Abstract
Background
The influence of comorbidities on the efficacy and safety of biologic therapies in psoriasis has not been rigorously explored.
Objective
To assess the incremental burden of comorbidities on clinical efficacy and safety of secukinumab vs. etanercept and placebo among patients with plaque psoriasis pooled from 4 phase 3 trials.
Methods
Efficacy was assessed at week 12 according to achievement of Psoriasis Area and Severity Index (PASI) and Investigator’s Global Assessment (IGA; modified 2011) responses. Efficacy comparisons between treatment arms stratified by comorbidity status were made using logistic regression analysis with nonresponder imputation. Relationships between baseline characteristics and clinical responses were evaluated by χ2 tests.
Results
Of 2401 patients, 1469 (61.2%) had ≥1 active baseline comorbidity. Regardless of comorbidity status, patients receiving secukinumab were more likely to achieve PASI and IGA responses than those receiving etanercept or placebo at week 12 (p < .05 for all comparisons). Body weight of ≥90 kg was consistently associated with a decreased likelihood of achieving PASI and IGA responses (p < .01 for all comparisons). Safety was comparable across treatment arms stratified by comorbidity.
Conclusions
Secukinumab improved clinical outcomes and was well tolerated in patients with concomitant baseline comorbid conditions.
Keywords:
Introduction
Psoriasis is a chronic inflammatory disease characterized in many patients by erythematous, scaly papules and plaques and affecting approximately 2% of the population (Citation1). Given the systemic nature of the disease, in addition to cutaneous symptoms, patients with psoriasis often experience a spectrum of extracutaneous pathologies, comorbidities, and complications (Citation1,Citation2). Comorbidities frequently associated with psoriasis include obesity (Citation3–5), metabolic syndrome (Citation6,Citation7), cardiovascular disease (Citation8–10), and psoriatic arthritis (PsA) (Citation11,Citation12), and these comorbidities are factors in selection of the ideal therapy. A holistic approach is required to address the varied and systemic manifestations of psoriatic disease.
The presence of comorbidities represents a clinical challenge for the management of psoriasis, especially when initiation of a systemic therapy is considered. Although systemic therapies may improve comorbidities of psoriasis by reducing overall inflammation, the potential effect of psoriasis treatments on comorbidities remains poorly understood, and treatment-emergent adverse events (TEAEs) may adversely interfere with some comorbidities (Citation2). Biologics selectively targeting interleukin 17A (IL-17A) are increasingly prescribed due to established efficacy in skin and joints, as well as a favorable safety profile compared with tumor necrosis factor inhibitors (TNFis) (Citation13,Citation14). Secukinumab, a fully human monoclonal antibody that selectively binds to and neutralizes IL-17A, has shown long-lasting efficacy and safety in treating the complete spectrum of manifestations of psoriatic disease, including psoriasis vulgaris (Citation14,Citation15); palmoplantar (Citation16), nail (Citation17), and scalp psoriasis (Citation18); all PsA disease domains, including enthesitis and dactylitis (Citation19–23); and ankylosing spondylitis (Citation24,Citation25). Preliminary results from ongoing studies confirm the effectiveness, persistence, and safety of secukinumab in the real world for up to 2 years (Citation26–28). However, the safety profile and effect of biologic treatments on disease activity in patients with specific comorbidities may not be similar to those for all patients with psoriasis (Citation2), as randomized clinical trials often exclude patients with relevant comorbidities. In the phase 3 clinical trials ERASURE (Citation14), FIXTURE (Citation14), FEATURE (Citation29), and JUNCTURE (Citation30), the efficacy and safety of secukinumab were characterized in patients with moderate to severe psoriasis. However, the effect of active baseline comorbidities on these parameters was not studied.
In this secondary analysis of patients pooled from these 4 phase 3 studies, the efficacy and safety of secukinumab were compared with those of etanercept and placebo in patients with and without active baseline comorbidities. Relationships between patient characteristics and comorbidities, as well as the effect of comorbidities on treatment response, were also observed.
Patients and methods
Study design and population
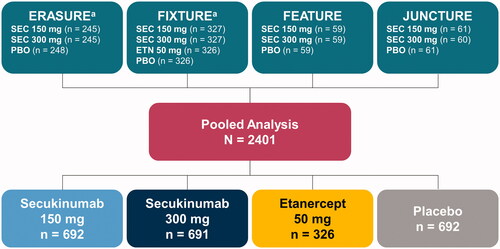
This analysis included pooled data from patients treated with secukinumab, etanercept, or placebo in 4 randomized, double-blind, placebo-controlled, phase 3 trials: ERASURE (NCT01365455) (Citation14), FIXTURE (NCT01358578) (Citation14), FEATURE (NCT01555125) (Citation29), and JUNCTURE (NCT01636687) (Citation30) (). All studies included adult patients with plaque psoriasis that was poorly controlled with topical therapy, phototherapy, and/or systemic therapy and had been diagnosed ≥6 months prior to randomization. All patients had moderate to severe psoriasis as defined by a composite Psoriasis Area and Severity Index (PASI) score of ≥12, an Investigator’s Global Assessment modified 2011 (IGA mod 2011) score of ≥3, and total affected body surface area of ≥10% at baseline. In the trials pooled for this analysis, patients were not randomized on the basis of the presence of comorbidities. For this study, one patient each from ERASURE and FIXTURE was excluded from the analysis due to protocol deviations; both patients signed the corresponding informed consent form after starting the study procedures.
Figure 1. Study population for post hoc analysis. ETN: etanercept; PBO: placebo; SEC: secukinumab. aOne patient from each study was excluded from the pooled analysis as a result of protocol deviations.
Patients were assigned to cohorts based on the presence or absence of active baseline comorbidities. Those patients with active baseline comorbidities had ≥1 of the following common comorbid conditions: angina pectoris, arthritis, cardiac failure, coronary artery disease, depression, diabetes, gout, hyperlipidemia, hypertension, osteoarthritis, and rheumatoid arthritis as defined by Medical Dictionary for Regulatory Activities questionnaires; obesity, defined as a medical history of obesity or baseline body mass index (BMI) of ≥30 kg/m2; or PsA, as reported by the patient and indicated by the principal investigator in the electronic case report form. The studies from which these data were pooled were conducted in accordance with the Declaration of Helsinki principles and were approved by institutional review boards or independent ethics committees.
Study objectives
The objective of this secondary analysis was to assess the incremental burden of comorbidities on clinical efficacy and safety of secukinumab vs. etanercept after 12 weeks of treatment among patients with moderate to severe plaque psoriasis pooled from 4 phase 3 trials (Citation14,Citation29,Citation30). Additionally, this analysis assessed the association between comorbidities and baseline demographics, as well as the effect of specific baseline characteristics on overall treatment response.
Statistical analysis
Efficacy comparisons between secukinumab 150 mg and secukinumab 300 mg vs. etanercept and placebo within patient groups stratified by presence or absence of comorbidities were made using logistic regression analysis with nonresponder imputation for missing patient data. Efficacy was assessed at week 12 according to the proportion of patients who achieved PASI75, PASI90, and PASI100, and IGA mod 2011 0 (clear) and 0/1 (clear/almost clear). Relationships between baseline demographics and clinical responses were evaluated by χ2 tests. The frequencies of TEAEs, treatment-emergent serious adverse events, and TEAEs leading to discontinuation were summarized for each treatment arm stratified by presence of active baseline comorbidities.
Patients pooled in this secondary analysis were not randomized by presence vs. absence of baseline comorbidities in the original phase 3 trials. Statistical comparisons are for hypothesis generation only; analyses were not adjusted for multiple comparisons.
Results
Patient population and comorbidities at baseline
Of the 2401 patients included in this analysis, 1469 (61.2%) had ≥1 active baseline comorbidity (). Across all treatment arms, the most frequent diagnosed comorbidities included obesity (37.2% of patients), hypertension (24.6%), PsA (18.0%), hyperlipidemia (15.9%), and diabetes (8.4%) (). Other comorbidities affecting < 6% of the overall patient population included depression, osteoarthritis, coronary artery disease, gout, arthritis, rheumatoid arthritis, cardiac failure, and angina pectoris.
Table 1. Patients with active baseline comorbidities of psoriasis pooled from the ERASURE, FIXTURE, FEATURE, and JUNCTURE studies (full-analysis set).
Relationships among comorbidities and baseline characteristics
Baseline disease characteristics were balanced across treatment arms, as previously published, and all patients had moderate to severe psoriasis regardless of comorbidity status () (Citation14,Citation29,Citation30). As patients were not randomized by presence vs. absence of baseline comorbidities in the original studies, imbalances existed between baseline characteristics of patients with and without active baseline comorbidities. Compared with patients with no active baseline comorbidities, those with active baseline comorbidities were older, heavier (as measured by weight and BMI), more likely to be biologic experienced, and more likely to have lived with a diagnosis of psoriasis for a longer time (). Specific associations were found between individual comorbidities and imbalances in baseline characteristics. Linear regression analysis identified that both higher weight and older age were associated with the presence of specific comorbidities, including obesity, hypertension, hyperlipidemia, and diabetes (p < .05 for all comparisons) (). Additionally, patients with concomitant PsA were more likely to have previous exposure to biologics (p < .05) (). These relationships may influence clinical response to secukinumab.
Table 2. Baseline demographics, treatment histories, and disease characteristics of patients with psoriasis stratified by presence vs. absence of active comorbiditiesa.
Table 3. Relationships between comorbidities and baseline demographics and treatment histories of patients with psoriasis pooled from the ERASURE, FIXTURE, FEATURE, and JUNCTURE studies.
Efficacy
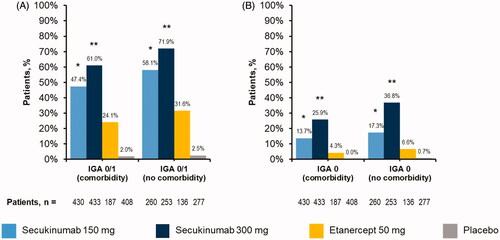
At week 12, secukinumab treatment significantly improved clinical outcomes regardless of comorbidity status in this cohort of patients with moderate to severe psoriasis. All patient groups receiving any dose of secukinumab were more likely to achieve PASI responses than patients with corresponding comorbidity status receiving etanercept or placebo (p < .05 for all comparisons) (). Similarly, all patient groups receiving any dose of secukinumab were more likely to achieve IGA mod 2011 0/1 or IGA mod 2011 0 responses than patients with corresponding comorbidity status receiving etanercept or placebo (p < .05 for all comparisons) (). Furthermore, patients treated with secukinumab 300 mg were more likely to achieve all PASI and IGA mod 2011 responses than those receiving secukinumab 150 mg, regardless of comorbidity status (p < .05 for all comparisons) ( and ). Numerical differences in achievement of PASI and IGA mod 2011 endpoints were observed between patients with and without comorbidities receiving any dose of secukinumab ( and ).
Figure 2. Efficacy as measured by (A) PASI75, (B) PASI90, and (C) PASI100 among patients with psoriasis stratified by presence or absence of active baseline comorbidities. PASI: Psoriasis Area and Severity Index. *p<.05 compared with etanercept and placebo. **p<.05 compared with secukinumab 150 mg, etanercept, and placebo.
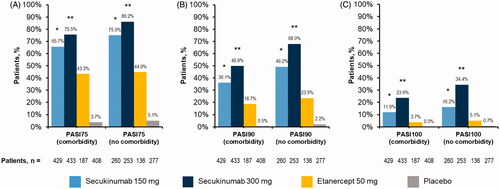
Figure 3. Efficacy as measured by (A) IGA mod 2011 0/1 or (B) IGA mod 2011 0 among patients with psoriasis stratified by presence or absence of active baseline comorbidities. IGA mod 2011, Investigator’s Global Assessment modified 2011. *p<.05 compared with etanercept and placebo. **p<.05 compared with secukinumab 150 mg, etanercept, and placebo.
The effect of comorbidities on clinical response to treatment was not significant by χ2 tests (). However, this same analysis identified that increased body weight (≥90 kg) was associated with a decreased likelihood of achieving all PASI and IGA mod 2011 clinical responses (p < .01) (). No other covariate was consistently associated with decreased likelihood of achieving all PASI and IGA mod 2011 responses, although previous exposure to biologics was associated with decreased likelihood of achieving PASI90 and IGA mod 2011 0/1, and greater age was associated with decreased likelihood of achieving PASI100 responses (p < .05 for all comparisons).
Table 4. Relationships between treatment response and baseline covariates across all patients with psoriasis pooled from the ERASURE, FIXTURE, FEATURE, and JUNCTURE studiesa.
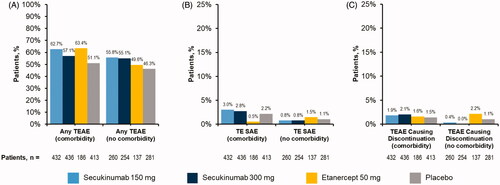
Safety
The frequency of TEAEs was comparable across treatment arms stratified by comorbidity (). Few patients experienced serious adverse events or TEAEs leading to discontinuation (), and no new safety signals were identified.
Discussion
In this post hoc analysis of patients pooled from 4 phase 3 studies of secukinumab in patients with moderate to severe psoriasis, the majority of patients had comorbidities at baseline. The top 5 most frequent comorbidities included obesity, hypertension, PsA, hyperlipidemia, and diabetes. Imbalances in baseline characteristics existed between patients with and without comorbidities. Those with comorbidities were older, heavier, and more biologic experienced than those without. Additionally, patients with PsA were more likely to have had previous exposure to biologic agents. Consistent with the primary analyses, patients receiving any dose of secukinumab were more likely to achieve PASI and IGA mod 2011 clinical responses compared with those receiving etanercept or placebo. Numerical differences in treatment response were observed between patients with and without comorbidities receiving secukinumab, although the effect of comorbidities on treatment was not found to be significant by χ2 tests. Body weight of ≥90 kg was consistently associated with decreased achievement of PASI and IGA mod 2011 clinical responses. The imbalances in baseline body weight between those with and without comorbidities likely explain the observed numerical differences in treatment response between these 2 groups. Secukinumab appeared to be safe in patients with comorbidities; the frequency and severity of TEAEs were comparable across all groups studied and were aligned with long-term safety data (Citation31–34). Secukinumab treatment was not associated with increased likelihood of TEAEs in patients with comorbidities.
In patients with psoriasis, body weight and BMI are known to influence clinical response to treatment with biologics, with increased BMI being a predictor of poorer response and reduction of body weight in obese patients associated with increased effectiveness of biologic therapy (Citation35). TNFis have been found to be less effective in patients with higher BMI than in those with normal BMI (Citation36,Citation37). Further, a meta-analysis of patients with immune-mediated inflammatory diseases, including psoriasis, found that obese patients were approximately 60% more likely than patients with normal BMI to experience TNFi failure (Citation38). Higher BMI or body weight also correlated with poorer outcomes and response to treatment with the IL-12/23 biologic ustekinumab, which is reflected by the approved dosing regimen that accounts for body weight: patients weighing ≤100 kg receive 45 mg of ustekinumab, and those weighing >100 kg receive 90 mg (Citation39). In pharmacokinetic studies, patients weighing >100 kg were found to have faster clearance and greater volume of distribution of ustekinumab than those weighing ≤100 kg (Citation40,Citation41). Additionally, one cohort study of Italian patients receiving a new systemic treatment for plaque psoriasis found an inverse relationship between BMI and achievement of PASI75 (Citation42), suggesting that these relationships may be common across systemic therapies for psoriasis. In general, increased doses of biologics are likely required to adequately treat patients with higher BMI (Citation43).
Although secukinumab is considered effective in patients regardless of body weight, relationships between effectiveness and patient BMI are beginning to emerge. In a pooled analysis of patients receiving secukinumab in the phase 3 ERASURE and FIXTURE trials stratified by BMI into quartiles, secukinumab 300 mg was effective in all quartiles as determined by the proportion of patients achieving PASI75, PASI90, and IGA mod 2011 0/1, although a numerical trend of decreasing effectiveness with increasing patient body weight was observed (Citation44). In the phase 3b OPTIMISE study, patients who achieved PASI75 but not PASI90 at week 24 of treatment with secukinumab were re-randomized to receive secukinumab 300 mg every 2 weeks or to continue receiving secukinumab 300 mg every 4 weeks (Citation45). Patients in whom secukinumab was uptitrated every 2 weeks were numerically more likely to achieve PASI90 at week 52 than those receiving secukinumab every 4 weeks (57.0% vs. 46.5%) (Citation45). The numerical benefit of decreased dosing intervals was greatest in patients weighing ≥90 kg (Citation45).
Explanations for these observations may be rooted in both the pathophysiological mechanisms of psoriasis, including the potential inflammatory role of adipose tissue, and the pharmacokinetics of the biologic drugs themselves. Associations have been found among white adipose tissue, circulating levels of inflammatory adipokines released by adipose tissue, and the presence and severity of psoriasis (Citation3,Citation46). In pediatric patients with psoriasis, obesity seems to be associated with development and severity of psoriasis (Citation47), with adiposity preceding the formation of psoriatic plaques (Citation48). An ongoing trial is investigating the role of genes modulating adipokine and adipocyte activity in systemic inflammation and response to secukinumab (NCT03055494). As patients with greater body mass may require higher doses of biologics to achieve therapeutic serum concentrations (Citation43), increased doses may be required to optimally treat heavier patients. A study of the safety and efficacy of higher doses of secukinumab in obese patients is currently underway (NCT03504852).
Studies comparing the effectiveness and safety of available biologic therapies in patients with comorbidities are important for informed shared decision-making in the treatment of psoriasis because relationships between psoriasis treatment and comorbidities remain poorly understood (Citation2). Methodological shortcomings exist in previous studies evaluating the association between comorbidities and psoriasis; many epidemiological studies of psoriasis comorbidities do not use a validated comorbidities index (Citation49). However, the presence of comorbidities has been found to be associated with more-advanced psoriatic disease, with the severity of comorbidities correlating directly to severity of psoriasis (Citation50).
Although rigorous controlled trials are required to assess the effect of individual comorbidities on treatment response and safety of secukinumab, results presented here suggesting that secukinumab is safe and effective in patients with comorbidities are reinforced by evidence from clinical practice. Secukinumab has shown maintenance of therapeutic response over 5 years in an extension of the ERASURE and FIXTURE trials (NCT01544595), as well as the long-term monitoring study SCULPTURE (Citation31), and has been used clinically for >5 years for the treatment of moderate to severe psoriasis. The safety of secukinumab has been demonstrated in patients with psoriasis, PsA, and ankylosing spondylitis over 5 years (Citation31–34). True long-term safety of secukinumab, as with any drug, can be established only through real-world registry data. Despite intrinsic differences between clinical trials and clinical practice, including adherence to drug regimens and patient baseline characteristics (Citation51), secukinumab retains a favorable safety profile in the real world (Citation52), with approximately 80% drug survival after 1 year (Citation27). As patients with psoriasis frequently experience comorbidities, the long-term use of secukinumab and its associated safety in real-world populations suggest that secukinumab is not widely contraindicated in patients with common comorbidities associated with psoriasis. The impact of secukinumab on comorbidities and in special populations should be considered as well (Citation36,Citation53).
Limitations
Some limitations are important to understand when considering the results of this study. Several common comorbidities of psoriasis were studied in this analysis, but others were not included, such as comorbid autoimmune diseases, demyelinating disease, inflammatory bowel disease, and concomitant chronic infection; these are additional factors that must be considered when selecting the optimal therapy for patients with psoriasis. In this post hoc analysis, patients were not randomized by presence or absence of specific comorbidities, and imbalances exist in baseline patient characteristics across treatment arms. The studies from which this patient population was pooled were not statistically powered to study the effects of individual comorbidities on treatment response, and analyses were not adjusted for multiple comparisons. As such, statistical comparisons presented here are for hypothesis generation only. Nonetheless, these findings suggest that the presence of comorbidities appears to have little effect on the efficacy and safety of secukinumab, although the increased body weight of obese patients may decrease the likelihood of achieving optimal treatment response.
Conclusions
In this post hoc analysis of patients with moderate to severe psoriasis, secukinumab improved clinical outcomes and was well tolerated in patients with and without baseline comorbid conditions. Although comorbidity status did not significantly affect achievement of clinical response in patients treated with secukinumab, body weight of ≥90 kg was associated with decreased likelihood of achievement of clinical response for all PASI and IGA mod 2011 endpoints measured. This association could underlie observed numerical differences in treatment responses between patients with and without comorbidities. Further research is needed to test the effect of individual comorbidities on response to secukinumab; studies of secukinumab dosing in obese patients are currently underway to ensure that psoriasis treatment is optimized in these patients.
Acknowledgements
The authors thank Richard Karpowicz, PhD, of Health Interactions, Inc, Hamilton, NJ, USA, for providing medical writing/editorial support, which was funded by Novartis Pharmaceuticals Corporation, East Hanover, NJ, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Disclosure statement
A.B. Gottlieb has received honoraria as an advisory board member and consultant for Avotres Therapeutics, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, Incyte, Janssen, LEO Pharma, Eli Lilly, Novartis, Sun Pharmaceutical, UCB, and XBiotech (only stock options that she has not used); and has received research/educational grants from Boehringer Ingelheim, Incyte, Janssen, Novartis, UCB, XBiotech, and Sun Pharmaceutical. J.J. Wu is or has been an investigator, consultant, and/or speaker for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Dermavant, Dermira, Dr Reddy’s Laboratories, Eli Lilly, Janssen, LEO Pharma, Novartis, Regeneron, Sanofi Genzyme, Sun Pharmaceutical, UCB, and Valeant. C.E.M. Griffiths has received honoraria or research grants from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, GSK, Janssen-Cilag, LEO Pharma, Merck Sharp & Dohme, Novartis, Pfizer, Sandoz, Trident, and UCB; is a National Institute for Health Research (NIHR) Emeritus Senior Investigator; and is supported in part by the NIHR Manchester Biomedical Research Centre. K. Marfo, E. Muscianisi, X. Meng, and J. Frueh are employees and stockholders of Novartis. M. Lebwohl is an employee of Icahn School of Medicine at Mount Sinai, which receives research funds from AbbVie, Amgen, Arcutis, Boehringer Ingelheim, Dermavant, Eli Lilly, Incyte, Janssen Research & Development, LEO Pharma, Ortho Dermatologics, Pfizer, and UCB; he is also a consultant for Aditum Bio, Allergan, Almirall, Arcutis, Avotres Therapeutics, BirchBioMed, BMD Skincare, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, Castle Biosciences, Corrona, Dermavant, Evelo, Facilitate International Dermatologic Education, Foundation for Research and Education in Dermatology, Inozyme Pharma, LEO Pharma, Meiji Seika Pharma, Menlo, Mitsubishi, NeuroDerm, Pfizer, Promius/Dr Reddy’s Laboratories, Serono, Theravance, and Verrica.
Additional information
Funding
References
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072.
- Carvalho AV, Romiti R, Souza CD, et al. Psoriasis comorbidities: complications and benefits of immunobiological treatment. An Bras Dermatol. 2016;91(6):781–789.
- Kirby B, Lynch M. Adipokines and psoriasis: the obesity link. Br J Dermatol. 2018;179(2):239.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:e54.
- Fleming P, Kraft J, Gulliver WP, et al. The relationship of obesity with the severity of psoriasis: a systematic review. J Cutan Med Surg. 2015;19(5):450–456.
- Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149(1):84–91.
- Brauchli YB, Jick SS, Meier CR. Psoriasis and the risk of incident diabetes mellitus: a population-based study. Br J Dermatol. 2008;159(6):1331–1337.
- Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31(8):1000–1006.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129(10):2411–2418.
- Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80(1):251–265.e19.
- Gladman DD, Antoni C, Mease P, et al. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl. 2):ii14–ii17.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–551.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis – results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338.
- Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400–409.
- Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76(1):70–80.
- Reich K, Sullivan J, Arenberger P, et al. Effect of secukinumab on the clinical activity and disease burden of nail psoriasis: 32-week results from the randomized placebo-controlled TRANSFIGURE trial. Br J Dermatol. 2019;181(5):954–966.
- Bagel J, Duffin KC, Moore A, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol. 2017;77(4):667–674.
- Orbai AM, McInnes IB, Coates LC, et al. Effect of secukinumab on the different GRAPPA-OMERACT core domains in psoriatic arthritis: a pooled analysis of 2049 patients. J Rheumatol. 2020;47(6):854–864.
- Kivitz AJ, Nash P, Tahir H, et al. Efficacy and safety of subcutaneous secukinumab 150 mg with or without loading regimen in psoriatic arthritis: results from the FUTURE 4 study. Rheumatol Ther. 2019;6(3):393–407.
- McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386(9999):1137–1146.
- Mease PJ, McInnes IB, Kirkham B, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med. 2015;373(14):1329–1339.
- Nash P, Mease PJ, McInnes IB, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther. 2018;20(1):47.
- Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis with high retention rate: 3-year results from the phase III trial, MEASURE 2. RMD Open. 2017;3(2):e000592.
- Baeten D, Sieper J, Braun J, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373(26):2534–2548.
- Papp K, Alavi A, Brassard D, et al. Secukinumab treatment results in improvement of disease characteristics in patients with moderate to severe psoriasis: 24 month follow-up data from the PURE registry. J Am Acad Dermatol. 2019;81(4(Suppl. 1)):AB274.
- Augustin M, Jullien D, Martin A, et al. Real-world evidence of secukinumab in psoriasis treatment – a meta-analysis of 43 studies. J Eur Acad Dermatol Venereol. 2020;34(6):1174–1185.
- Yiu ZZN, Mason KJ, Hampton PJ, et al. Drug survival of adalimumab, ustekinumab and secukinumab in patients with psoriasis: a prospective cohort study from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR). Br J Dermatol. 2020;183(2):294–302.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172(2):484–493.
- Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29(6):1082–1090.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32(9):1507–1514.
- Baraliakos X, Braun J, Deodhar A, et al. Long-term efficacy and safety of secukinumab 150 mg in ankylosing spondylitis: 5-year results from the phase III MEASURE 1 extension study. RMD Open. 2019;5(2):e001005.
- Deodhar A, Mease PJ, McInnes IB, et al. Long-term safety of secukinumab in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: integrated pooled clinical trial and post-marketing surveillance data. Arthritis Res Ther. 2019;21(1):111.
- Mease PJ, Kavanaugh A, Reimold A, et al. Secukinumab provides sustained improvements in the signs and symptoms in psoriatic arthritis: final 5 year efficacy and safety results from a phase 3 trial. Ann Rheum Dis. 2019;78(Suppl. 2):917. Abstract FRI0451.
- Al-Mutairi N, Nour T. The effect of weight reduction on treatment outcomes in obese patients with psoriasis on biologic therapy: a randomized controlled prospective trial. Expert Opin Biol Ther. 2014;14(6):749–756.
- Kaushik SB, Lebwohl MG. CME part I psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019;80(1):27–40.
- Menter A, Gordon KB, Leonardi CL, et al. Efficacy and safety of adalimumab across subgroups of patients with moderate to severe psoriasis. J Am Acad Dermatol. 2010;63(3):448–456.
- Singh S, Facciorusso A, Singh AG, et al. Obesity and response to anti-tumor necrosis factor-α agents in patients with select immune-mediated inflammatory diseases: a systematic review and meta-analysis. PLoS One. 2018;13(5):e0195123.
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371(9625):1675–1684.
- Thibodaux RJ, Triche MW, Espinoza LR. Ustekinumab for the treatment of psoriasis and psoriatic arthritis: a drug evaluation and literature review. Expert Opin Biol Ther. 2018;18(7):821–827.
- Zhu Y, Hu C, Lu M, et al. Population pharmacokinetic modeling of ustekinumab, a human monoclonal antibody targeting IL-12/23p40, in patients with moderate to severe plaque psoriasis. J Clin Pharmacol. 2009;49(2):162–175.
- Naldi L, Addis A, Chimenti S, et al. Impact of body mass index and obesity on clinical response to systemic treatment for psoriasis. Evidence from the Psocare project. Dermatology (Basel). 2008;217(4):365–373.
- Puig L. Obesity and psoriasis: body weight and body mass index influence the response to biological treatment. J Eur Acad Dermatol Venereol. 2011;25(9):1007–1011.
- Szepietowski J, Rich P, Loeffler J, et al. Secukinumab 300 mg shows superior efficacy across subject body weight groups: pooled analysis of phase 3 ERASURE and FIXTURE trials. J Am Acad Dermatol. 2015;72(5):AB248.
- Reich K, Puig L, Szepietowski JC, et al. Secukinumab dosing optimization in patients with moderate-to-severe plaque psoriasis: results from the randomized, open-label OPTIMISE study. Br J Dermatol. 2020;182(2):304–315.
- Lynch M, Ahern T, Sweeney CM, et al. Adipokines, psoriasis, systemic inflammation, and endothelial dysfunction. Int J Dermatol. 2017;56(11):1103–1118.
- Paller AS, Mercy K, Kwasny MJ, et al. Association of pediatric psoriasis severity with excess and central adiposity: an international cross-sectional study. JAMA Dermatol. 2013;149(2):166–176.
- Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150(5):573–574.
- Yang YW, Keller JJ, Lin HC. Medical comorbidity associated with psoriasis in adults: a population-based study. Br J Dermatol. 2011;165(5):1037–1043.
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149(10):1173–1179.
- Strober B, Karki C, Mason M, et al. Characterization of disease burden, comorbidities, and treatment use in a large, US-based cohort: results from the Corrona Psoriasis Registry. J Am Acad Dermatol. 2018;78(2):323–332.
- Strober BE, Germino R, Guana A, et al. US real-world effectiveness of secukinumab for the treatment of psoriasis: 6-month analysis from the Corrona Psoriasis Registry. J Dermatolog Treat. 2020;31(4):333–341.
- Kaushik SB, Lebwohl MG. CME part II psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80(1):43–53.