause irreversible joint damage (Citation21,Citation26). Specifically, diagnostic and treatment delays of more than 6 months contribute to peripheral joint erosions and poor functional outcomes (Citation27). When PsA is identified early, initiation of treatment with targeted therapies can significantly improve the signs and symptoms of PsA and prevent radiographic progression (Citation28–30).
The remainder of this review will provide dermatologists with practical insights into how to recognize the varied clinical manifestations of PsA, properly diagnose PsA, and interpret results from clinical trials of PsA.
Diagnosis of PsA
Inflammatory versus non-inflammatory arthritis
Recognizing subtle symptomatic differences between inflammatory and non-inflammatory arthritis is critical for proper diagnosis, as these diseases share similar characteristics such as pain and joint stiffness. In general, clinical characteristics of inflammatory arthritis include prolonged stiffness upon immobility, joint pain, joint tenderness, joint swelling, pain at entheses, swollen digits, and worsened back pain upon immobility (Citation9,Citation31). Patients with non-inflammatory arthritis typically present with normal acute-phase reactants, bony crepitus, asymmetric joint involvement, distal interphalangeal (DIP) joint involvement, a lack of prolonged morning stiffness, and bony outgrowths around the joints (Citation9,Citation31). Of note, non-inflammatory arthritis may be suspected in patients with PsA because inflammatory markers are normal in ∼50% of patients with PsA and DIP involvement in PsA may be confused with osteoarthritis on plain radiography (Citation31,Citation32). Questions to help practitioners identify inflammatory arthritis are presented in .
Table 1. Questions to ask patients to help identify the presence of inflammatory arthritis.
Clinical diagnosis of PsA
A simple mnemonic, ‘PSA,’ representing pain (in the joints), stiffness (>30 min after inactivity/sausage digit [dactylitis]), and axial (axial spine involvement/back pain associated with stiffness and pain that improves with activity), has been developed as a convenient method to help practitioners quickly recognize specific characteristics of PsA (Citation9). Enthesitis may present before symptoms of arthritis in individuals with PsA, and early enthesitis is not detectable by radiography. Thus, because enthesitis may be the only presenting musculoskeletal symptom of PsA, it is important for dermatologists to query patients regarding enthesitis symptoms. During clinical examinations, dermatologists can screen for visible signs of enthesitis such as redness and swelling at insertion sites (Citation33). Although enthesitis is typically evaluated by manual palpation, it is particularly challenging to assess because objective signs of inflammation such as redness and swelling are often absent and symptoms of enthesitis are known to mimic those of fibromyalgia, which is also associated with pain on digital palpation at tender points (Citation34–37). Evaluation of enthesitis is a challenge amongst dermatologists and rheumatologists due to these issues and additional education on techniques for evaluating enthesitis would be beneficial. We suggest to first ask about pain at the more commonly affected entheses (lateral epicondyle, medial femoral condyle, and Achilles tendon insertion) and then palpate and look for signs of inflammation (). Somatic symptoms and tender point counts can help differentiate PsA from fibromyalgia, in which patients have more tender points and greater enthesitis scores (Citation37). Detecting diffuse tenderness in soft tissue areas away from joints and entheses is also helpful in differentiating fibromyalgia from PsA. Patients with reactive arthritis can also exhibit enthesitis that commonly involves the Achilles tendon and plantar fascia (Citation38). Other factors that can help to differentiate reactive arthritis from PsA include younger age at onset, sacroiliitis, and articular symptoms primarily affecting the lower limbs and the presence of acute anterior uveitis, dysuria, or diarrhea/inflammatory bowel disease (Citation38). Being cognizant of these differences, a diagnosis of reactive arthritis or PsA requires rheumatologic evaluation and the dermatologist will have benefited the patient greatly even if the diagnosis is reactive arthritis rather than PSA.
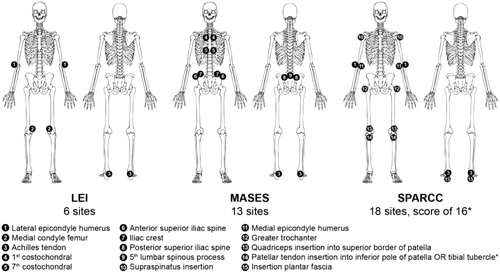
Figure 1. Enthesitis sites evaluated by clinical assessment instruments. LEI: Leeds Enthesitis Index; MASES: Maastricht Ankylosing Spondylitis Enthesitis Score; SPARCC: Spondyloarthritis Research Consortium of Canada. *For scoring purposes, the inferior patella and tibial tuberosity are considered one site because of their anatomical proximity. Image from Mease 2017 (Citation178).
Although PsA is a type of inflammatory arthritis, it may be challenging to identify, because its symptoms may mimic non-inflammatory arthritis. More specifically, in patients with PsA, the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level are within the normal range nearly 50% of the time, joints are affected asymmetrically, DIP joint involvement may be present, and radiographic manifestations include new bone formation and concurrent bone loss as opposed to only bony erosion (Citation13,Citation31,Citation32,Citation39,Citation40). Characteristics that can help dermatologists differentiate PsA from non-inflammatory arthritis (osteoarthritis), inflammatory arthritis (gout and rheumatoid arthritis [RA]), and pain (fibromyalgia) are described in . These characteristics, however, can be challenging to differentiate when two or more diseases are present simultaneously, and osteoarthritis and/or fibromyalgia can present with PsA (Citation41,Citation42). Practitioners should also be aware that the risk of gout is significantly increased in patients with concurrent PsA and psoriasis (Citation43).
Table 2. Differential diagnosis of PsA.
To address the need for early screening tools to identify PsA in patients with psoriasis, three validated screening tools exist: the psoriasis and epidemiology screening tool (PEST), the psoriatic arthritis screening and evaluation (PASE), and the Toronto psoriatic arthritis screening (ToPAS) (Citation44–48). PEST is a patient self-assessment tool consisting of five questions and a drawing of a mannequin diagram on which patients indicate locations of stiff, swollen, or painful joints (Citation49,Citation50). PASE is a questionnaire of 15 items designed specifically for use in dermatology clinics to identify patients with psoriasis and symptoms of inflammatory musculoskeletal disease (Citation44,Citation51,Citation52). ToPAS is a 12-item questionnaire in which patients use pictures of psoriatic skin, nail disease, inflamed joints, and dactylitis to identify symptoms (Citation49). In a comparison of these tools, PEST, the only nonproprietary screening tool and available on the free GRAPPA mobile phone app, and ToPAS had slightly better sensitivity than PASE in detecting PsA in patients with psoriasis; however, all questionnaires had high false-positive rates, resulting in low specificity (Citation53).
Although screening tools can be useful in some circumstances, they are often inconvenient and challenging to include during routine office visits with patients (Citation9). Additionally, it is important for dermatologists to first recognize the presence of inflammatory arthritis before attempting differential diagnosis for PsA so that other causes of joint pain are not overlooked.
The ClASsification criteria for Psoriatic ARthritis (CASPAR) criteria, which were developed to standardize enrollment in PsA clinical trials, can help dermatologists identify patients with PsA but are not required for diagnosis (Citation54). The diagnosis of PsA is clinical and based on patient history and physical examination and supported by imaging and laboratory evaluation. Patients can fulfill the CASPAR criteria to be diagnosed with PsA and dermatologists may find these criteria useful when evaluating patients for possible signs of PsA (Citation55). These classification criteria consist of two groupings: the stem (or required criteria) and criteria associated with a numerical value (Citation54,Citation55). To fulfill the CASPAR criteria, a patient must present with at least one of the stem components and ≥3 points from the criteria listed in .
Table 3. CASPAR criteria.
The stem criteria of CASPAR are defined under the broad term ‘inflammatory articular disease,’ which consists of inflammatory joint (peripheral) disease, enthesitis, and inflammatory axial disease. Inflammatory joint disease is generally recognized by joint swelling, erythema, and pain (even at rest) (Citation55).
As part of the clinical examination for peripheral arthritis (part of the stem criteria of CASPAR), dermatologists should evaluate patients for signs of swelling, which is indicative of active synovitis, and tenderness, which is another sign of inflammation. Practical assessments can be conducted by applying light pressure (e.g. enough to blanch the tip of a fingernail) at the joint line. Key joints to evaluate include DIP joints, proximal interphalangeal joints, metacarpophalangeal and metatarsophalangeal joints, and wrist, elbow, shoulder, sternoclavicular, temporomandibular, hip, knee, ankle, and midtarsal joints (Citation56). Novel educational tools are being developed to help train dermatologists on how to accurately perform a musculoskeletal examination to screen for PsA.
Axial inflammatory articular disease (part of the stem criteria of CASPAR) is typically characterized by slow-developing back pain persisting for >3 months before age 40 years, alternating buttock pain (caused by sacroiliitis), pain causing waking from sleep during the second half of the night and prolonged morning stiffness or stiffness upon immobility (Citation57,Citation58). Additionally, treatments that are effective for other PsA domains may not improve axial disease.
The numerically valued criteria of CASPAR include psoriasis or a personal/family history of psoriasis, psoriatic nail dystrophy, a negative test result for the presence of rheumatoid factor (RF), current dactylitis or history of dactylitis (recorded by a rheumatologist), and juxta-articular new-bone formation (Citation54).
Among the CASPAR criteria, ongoing psoriasis (which is weighted more than all other criteria) or a personal or family history of psoriasis is encompassed within one category, whereas nail dystrophy is counted as a separate category. Of the remaining criteria, dactylitis requires the most careful clinical attention, because it may occur in any digit, but most frequently manifests in the toes (Citation59). A dactylitic digit, which is very specific to PsA, may be red, hot, and tender (acute dactylitis) or swollen without acute inflammatory changes (chronic dactylitis) and may occur in isolation or as one of several digits affected (Citation6,Citation59,Citation60). During clinical examinations, dermatologists can identify dactylitis by screening for ‘sausage-shaped’ digits, which present with uniform inflammation such that soft tissue between the metacarpophalangeal and proximal interphalangeal, proximal and DIP, and/or DIP and digital tuft are diffusely swollen (Citation61). The entire digit is affected and swelling at each joint cannot be independently recognized, and flexion is difficult or impossible (Citation62). The presence of RF, a type of autoantibody first found to be associated with RA and not typically present in PsA, is determined through a blood test (Citation63). Although negative RF is one of the numerically valued CASPAR criteria, RF is present in approximately 13% of patients with PsA, and the incidence of positive RF increases with age (Citation21,Citation64). Anti-cyclic citrullinated peptide antibodies are an additional negative marker of PsA (not included in the CASPAR criteria); however, roughly 13% of patients with PsA present with these antibodies (Citation65). When positive in patients with PsA, both RF and anti-cyclic citrullinated peptide antibodies typically have a low titer, especially in comparison to patients with RA (Citation66,Citation67). Finally, the last numerically valued CASPAR criteria, juxta-articular new bone formation, is not typically a feature of early PsA.
Psoriasis subtypes and PsA
Although psoriasis can occur in a variety of locations on the body, specific manifestations are clinical predictors of PsA (Citation68). Several studies have found an increased risk of PsA has correlations with psoriatic scalp lesions, intertriginous/inverse psoriasis, and nail dystrophy (Citation68–71). The hazard ratios for PsA in patients with scalp, intergluteal/perianal, and nail psoriasis are 3.89, 2.35, and 2.93, respectively (Citation68), and in mild psoriasis, 83% of patients with scalp and nail psoriasis, 40% of patients with intergluteal/perianal lesions, and 37% of patients with isolated scalp psoriasis met CASPAR criteria for PsA (Citation69). Similarly, the incidences of scalp, intergluteal, and nail psoriasis were higher in patients with PsA (100, 83, and 64%, respectively) than in patients with psoriasis alone (67, 25, and 40%, respectively) (Citation70). Recognition of inverse psoriasis is an important clue for informing a diagnosis of PsA that is commonly missed during physical examination. Inverse psoriasis has typically been considered uncommon but contemporary findings report a prevalence of 21–30% for inverse psoriasis in patients with psoriasis (Citation72).
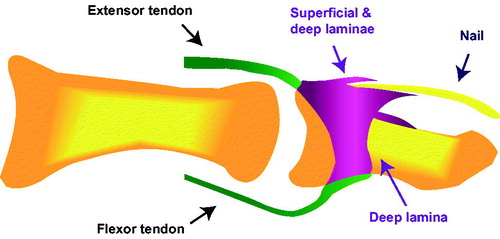
Although nail psoriasis is common in moderate-to-severe psoriasis without PsA, it is even more prevalent in PsA (over 80% of patients affected) and is hypothesized to indicate involvement of the distal phalanx in PsA (Citation55,Citation68,Citation73). This unique link between nail dystrophy and PsA can be explained by the physiological relationship between nails and entheses. Histological studies have demonstrated that the extensor tendon, which is attached to the terminal phalanx, extends distally and connects with the nail root, revealing that the fascia of the nail root is an extension of the enthesis and providing evidence of how inflammation of these entheses often gives rise to nail pitting () (Citation74). The link between nail disease and adjacent enthesitis has further been demonstrated through magnetic resonance imaging (MRI) and ultrasound studies (Citation75,Citation76). Therefore, although nails are developmentally related to the skin, they are functionally linked with entheses, underscoring the correlation between nail disease/osteolysis and PsA – particularly in DIP joints (Citation77,Citation78).
Figure 2. The fascia of the nail root is an extension of the entheses. Image from McGonagle 2009 (Citation179).
Overall, these observations indicate that dermatologists should pay particular attention for PsA manifestations in patients with psoriasis affecting the scalp, intergluteal/perianal areas, and nails (Citation79).
Treatment of PsA
The European League Against Rheumatism (EULAR) and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) have developed recommendations for the treatment of PsA, with guidelines for appropriate use of nonsteroidal anti-inflammatory drugs (NSAIDs), traditional disease-modifying anti-rheumatic drugs (DMARDs; e.g. methotrexate, sulfasalazine, leflunomide, cyclosporine), biologics, and small-molecule inhibitors (Citation80,Citation81). These recommendations provide specific guidance for treatment by PsA clinical domain. Choice of therapy should be optimized to target the PsA symptoms that are the most burdensome to each patient, with the overarching goals of maximizing long-term health-related quality of life and preventing irreversible joint structural damage (Citation80,Citation81). Briefly, NSAIDs are recommended to control pain and inflammation in patients with peripheral arthritis, axial disease, and enthesitis, and traditional DMARDs are recommended as treatments for peripheral arthritis, dactylitis, and skin/nail disease. Guidelines clarify that the term ‘DMARD’ to describe these agents was chosen for historical purposes and that these agents do not provide any disease-modifying effects on radiographic damage. Biologics that inhibit TNF-α, IL-17A, or IL-12/23 are recommended across all PsA domains, and the small-molecule phosphodiesterase-4 inhibitor, apremilast, is recommended for all domains except axial disease (Citation80,Citation81). These guidelines were developed in 2015, when data were available for the TNF-α inhibitors, etanercept, adalimumab, infliximab, certolizumab pegol, and golimumab, and for the IL-12/23 inhibitor, ustekinumab (Citation80,Citation81). Since then, additional data have been published for apremilast, the IL-17A inhibitors secukinumab and ixekizumab, the Janus kinase inhibitor tofacitinib, and the T-cell inhibitor abatacept, all of which are now approved for adults with active PsA. The IL-23 inhibitor guselkumab is approved for moderate-to-severe plaque psoriasis and is in late-stage development for PsA. Additionally, NSAIDs, adalimumab, certolizumab pegol, etanercept, golimumab, infliximab, and secukinumab are approved for the treatment of ankylosing spondylitis (AS), a condition related to PsA.
Domain-specific evaluation of PsA in clinical trials
Dermatologists should be particularly aware of measures used to assess PsA, as the International Dermatology Outcome Measures (IDEOM) group has recently encouraged screening and subsequent measurement of PsA symptoms in all psoriasis clinical trials (Citation82). The IDEOM group evaluated the patient global (PG)-arthritis, routine assessment patient index data (RAPID)-3, and Psoriatic Arthritis Impact of Disease (PsAID)-9 tools for PsA symptom evaluation and recommended that PsAID9 and RAPID3 are potentially more appropriate measures of PsA symptoms than PG-arthritis (Citation82). In PsA clinical trials, various measures () are used to assess peripheral arthritis, dactylitis, enthesitis, skin/nail disease, and radiographic outcomes. Additionally, several different instruments are used to evaluate patient health status (patient-reported outcomes [PROs]), and several individual clinical and PRO measures have been integrated into composite indices to create more comprehensive measures.
Table 4. Indices used to measure PsA activity by clinical domain (Citation56,Citation89,Citation96–99,Citation121,Citation151–158).
Peripheral arthritis measures
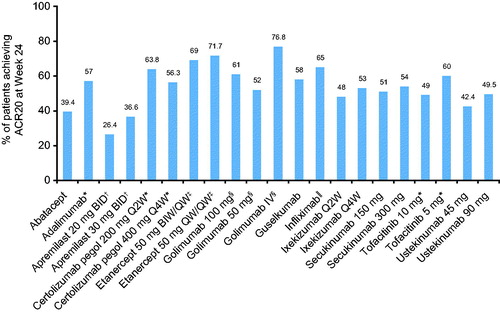
Peripheral arthritis is predominantly assessed by indices, including the 68 tender and 66 swollen joint count, the 28-joint Disease Activity Score (DAS28), a composite measure that assesses 28 peripheral joints along with a measure of ESR or CRP (in the DAS28-CRP measure), and the American College of Rheumatology (ACR) criteria, another composite measure that specifies a percentage improvement (i.e. 20, 50, or 70%) in tender or swollen joint counts, as well as 3 of 5 additional measures () (Citation56,Citation83). These measures include the patient’s assessment of pain, the patient’s global assessment of disease activity, the physician’s assessment of physical function, the patient’s assessment of physical function, and acute-phase reactant value. Furthermore, when trials last longer than 1 year and agents are being tested as DMARDs, ACR includes radiography or another imaging technique (Citation83,Citation84). Across PsA clinical trials, 20% improvement in ACR criteria (ACR20) has been used as a primary endpoint to assess the efficacy of biologics and small-molecule inhibitors for the treatment of articular symptoms. Results from these trials are summarized in . However, because DAS28 was originally developed for RA, it does not evaluate some of the most frequently involved joints in PsA (particularly in the lower extremities) and all PsA domains. Although ACR response is used as the primary endpoint in PsA clinical trials, it is not employed in clinical practice and does not evaluate all PsA domains.
Figure 3. ACR20 response rates reported at week 24 in PsA trials of biologics and small-molecule inhibitors (Citation86,Citation102,Citation103,Citation132,Citation134–136,Citation138,Citation139,Citation159–161,Citation180). For agents without a dose specified, results are presented for the approved dosage. Figure is for visualization purposes only and should not be used to make direct comparisons of efficacy between therapies. Data values have not been statistically corrected. Unless otherwise indicated, the proportion of patients with ACR20 at week 24 was the primary endpoint of the study. ACR: American College of Rheumatology; BID: two times daily; BIW: twice weekly; PsA: psoriatic arthritis; Q2W: every 2 weeks; Q4W; every 4 weeks; QW: once weekly. *Primary endpoint: ACR20 at week 12; †Primary endpoint: ACR20 at week 16; ‡ACR20 at week 24 is a secondary endpoint; §Primary endpoint: ACR20 at week 14; ‖‖Results reported at week 16 (primary endpoint).
Dactylitis measures
Dactylitis is also measured using multiple approaches (). The simplest assessment is counting dactylitic digits (tender and non-tender or just tender) (Citation85). Alternative clinical indices include a 0–3 scale of physician-rated severity to assess all 20 digits (Citation86), the Leeds Dactylitis Index (LDI), and LDI-Basic (Citation87,Citation88). The LDI and LDI-Basic multiply the tenderness score of each affected digit (based on the Ritchie index – graded 0–3 for LDI or binary score for LDI-Basic) by the ratio of the circumference of the affected digit to the circumference of the digit on the opposite hand or foot. Results from clinical trials using these indices to evaluate the effects of treatment on dactylitis are summarized in . Drugs that have shown statistically significant improvements in dactylitis indices compared with placebo include infliximab, certolizumab pegol, intravenous golimumab, subcutaneous golimumab 100 mg, ustekinumab, guselkumab, secukinumab, and ixekizumab. Statistical significance was not reached or was not tested for in trials of adalimumab, etanercept, tofacitinib, apremilast, and abatacept. It is not possible to indirectly compare results across trials because different clinical indices and different reporting methods were used to assess dactylitis across studies. At present, there is no consensus on the best method for assessing dactylitis in PsA clinical trials, and minimal clinically important difference (MCID) thresholds have not been established for available dactylitis indices.
Table 5. Results of dactylitis assessments by clinical index from trials of biologics and small-molecule inhibitors in PsA.
Enthesitis measures
Enthesitis can be measured with imaging techniques, such as ultrasound and MRI; however, in dermatology clinics, use of domain-specific clinical indices is more practical. Instruments available for the assessment of enthesitis include the Leeds Enthesitis Index (LEI), the Maastricht Ankylosing Spondylitis Enthesitis Score (MASES), the 4-point enthesitis measure, the Spondyloarthritis Research Consortium of Canada (SPARCC) enthesitis measure, the Berlin index, and the San Francisco index () (Citation56).
As with dactylitis, there is currently no consensus on which enthesitis index is best; therefore, PsA trials of biologics and small-molecule inhibitors have used different instruments. As shown in , even when the same instrument was used in different studies, differences in reporting methods and study designs restrict the ability to indirectly compare results across studies. Furthermore, the clinical significance of improvements on different indices is not well established, and MCID is not available for any enthesitis measure (Citation89,Citation90). However, it is noteworthy that statistically significant improvements in enthesitis have been reported compared with placebo in phase 3 studies of infliximab, certolizumab pegol, golimumab (subcutaneous and intravenous formulations), secukinumab, ustekinumab, and apremilast, and in a phase 2 study of guselkumab ().
Table 6. Results of enthesitis assessments by clinical index from trials of biologics and small-molecule inhibitors in PsA.
Skin and nail psoriasis measures
Psoriasis is measured by tools frequently used in dermatology trials, including the psoriasis area and severity index (PASI), body surface area (BSA), physicians global assessment (PGA), Investigator’s Global Assessment (IGA), or IGA modified 2011 () (Citation56,Citation91). The primary endpoint in psoriasis trials and the key skin endpoint in PsA trials are PASI responses (usually percentage of patients achieving at least 75% improvement in PASI scores [PASI75]). Nail psoriasis is assessed using the nail psoriasis severity index (NAPSI) or modified NAPSI () (Citation56).
The National Psoriasis Foundation recently defined acceptable response as either BSA ≤3% or ≥75% BSA improvement from baseline and target response as BSA ≤1% for patients with psoriasis (Citation92). Additionally, the product of PGA and BSA (PGAxBSA) is a simple tool for assessing psoriasis severity and response to treatment with concordance to PASI response that can evaluate treatment targets for psoriasis (Citation93–95). As use of newer biologics and small-molecule inhibitors will be needed to meet these goals, it is critical that dermatologists understand the impact that these drugs have on PsA and other domains of psoriatic disease so that patients are appropriately treated.
Treatment of skin disease by dermatologists is an important component of comprehensive PsA disease management. While PsA clinical trials have included skin assessments, results from psoriasis trials can provide the most specific and focused information on the effects of different therapies on clearance of skin and nail lesions. summarizes PASI and NAPSI response rates for biologic and small-molecule inhibitor therapies approved for the treatment of moderate-to-severe plaque psoriasis, including etanercept, infliximab, adalimumab, ustekinumab, guselkumab, secukinumab, ixekizumab, and apremilast.
Table 7. Results of psoriasis assessments from trials of biologics and small-molecule inhibitors in chronic plaque psoriasis.
Radiographic measures
As shown in , the most common radiographic scoring methods for PsA clinical trials are modified from RA scoring methods and include the modified Steinbrocker, the modified Sharp method for PsA, the modified Sharp/van der Heijde method for PsA (also reported as the van der Heijde-modified total Sharp score [mTSS]), and the Psoriatic Arthritis Ratingen Score (PARS) (Citation96). Although these tools all analyze joints in the hands and feet, scoring criteria vary between methods. For example, both modified Sharp and mTSS separately assess erosions and joint space narrowing (a product of cartilage destruction), whereas the PARS method separately assesses erosions and bony proliferations. Further, the modified Steinbrocker uses only one scale that considers erosions, joint space narrowing, and ankylosis. GRAPPA consensus is that the Sharp/van der Heijde method for PsA is the optimal tool for use in randomized controlled trials (Citation97).
Radiographic progression results from PsA trials are summarized in . All drugs, except abatacept, were associated with statistically significant differences compared with placebo in mTSS/modified Sharp/van der Heijde score (not reported for guselkumab, apremilast, or tofacitinib). However, differences between active treatment and placebo were below the MCID based on the smallest detectable difference observed in RA studies (Citation98,Citation99). In many cases, most (>80%) patients in both placebo and active-treatment groups had a change in Sharp/van der Heijde score that was below the smallest detectable change, indicating that no measurable progression was observed in the majority of patients during the study period (Citation29,Citation100–102). This lack of progression has made it difficult to detect meaningful treatment differences in radiographic progression (Citation103). Of the drugs available for PsA, approval for inhibition of radiographic progression is given for adalimumab, etanercept, certolizumab pegol, infliximab, ixekizumab, secukinumab, and abatacept by both the FDA and EMA (Citation104–117). For golimumab, ustekinumab, and tofacitinib, only the EMA gives approval for inhibition of radiographic progression (Citation118–120). Apremilast does not have approval for inhibition of radiographic progression from either agency.
Table 8. Results of radiographic progression assessments from trials of biologics and small-molecule inhibitors in PsA.
Spine measures
The most commonly used indices for assessment of spondylitis include the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and the Bath Ankylosing Spondylitis Functional Index (BASFI) (). Components of the BASDAI and BASFI, along with patient assessments of spinal pain and global impression of disease activity rated on a 0–10 cm visual analog scale, are included in the composite Assessment of SpondyloArthritis international Society (ASAS) improvement criteria (Citation121), which is the most common primary endpoint in recent AS clinical trials.
While axial disease is an important clinical domain of PsA, fewer than 5% of patients have isolated spinal joint involvement (Citation26). Thus, because not all patients with PsA present with spinal involvement and use of MRI in clinical trials is expensive, formal assessment of spondylitis has not been performed in randomized controlled PsA trials. However, the effects of biologic and small-molecule inhibitor therapies on axial disease in PsA can be inferred from results of controlled clinical trials of these agents in patients with AS. All TNF-α inhibitors approved for the treatment of active PsA (etanercept, adalimumab, infliximab, golimumab, and certolizumab pegol) are also approved for the treatment of AS. In AS clinical trials, ASAS20 response rates were similar to all TNF-α inhibitors, ranging from 58 to 61% (Citation122). In the MEASURE 1 and MEASURE 2 studies of the IL-17A inhibitor secukinumab in patients with AS, ASAS20 response rates at week 16 were 61 and 61%, respectively, with the 150-mg dose and 60 and 41%, respectively, with the 75-mg dose (Citation123). ASAS20 response rates were significantly higher with tofacitinib 5 mg (80.8%) compared with placebo (41.2%; p < .001) in a phase 2 dose-ranging study in AS (Citation124). In a small open-label pilot study of abatacept in AS, 27% of TNF inhibitor-naïve and 20% of TNF inhibitor-experienced patients achieved ASAS20 responses, suggesting limited efficacy of abatacept for this indication (Citation125). Methotrexate has not been demonstrated to show benefit in patients with AS (Citation126). A clinical trial of ixekizumab (NCT02696798) is ongoing in AS.
PRO measures
PRO measures were developed to assess patients’ perspectives of various quality-of-life domains. These measures include the pain visual analog scale (VAS), the health assessment questionnaire disability index (HAQ-DI), the EuroQoL 5 dimension questionnaire (EQ-5D), and the 36-item short form survey (SF-36), which is broken into the physical component summary (PCS) and mental component summary (MCS) (Citation56). Changes from baseline of ≥10 mm for pain VAS and ≥0.30–0.35 for HAQ-DI have been used as MCID in PsA trials, and an MCID for change from baseline in SF-36 has been defined as 5–10 (2.5–5 for PCS and MCS individually) in PsA (Citation101,Citation127–130). Clinically important improvements in these PROs have been reported in PsA clinical trials of adalimumab, etanercept, infliximab, golimumab (subcutaneous and intravenous), certolizumab pegol, ustekinumab, guselkumab, secukinumab, ixekizumab, apremilast, tofacitinib, and abatacept (Citation100–103,Citation131–139). Additionally, the PsAID12, which is available on the GRAPPA mobile-phone app, and the RAPID3 questionnaires are useful for dermatologists to use in clinical practice to evaluate the impact of PsA symptoms on patients (Citation140,Citation141).
Additional composite measures
Composite indices, such as the Psoriatic Arthritis Disease Activity Score (PASDAS), have been developed as a means of integrating information from a variety of individual outcome indices, including physician’s global assessment scores, disease activity indices, and PROs into one meaningful measure of PsA (Citation142). Minimal disease activity (MDA), a measure that encompasses all aspects of PsA, is different from PASDAS and similar composite indices because it was created to be an objective target for treatment. When MDA criteria are met, PsA is considered to be in a satisfactory state of disease activity for both patients and physicians. Patients are classified as achieving MDA when they meet 5 of 7 criteria, including tender joint count ≤1; swollen joint count ≤1; PASI ≤1 or body surface area ≤3%; patient pain VAS ≤15; patient global disease activity VAS ≤20; health assessment questionnaire ≤0.5; and tender entheseal points ≤1 (Citation143). The value of using MDA as a treatment target was demonstrated in the TIght COntrol of Psoriatic Arthritis (TICOPA) trial (Citation144). In this study, MDA was successfully used as an objective goal in a treat-to-target approach as patients with early PsA treated with this tight control goal had significantly improved joint outcomes compared with patients receiving standard care.
Summary
PsA is a heterogeneous, rapidly developing disease with potentially deleterious effects and frequently occurs within 10 years following the appearance of psoriasis (Citation21). We consider PsA to be the major comorbidity of psoriasis due to the likelihood of PsA to result in permanent disability if left untreated. Early PsA diagnosis and initiation of treatment can prevent possibly adverse consequences, whereas a diagnostic delay of more than 6 months contributes to poor radiographic and functional outcomes (Citation27). Therefore, while providing important care to manage the skin symptoms of psoriasis, dermatologists have the unique ability to recognize early signs of PsA in patients with psoriasis and be the first healthcare practitioners to detect PsA. Dermatologists can then initiate treatment with agents that are effective for both psoriasis and PsA. There are currently available PsA drugs that block TNF-α and IL-17A. These medications can inhibit radiographic progression, control signs and symptoms, improve physical function, prevent disability, and improve quality of life. Thus, dermatologists, by detecting PsA early, are key to preventing disability in these patients. Treatments should be selected that target the most burdensome or progressive PsA clinical domain(s) for each patient based on the efficacy profiles of available treatments for these domains.
Acknowledgments
Technical assistance with editing and styling of the manuscript for submission was provided by Oxford PharmaGenesis Inc. and was funded by Novartis Pharmaceuticals Corporation. The authors were fully responsible for all content and editorial decisions and received no financial support or other form of compensation related to the development of this manuscript.
Disclosure statement
Alice Gottlieb: Consultant/advisory board agreements for Janssen, Celgene, Bristol-Myers Squibb, AbbVie, UCB, Novartis, Incyte, Lilly, Reddy Labs, Valeant, Dermira, Allergan, Sun, XBiotech, and Leo. Pharmaceutical industry research/educational grants from Janssen, Incyte, UCB, Novartis, and Lilly, and XBiotech.
Joseph F. Merola: Consultant and/or investigator for Merck Research Laboratories, Abbvie, Eli Lilly and Company, Novartis, Janssen, UCB, Samumed, Celgene, Sanofi Regeneron, GSK, Almirall, Sun Pharma, Biogen, Pfizer, Incyte, Aclaris, and Leo Pharma.
Additional information
Funding
References
- Acosta Felquer ML, FitzGerald O. Peripheral joint involvement in psoriatic arthritis patients. Clin Exp Rheumatol. 2015;33:S26–S30.
- Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013;69:729–735.
- Kavanaugh A, Helliwell P, Ritchlin CT. Psoriatic arthritis and burden of disease: patient perspectives from the population-based multinational assessment of psoriasis and psoriatic arthritis (MAPP) survey. Rheumatol Ther. 2016;3:91–102.
- Coates LC, Hodgson R, Conaghan PG, et al. MRI and ultrasonography for diagnosis and monitoring of psoriatic arthritis. Best Pract Res Clin Rheumatol. 2012;26:805–822.
- Terslev L, Naredo E, Iagnocco A, et al. Defining enthesitis in spondyloarthritis by ultrasound: results of a Delphi process and of a reliability reading exercise. Arthritis Care Res. 2014;66:741–748.
- Gladman DD. Clinical features and diagnostic considerations in psoriatic arthritis. Rheum Dis Clin North Am. 2015;41:569–579.
- Polachek A, Li S, Chandran V, et al. Clinical enthesitis in a prospective longitudinal psoriatic arthritis cohort: incidence, prevalence, characteristics, and outcome. Arthritis Care Res. 2017;69:1685–1691.
- Liu JT, Yeh HM, Liu SY, et al. Psoriatic arthritis: epidemiology, diagnosis, and treatment. WJO. 2014;5:537–543.
- Cohen JM, Husni ME, Qureshi AA, et al. Psoriatic arthritis: it’s as easy as “PSA”. J Am Acad Dermatol. 2015;72:905–906.
- Eder L, Chandran V, Gladman DD. What have we learned about genetic susceptibility in psoriasis and psoriatic arthritis? Curr Opin Rheumatol. 2015;27:91–98.
- Eder L, Chandran V, Pellet F, et al. Human leucocyte antigen risk alleles for psoriatic arthritis among patients with psoriasis. Ann Rheum Dis. 2012;71:50–55.
- Rahman P, Roslin NM, Pellett FJ, et al. High resolution mapping in the major histocompatibility complex region identifies multiple independent novel loci for psoriatic arthritis. Ann Rheum Dis. 2011;70:690–694.
- Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376:957–970.
- FitzGerald O, Haroon M, Giles JT, et al. Concepts of pathogenesis in psoriatic arthritis: genotype determines clinical phenotype. Arthritis Res Ther. 2015;17:115.
- Gladman DD, Cheung C, Ng CM, et al. HLA-C locus alleles in patients with psoriatic arthritis (PsA). Hum Immunol. 1999;60:259–261.
- Chandran V, Schentag CT, Brockbank JE, et al. Familial aggregation of psoriatic arthritis. Ann Rheum Dis. 2009;68:664–667.
- Karason A, Love TJ, Gudbjornsson B. A strong heritability of psoriatic arthritis over four generations—the Reykjavik Psoriatic Arthritis Study. Rheumatology. 2009;48:1424–1428.
- Fitzgerald O, Winchester R. Psoriatic arthritis: from pathogenesis to therapy. Arthritis Res Ther. 2009;11:214.
- Suzuki E, Mellins ED, Gershwin ME, et al. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev. 2014;13:496–502.
- Lebwohl MG, Kavanaugh A, Armstrong AW, et al. US Perspectives in the management of psoriasis and psoriatic arthritis: patient and physician results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) Survey. Am J Clin Dermatol. 2016;17:87–97.
- Gladman DD, Antoni C, Mease P, et al. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64:ii14–ii17.
- Catanoso M, Pipitone N, Salvarani C. Epidemiology of psoriatic arthritis. Reumatismo. 2012;64:66–70.
- Gottlieb AB, Kircik L, Eisen D, et al. Use of etanercept for psoriatic arthritis in the dermatology clinic: the experience diagnosing, understanding care, and treatment with etanercept (EDUCATE) study. J Dermatolog Treat. 2006;17:343–352.
- Husted JA, Thavaneswaran A, Chandran V, et al. Cardiovascular and other comorbidities in patients with psoriatic arthritis: a comparison with patients with psoriasis. Arthritis Care Res (Hoboken). 2011;63:1729–1735.
- de Oliveira M, de Oliveira Rocha B, Vieira Duarte G. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90:9–20.
- Gottlieb A, Korman NJ, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol. 2008;58:851–864.
- Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74:1045–1050.
- Gladman DD. Recent advances in understanding and managing psoriatic arthritis. F1000Res. 2016;5:2670.
- Kavanaugh A, Ritchlin C, Rahman P, et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann Rheum Dis. 2014;73:1000–1006.
- Merola JF, Lockshin B, Mody EA. Switching biologics in the treatment of psoriatic arthritis. Semin Arthritis Rheum. 2017;47:29–37.
- Mease PJ. Distinguishing inflammatory from noninflammatory arthritis, enthesitis, and dactylitis in psoriatic arthritis: a report from the GRAPPA 2010 annual meeting. J Rheumatol. 2012;39:415–417.
- Punzi L, Podswiadek M, Oliviero F, et al. Laboratory findings in psoriatic arthritis. Reumatismo. 2007;59(1):52–55.
- Sakkas LI, Alexiou I, Simopoulou T, et al. Enthesitis in psoriatic arthritis. Semin Arthritis Rheum. 2013;43:325–334.
- Karreman MC, Weel AE, van der Ven M, et al. Prevalence of psoriatic arthritis in primary care patients with psoriasis. Arthritis Rheumatol. 2016;68:924–931.
- Almodóvar R, Carmona L, Zarco P, et al. Fibromyalgia in patients with ankylosing spondylitis: prevalence and utility of the measures of activity, function and radiological damage. Clin Exp Rheumatol. 2010;28:S33–S39.
- Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62:600–610.
- Marchesoni A, Atzeni F, Spadaro A, et al. Identification of the clinical features distinguishing psoriatic arthritis and fibromyalgia. J Rheumatol. 2012;39:849–855.
- Stavropoulos PG, Soura E, Kanelleas A, et al. Reactive arthritis. J Eur Acad Dermatol Venereol. 2015;29:415–424.
- Cervini C, Leardini G, Mathieu A, et al. Psoriatic arthritis: epidemiological and clinical aspects in a cohort of 1.306 Italian patients. Reumatismo. 2005;57:283–290.
- Mensah KA, Schwarz EM, Ritchlin CT. Altered bone remodeling in psoriatic arthritis. Curr Rheumatol Rep. 2008;10:311–317.
- McGonagle D, Hermann KG, Tan AL. Differentiation between osteoarthritis and psoriatic arthritis: implications for pathogenesis and treatment in the biologic therapy era. Rheumatology. 2015;54:29–38.
- Brikman S, Furer V, Wollman J, et al. The effect of the presence of fibromyalgia on common clinical disease activity indices in patients with psoriatic arthritis: a cross-sectional study. J Rheumatol. 2016;43:1749–1754.
- Merola JF, Wu S, Han J, et al. Psoriasis, psoriatic arthritis and risk of gout in US men and women. Ann Rheum Dis. 2015;74:1495–1500.
- Husni ME, Meyer KH, Cohen DS, et al. The PASE questionnaire: pilot-testing a psoriatic arthritis screening and evaluation tool. J Am Acad Dermatol. 2007;57:581–587.
- Ibrahim GH, Buch MH, Lawson C, et al. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol. 2009;27:469–474.
- Chandran V, Gladman DD. Toronto Psoriatic Arthritis Screening (ToPAS) questionnaire: a report from the GRAPPA 2009 annual meeting. J Rheumatol. 2011;38:546–547.
- Karreman MC, Weel A, van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology. 2017;56:597–602.
- Boehncke WH, Qureshi A, Merola JF, et al. Diagnosing and treating psoriatic arthritis: an update. Br J Dermatol. 2014;170:772–786.
- Dominguez P, Gladman DD, Helliwell P, et al. Development of screening tools to identify psoriatic arthritis. Curr Rheumatol Rep. 2010;12:295–299.
- Walsh JA, Callis Duffin K, Krueger GG, et al. Limitations in screening instruments for psoriatic arthritis: a comparison of instruments in patients with psoriasis. J Rheumatol. 2013;40:287–293.
- Merola JF, Husni ME, Qureshi AA. Screening instruments for psoriatic arthritis. J Rheumatol. 2013;40:1623.
- Husni ME, Qureshi AA, Koenig AS, et al. Utility of the PASE questionnaire, psoriatic arthritis (PsA) prevalence and PsA improvement with anti-TNF therapy: results from the PRISTINE trial. J Dermatolog Treat. 2014;25:90–95.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802–807.
- Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665–2673.
- Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs. 2014;74:423–441.
- Mease PJ. Measures of psoriatic arthritis: tender and Swollen Joint Assessment, Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Modified Nail Psoriasis Severity Index (mNAPSI), Mander/Newcastle Enthesitis Index (MEI), Leeds Enthesitis Index (LEI), Spondyloarthritis Research Consortium of Canada (SPARCC), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Leeds Dactylitis Index (LDI), Patient Global for Psoriatic Arthritis, Dermatology Life Quality Index (DLQI), Psoriatic Arthritis Quality of Life (PsAQOL), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Psoriatic Arthritis Response Criteria (PsARC), Psoriatic Arthritis Joint Activity Index (PsAJAI), Disease Activity in Psoriatic Arthritis (DAPSA), and Composite Psoriatic Disease Activity Index (CPDAI). Arthr Care Res. 2011;63:S64–S85.
- Strand V, Singh JA. Evaluation and management of the patient with suspected inflammatory spine disease. Mayo Clin Proc. 2017;92:555–564.
- Golder V, Schachna L. Ankylosing spondylitis: an update. Aust Fam Physician. 2013;42:780–784.
- Brockbank JE, Stein M, Schentag CT, et al. Dactylitis in psoriatic arthritis: a marker for disease severity? Ann Rheum Dis. 2005;64:188–190.
- Kaeley GS, Eder L, Aydin SZ, et al. Dactylitis: a hallmark of psoriatic arthritis. Semin Arthritis Rheum. 2018;48:263–273.
- Rothschild BM, Pingitore C, Eaton M. Dactylitis: implications for clinical practice. Semin Arthritis Rheum. 1998;28:41–47.
- Olivieri I, Padula A, Scarano E, et al. Dactylitis or “sausage-shaped” digit. J Rheumatol. 2007;34:1217–1222.
- Falkenburg WJ, van Schaardenburg D, Ooijevaar-de Heer P, et al. IgG subclass specificity discriminates restricted IgM rheumatoid factor responses from more mature anti-citrullinated protein antibody-associated or isotype-switched IgA responses. Arthritis Rheumatol. 2015;67:3124–3134.
- Waller M, Toone EC, Vaughan E. Study of rheumatoid factor in a normal population. Arthritis Rheum. 1964;7:513–520.
- Inanc N, Dalkilic E, Kamali S, et al. Anti-CCP antibodies in rheumatoid arthritis and psoriatic arthritis. Clin Rheumatol. 2007;26:17–23.
- Candia L, Marquez J, Gonzalez C, et al. Low frequency of anticyclic citrullinated peptide antibodies in psoriatic arthritis but not in cutaneous psoriasis. J Clin Rheumatol. 2006;12:226–229.
- Popescu C, Zofotă S, Bojincă V, et al. Anti-cyclic citrullinated peptide antibodies in psoriatic arthritis—cross-sectional study and literature review. J Med Life. 2013;6:376–382.
- Wilson FC, Icen M, Crowson CS, et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis Rheum. 2009;61:233–239.
- Patrizi A, Venturi M, Scorzoni R, et al. Nail dystrophies, scalp and intergluteal/perianal psoriatic lesions: risk factors for psoriatic arthritis in mild skin psoriasis? G Ital Dermatol Venereol. 2014;149:177–184.
- Takata T, Takahashi A, Taniguchi Y, et al. Detection of asymptomatic enthesitis in psoriasis patients: an onset of psoriatic arthritis? J Dermatol. 2016;43:650–654.
- Papadavid E, Katsimbri P, Kapniari I, et al. Prevalence of psoriatic arthritis and its correlates among patients with psoriasis in Greece: results from a large retrospective study. J Eur Acad Dermatol Venereol. 2016;30:1749–1752.
- Merola JF, Li T, Li WQ, et al. Prevalence of psoriasis phenotypes among men and women in the USA. Clin Exp Dermatol. 2016;41:486–489.
- Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis—clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43:790–794.
- Tan AL, Benjamin M, Toumi H, et al. The relationship between the extensor tendon enthesis and the nail in distal interphalangeal joint disease in psoriatic arthritis—a high-resolution MRI and histological study. Rheumatology (Oxford). 2006;46:253–256.
- Aydin SZ, Castillo-Gallego C, Ash ZR, et al. Ultrasonographic assessment of nail in psoriatic disease shows a link between onychopathy and distal interphalangeal joint extensor tendon enthesopathy. Dermatology. 2012;225:231–235.
- Tan AL, Grainger AJ, Tanner SF, et al. A high-resolution magnetic resonance imaging study of distal interphalangeal joint arthropathy in psoriatic arthritis and osteoarthritis: are they the same? Arthritis Rheum. 2006;54:1328–1333.
- McGonagle D. Enthesitis: an autoinflammatory lesion linking nail and joint involvement in psoriatic disease. J Eur Acad Dermatol Venereol. 2009;23: 9–13.
- Sobolewski P, Walecka I, Dopytalska K. Nail involvement in psoriatic arthritis. Reumatologia. 2017;55:131–135.
- Rouzaud M, Sevrain M, Villani AP, et al. Is there a psoriasis skin phenotype associated with psoriatic arthritis? Systematic literature review. J Eur Acad Dermatol Venereol. 2014;28: 17–26.
- Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060–1071.
- Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499–510.
- Perez-Chada LM, Cohen JM, Gottlieb AB, et al. Achieving international consensus on the assessment of psoriatic arthritis in psoriasis clinical trials: an International Dermatology Outcome Measures (IDEOM) initiative. Arch Dermatol Res. 2018;310:701–710.
- Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38:727–735.
- Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum. 1993;36:729–740.
- Clegg DO, Reda DJ, Mejias E, et al. Comparison of sulfasalazine and placebo in the treatment of psoriatic arthritis. A department of veterans affairs cooperative study. Arthritis Rheum. 1996;39:2013–2020.
- Antoni CE, Kavanaugh A, Kirkham B, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum. 2005;52:1227–1236.
- Helliwell PS, Firth J, Ibrahim GH, et al. Development of an assessment tool for dactylitis in patients with psoriatic arthritis. J Rheumatol. 2005;32:1745–1750.
- Ferguson EG, Coates LC. Optimisation of rheumatology indices: dactylitis and enthesitis in psoriatic arthritis. Clin Exp Rheumatol. 2014;32:S-113–S-117.
- Healy PJ, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum. 2008;59:686–691.
- Maksymowych WP, Mallon C, Morrow S, et al. Development and validation of the Spondyloarthritis Research Consortium of Canada (SPARCC) Enthesitis index. Ann Rheum Dis. 2009;68:948–953.
- Langley RG, Feldman SR, Nyirady J, et al. The 5-point Investigator’s Global Assessment (IGA) scale: a modified tool for evaluating plaque psoriasis severity in clinical trials. J Dermatolog Treat. 2015;26:23–31.
- Armstrong AW, Siegel MP, Bagel J, et al. From the medical board of the national psoriasis foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290–298.
- Gottlieb AB, Merola JF, Chen R, et al. Assessing clinical response and defining minimal disease activity in plaque psoriasis with the Physician Global Assessment and body surface area (PGA x BSA) composite tool: an analysis of apremilast phase 3 ESTEEM data. J Am Acad Dermatol. 2017;77:1178–1180.
- Merola JF, Amato DA, See K, et al. Evaluation of sPGA × BSA as an outcome measure and treatment target for clinical practice. J Invest Dermatol. 2018;138:1955–1961.
- Walsh JA, McFadden M, Woodcock J, et al. Product of the physician global assessment and body surface area: a simple static measure of psoriasis severity in a longitudinal cohort. J Am Acad Dermatol. 2013;69:931–937.
- Wassenberg S. Radiographic scoring methods in psoriatic arthritis. Clin Exp Rheumatol. 2015;33:S55–S59.
- Tillett W, Jadon D, Shaddick G, et al. Feasibility, reliability, and sensitivity to change of four radiographic scoring methods in patients with psoriatic arthritis. Arthritis Care Res. 2014;66:311–317.
- van der Heijde D, Lassere M, Edmonds J, et al. Minimal clinically important difference in plain films in RA: group discussions, conclusions, and recommendations. OMERACT Imaging Task Force. J Rheumatol. 2001;28:914–917.
- Bruynesteyn K, van der Heijde D, Boers M, et al. Determination of the minimal clinically important difference in rheumatoid arthritis joint damage of the Sharp/van der Heijde and Larsen/Scott scoring methods by clinical experts and comparison with the smallest detectable difference. Arthritis Rheum. 2002;46:913–920.
- van der Heijde D, Kavanaugh A, Gladman DD, et al. Infliximab inhibits progression of radiographic damage in patients with active psoriatic arthritis through one year of treatment: results from the induction and maintenance psoriatic arthritis clinical trial 2. Arthritis Rheum. 2007;56:2698–2707.
- Kavanaugh A, van der Heijde D, McInnes IB, et al. Golimumab in psoriatic arthritis: one-year clinical efficacy, radiographic, and safety results from a phase III, randomized, placebo-controlled trial. Arthritis Rheum. 2012;64:2504–2517.
- Kavanaugh A, Husni ME, Harrison DD, et al. Safety and efficacy of intravenous golimumab in patients with active psoriatic arthritis: results through week twenty-four of the GO-VIBRANT study. Arthritis Rheumatol. 2017;69:2151–2161.
- Mease PJ, Gottlieb AB, van der Heijde D, et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis. 2017;76:1550–1558.
- Humira (adalimumab) [prescribing information]. North Chicago (IL): AbbVie Inc.; 2018.
- Humira [summary of product characteristcs]. Ludwigshafen, Germany: AbbVie Deutschland GmbH & Co. KG.; 2017.
- Remicade (infliximab) [prescribing information]. Horsham (PA): Janssen Biotech, Inc.; 2018.
- Remicade [summary of product characteristics]. Leiden, The Netherlands: Janssen Biologics B.V.; 2018.
- Enbrel (etanercept) [prescribing information]. Thousand Oaks (CA): Immunex Corporation; 2017.
- Enbrel [summary of product characteristics]. Kent (UK): Pfizer Limited; 2014.
- Cimzia (certolizumab pegol) [prescribing information]. Smyrna (GA): UCB, Inc.; 2017.
- Cimzia [summary of product characteristics]. Bruxelles, Belgium: UCB Pharma S.A.; 2019.
- Taltz (ixekizumab) [prescribing information]. Indianapolis (IN): Eli Lilly and Company; 2018.
- Taltz [summary of product characteristics]. Utrecht, The Netherlands: Eli Lilly Nederland B.V.; 2016.
- Cosentyx (secukinumab) [prescribing information]. East Hanover (NJ): Novartis Pharmaceuticals Corporation; 2018.
- Cosentyx [summary of product characteristics]. Camberley (UK): Novartis Europharm Limited; 2016.
- Orencia (abatacept) [prescribing information]. Princeton (NJ): Bristol-Myers Squibb Company; 2017.
- Orencia [summary of product characteristics]. Uxbridge (UK): Bristol-Myers Squibb Pharma EEIG; 2019.
- Simponi [summary of product characteristics]. Leiden, The Netherlands: Janssen Biologics B.V.; 2019.
- Stelara [summary of product characteristics]. Beerse, Belgium: Janssen-Cilag International NV.; 2016.
- Xeljanz [summary of product characteristics]. Kent (UK): Pfizer Limited; 2018.
- Sieper J, Rudwaleit M, Baraliakos X, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis. 2009;68: ii1–i44.
- Toussirot E. Biologics in spondyloarthritis: TNFα inhibitors and other agents. Immunotherapy. 2015;7:669–681.
- Baeten D, Sieper J, Braun J, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373:2534–2548.
- van der Heijde D, Deodhar A, Wei JC, et al. Tofacitinib in patients with ankylosing spondylitis: a phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann Rheum Dis. 2017;76:1340–1347.
- Song IH, Heldmann F, Rudwaleit M, et al. Treatment of active ankylosing spondylitis with abatacept: an open-label, 24-week pilot study. Ann Rheum Dis. 2011;70:1108–1110.
- Chen J, Veras MM, Liu C, et al. Methotrexate for ankylosing spondylitis. Cochrane Database Syst Rev. 2013;CD004524.
- Mease PJ, Woolley JM, Bitman B, et al. Minimally important difference of Health Assessment Questionnaire in psoriatic arthritis: relating thresholds of improvement in functional ability to patient-rated importance and satisfaction. J Rheumatol. 2011;38:2461–2465.
- Strand V, Sharp V, Koenig AS, et al. Comparison of health-related quality of life in rheumatoid arthritis, psoriatic arthritis and psoriasis and effects of etanercept treatment. Ann Rheum Dis. 2012;71:1143–1150.
- Gladman D, Fleischmann R, Coteur G, et al. Effect of certolizumab pegol on multiple facets of psoriatic arthritis as reported by patients: 24-week patient-reported outcome results of a phase III, multicenter study. Arthritis Care Res. 2014;66:1085–1092.
- Busse JW, Bartlett SJ, Dougados M, et al. Optimal strategies for reporting pain in clinical trials and systematic reviews: recommendations from an OMERACT 12 workshop. J Rheumatol. 2015;42:1962–1970.
- Gladman DD, Mease PJ, Ritchlin CT, et al. Adalimumab for long-term treatment of psoriatic arthritis: forty-eight week data from the adalimumab effectiveness in psoriatic arthritis trial. Arthritis Rheum. 2007;56:476–488.
- Deodhar A, Gottlieb A, Boehncke WH, et al. OP0218 Efficacy and safety results of guselkumab, and anti-IL23 monoclonal antibody, in patients with active psoriatic arthritis over 24 weeks: a phase 2a, randomized, double-blind, placebo-controlled study [abstract]. Ann Rheum Dis. 2017;76:142–143.
- Mease PJ, McInnes IB, Kirkham B, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med. 2015;373:1329–1339.
- Gladman D, Rigby W, Azevedo VF, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med. 2017;377:1525–1536.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020–1026.
- Mease PJ, van der Heijde D, Ritchlin CT, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis. 2017;76:79–87.
- Mease PJ, Kivitz AJ, Burch FX, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum. 2004;50:2264–2272.
- McInnes IB, Kavanaugh A, Gottlieb AB, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382:780–789.
- Mease PJ, Fleischmann R, Deodhar AA, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis. 2014;73:48–55.
- Holland R, Tillett W, Korendowych E, et al. Validation of the Psoriatic Arthritis Impact of Disease (PsAID) Questionnaire and its potential as a single-item outcome measure in clinical practice. Ann Rheum Dis. 2018;77:343–347.
- Coates LC, Tillett W, Shaddick G, et al. Value of the Routine Assessment of Patient Index Data 3 in patients with psoriatic arthritis: results from a tight-control clinical trial and an observational cohort. Arthritis Care Res. 2018;70:1198–1205.
- Helliwell PS, FitzGerald O, Fransen J, et al. The development of candidate composite disease activity and responder indices for psoriatic arthritis (GRACE project). Ann Rheum Dis. 2013;72:986–991.
- Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis. 2010;69:48–53.
- Coates LC, Moverley AR, McParland L, et al. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open-label, randomised controlled trial. Lancet. 2015;386:2489–2498.
- Taylor PC, Maini RN. Biologic markers in the diagnosis and assessment of rheumatoid arthritis. In: O’Dell JR, editor. UpToDate. Waltham, MA: UpToDate. [cited 28 Nov 2018].
- Doherty M, Abhishek A. Clinical manifestations and diagnosis of osteoarthritis. In: Hunter D, editor. UpToDate. Waltham (MA): UpToDate. [cited 28 Nov 2018].
- Goldenberg DLG. Clinical manifestations and diagnosis of fibromyalgia in adults. In: Schur PH, editor. UpToDate. Waltham (MA): UpToDate. [cited 28 Nov 2018].
- Singh JA. Racial and gender disparities among patients with gout. Curr Rheumatol Rep. 2013;15:307.
- Rymal E, Rizzolo D. Gout: a comprehensive review. JAAPA. 2014;27:26–31.
- Mies Richie A, Francis ML. Diagnostic approach to polyarticular joint pain. Am Fam Physician. 2003;68:1151–1160.
- van der Heijde D, Braun J, Deodhar A, et al. Comparison of three enthesitis indices in a multicentre, randomized, placebo-controlled trial of golimumab in ankylosing spondylitis (GO-RAISE). Rheumatology. 2013;52:321–325.
- Rahman P, Gladman DD, Cook RJ, et al. Radiological assessment in psoriatic arthritis. Br J Rheumatol. 1998;37:760–765.
- Wassenberg S, Fischer-Kahle V, Herborn G, et al. A method to score radiographic change in psoriatic arthritis. Z Rheumatol. 2001;60:156–166.
- Mattei PL, Corey KC, Kimball AB. Psoriasis Area Severity Index (PASI) and the Dermatology Life Quality Index (DLQI): the correlation between disease severity and psychological burden in patients treated with biological therapies. J Eur Acad Dermatol Venereol. 2014;28:333–337.
- Carlin CS, Feldman SR, Krueger JG, et al. A 50% reduction in the Psoriasis Area and Severity Index (PASI 50) is a clinically significant endpoint in the assessment of psoriasis. J Am Acad Dermatol. 2004;50:859–866.
- Ward MM, Guthrie LC, Alba MI. Brief report: rheumatoid arthritis response criteria and patient-reported improvement in arthritis activity: is an American College of Rheumatology twenty percent response meaningful to patients? Arthritis Rheumatol. 2014;66:2339–2343.
- Zochling J. Measures of symptoms and disease status in ankylosing spondylitis: Ankylosing Spondylitis Disease Activity Score (ASDAS), Ankylosing Spondylitis Quality of Life Scale (ASQoL), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI), Bath Ankylosing Spondylitis Global Score (BAS-G), Bath Ankylosing Spondylitis Metrology Index (BASMI), Dougados Functional Index (DFI), and Health Assessment Questionnaire for the Spondylarthropathies (HAQ-S). Arthritis Care Res. 2011;63:S47–S58.
- Englbrecht M, Wang Y, Ronneberger M, et al. Measuring joint involvement in polyarticular psoriatic arthritis: an introduction of alternatives. Arthritis Care Res. 2010;62:977–983.
- Mease PJ, Gladman DD, Ritchlin CT, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52:3279–3289.
- Sterry W, Ortonne JP, Kirkham B, et al. Comparison of two etanercept regimens for treatment of psoriasis and psoriatic arthritis: PRESTA randomised double blind multicentre trial. BMJ. 2010;340:c147.
- Kavanaugh A, McInnes I, Mease P, et al. Golimumab, a new human tumor necrosis factor α antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum. 2009;60:976–986.
- Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014–2022.
- Ortonne JP, Paul C, Berardesca E, et al. A 24-week randomized clinical trial investigating the efficacy and safety of two doses of etanercept in nail psoriasis. Br J Dermatol. 2013;168:1080–1087.
- Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366:1367–1374.
- Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58:106–115.
- Thaci D, Unnebrink K, Sundaram M, et al. Adalimumab for the treatment of moderate to severe psoriasis: subanalysis of effects on scalp and nails in the BELIEVE study. J Eur Acad Dermatol Venereol. 2015;29:353–360.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665–1674.
- Rich P, Bourcier M, Sofen H, et al. Ustekinumab improves nail disease in patients with moderate-to-severe psoriasis: results from PHOENIX 1. Br J Dermatol. 2014;170:398–407.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis-results of two phase 3 trials. N Engl J Med. 2014;371:326–338.
- Reich K, Arenberger P, Mrowietz U, et al. Secukinumab shows high and sustained efficacy in nail psoriasis: 1.5 year results from the TRANSFIGURE study. Presented at: The 2017 American Academy of Dermatology Annual Meeting; 2017 March 3–7; Orlando, FL.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541–551.
- Dennehy EB, Zhang L, Amato D, et al. Ixekizumab is effective in subjects with moderate to severe plaque psoriasis with significant nail involvement: results from UNCOVER 3. J Drugs Dermatol. 2016;15:958–961.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37–49.
- Rich P, Gooderham M, Bachelez H, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with difficult-to-treat nail and scalp psoriasis: results of 2 phase III randomized, controlled trials (ESTEEM 1 and ESTEEM 2). J Am Acad Dermatol. 2016;74:134–142.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405–417.
- Mease P, van der Heijde D, Landewé R, et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheum Dis. 2018;77:890–897.
- van der Heijde D, Fleischmann R, Wollenhaupt J, et al. Effect of different imputation approaches on the evaluation of radiographic progression in patients with psoriatic arthritis: results of the RAPID-PsA 24-week phase III double-blind randomised placebo-controlled study of certolizumab pegol. Ann Rheum Dis. 2014;73:233–237.
- Mease PJ, Van den Bosch F, Sieper J, et al. Performance of 3 ethesitis indices in patients with peripheral spondyloarthritis during treatment with adalimumab. J Rheumatol. 2017;44:599–608.
- McGonagle D, Tan AL, Benjamin M. The nail as a musculoskeletal appendage-implications for an improved understanding of the link between psoriasis and arthritis. Dermatology. 2009;218:97–102.
- McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137–1146.

