Abstract
Background
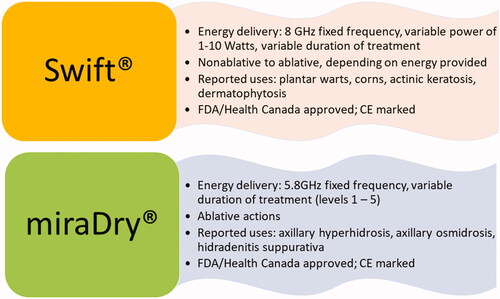
Microwaves are used in medicine for diagnostics, and treatment of cancer. Recently, novel microwave devices (Swift®, Emblation Ltd, UK and miraDry®, Miramar Labs Inc., CA) have been cleared by the FDA and Health Canada for various dermatological conditions.
Objective and methods
To review the dermatological use of microwave-based treatments (plantar warts, corns, actinic keratosis, dermatophytosis, axillary hyperhidrosis, osmidrosis, and hidradenitis suppurativa). Clinical trials, case reports, or in vitro studies for each condition are summarized.
Results and conclusion
Microwaves are a promising alternative therapy for cutaneous warts, actinic keratosis, axillary hyperhidrosis, and osmidrosis, with favorable safety profiles. However, patients with hidradenitis suppurativa have had negative clinical outcomes. Limited treatment of corns showed good pain reduction but did not resolve hyperkeratosis. A preliminary in vitro study indicated that microwave treatment inhibits the growth of T. rubrum. We present the first case of toenail onychomycosis successfully treated with microwaves. Despite the advancements in the use of microwaves, the mechanism of action in non-ablative treatment is not well understood; further research is needed. More high-quality randomized clinical trials with larger groups and long follow-up periods are also required to evaluate the clinical benefits and possible adverse effects of microwaves in treating dermatological conditions.
1. Introduction
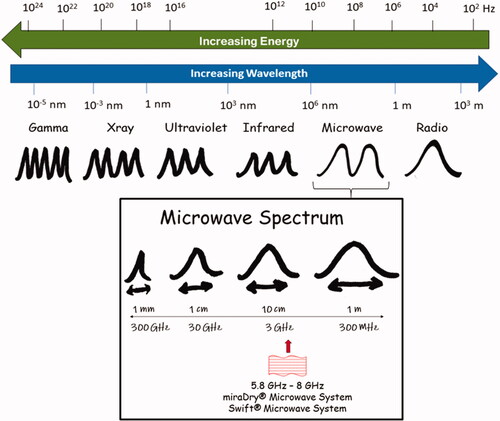
Microwaves are a part of the electromagnetic spectrum (), widely used in industries such as food processing, communications, medical research, and biosensor diagnostics. The wavelengths range from 1 meter to 1 millimeter in free space, and frequencies range from 300 MHz to 300 GHz (Citation1). Microwaves were developed for communication and satellite navigation in the 1950s, utilizing their electromagnetic wave properties. Microwaves were used in domestic appliances in the late 1970s to reduce microorganisms in food through targeted thermal deactivation or killing.
The Industrial Scientific and Medical (ISM)-approved frequency range for domestic purposes is 2.45 GHz (Citation2). However, higher frequencies have lower penetration ability and microwave systems with wavelengths of 5.8–8 GHz range have proven useful for some dermatological applications () (Citation3).
Microwaves may produce both thermal and non-thermal effects which can be utilized for a variety of medical applications (Citation3). Thermal heating is generated locally through molecular dipolar rotation (particularly through polar water molecules) and ionic conduction within the area where the microwave’s electromagnetic fields are applied (Citation2). Thermal effects may vary depending on the temperature generated: diathermia (up to 41 °C) increases blood flow for tissue warming in physiotherapy/pain reduction applications; hyperthermia (from 41 to 45 °C) can stimulate cell apoptosis; thermoablation (>45 °C) destroys tissue (Citation3). Direct tissue ablation is not always necessary; non-ablative heating can promote healing through stimulation of the immune system (Citation4), and cooling agents should not be applied to the treatment area after non-ablative treatment.
Since the early 1980s, these effects have been utilized in a variety of medical applications in physiotherapy, oncology and surgery (Citation3). Scanning with microwaves can be useful for visualization of tumors and transmission of microwaves allows for communications such as data telemetry from an implanted medical device (e.g. pacemaker) to an external device (data telemetry).
Herein, we summarize the current literature regarding the current and prospective use of microwaves as a therapeutic modality across dermatological conditions. We also report the first case of using microwaves to successfully treat toenail onychomycosis (dermatophytoma).
2. Use of microwaves in dermatology
2.1. Plantar warts
The clinical appearance of warts may vary depending on the type of HPV and the anatomical site (Citation5,Citation6). There are about 150 different forms of HPV, with HPV types 1, 2, 4, 27, or 57 causing the majority of verruca vulgaris lesions (Citation7).
The most common topical preparation used is salicylic acid (SA) (Citation7). Other topical agents include 5-fluorouracil, podophyllin, and silver nitrate (Citation5). Cryotherapy with liquid nitrogen can be considered if topical treatments have been ineffective or contraindicated. Additionally, cryotherapy can be an adjunct to topical therapy. Cryotherapy causes tissue destruction; in a meta-analysis of RCTs, this therapy had low efficacy to manage common warts (with a mean clearance on all sites of 49%) (Citation8).
The common treatment endpoint is the clinical cure, defined as the complete disappearance of elevated/warty skin (Citation5). Warts that relapse or are resistant to primary treatment may be treated with systemic immunotherapy, targeting the viral life cycle, or surgical excision of the infected skin (Citation5). Even with an armamentarium of treatments available, warts remain a persistent problem.
Microwaves are a new treatment modality introduced in North America in January 2019 for plantar warts. Swift® is a novel microwave system that is FDA-cleared, Health Canada approved, and CE marked. This microwave device uses a specialized probe to deliver low-dose microwave energy to the infected tissue at a depth of 2–4 mm, heating the tissue cells to the 42–45 °C range. This heating of the infected tissue triggers an immunological response, allowing the body to detect and eliminate the HPV virus (Citation9). Subsequently, the infected tissue is repaired, replaced, and regenerated.
2.1.1. Microwave treatment protocol
For plantar warts, a typical treatment consists of a 7 mm diameter microwave applicator (hand-held device, 8GHz) that emits 1–10 Watts of microwave energy directly to the affected area. An average treatment session involves 3–5 applications, each lasting 2 s, spaced one second apart (Citation9) and 3–4 sessions each a month apart.
Ongoing research has observed the response to localized topical microwave energy in HPV16 positive cervical tumor cells of 3 D organotypic raft cultures. Although oncogenic in HPV type, and of different tissue types, it demonstrates the same principles that may underlie external dermatology applications. This in vitro work shows inhibited cell proliferation (Ki67); activated apoptosis (caspase 3) and autophagy (LC3B); increase of heat shock proteins (HSP70), and translational stress (G3BP and PABP) in cervical cancer tissues (Citation10).
2.1.2. Clinical evidence
The efficacy of locally delivered microwaves in treating cutaneous viral warts has been presented in an uncontrolled phase I study and a case report () (Citation11,Citation12). The study design, patient characteristics, treatment regimen, clinical endpoint, and adverse events of patients with warts and corns treated with microwaves are listed in (Citation11,Citation12). This study reported that 75.9% of recalcitrant plantar warts resolved, with an average of 3 treatment sessions. The study also observed activation of dendritic cells (CD80, CD86, CD40) and enhancement of anti-HPV responses by CD8+ T cells induced by microwave-treated keratinocytes (Citation11).
Table 1. Clinical outcomes of microwave therapy for plantar warts and corns.
Post-marketing surveillance of this therapy in the UK, based on an online survey, reported that the efficacy rates were 79.2% (65.9–87.5%) for plantar and 82.3% (71.4–100%) for common warts. There was complete resolution of warts with three treatments on average. On a 10-point scale, patients were "very satisfied" (Citation13).
2.1.3. Adverse events
For a typical 5-s treatment, patients generally reported moderate discomfort for about 2 s, which subsided after the treatment. There was also a common observation that discomfort decreased with subsequent treatments (Citation11). The lesions were described as painful and assessed using a numerical scale (scored out of 10). There were reductions in pain immediately following the first session (Citation11). Feedback from early post-market surveillance found a shortened treatment time of 2 s would be used without compromising on efficacy. Further post-marketing surveillance of this lower dose microwave treatment in the UK reported a few adverse events, including blistering, superficial ulceration, and delayed healing (n = 7), based on an online 79-item survey (Citation13). Even though there is limited evidence, this microwave technology could be an effective and safe treatment for cutaneous warts.
The microwave energy vibrates water molecules, generating heat, without damaging DNA. The microwaves do not break the skin, thus lowering the risk of infection (Citation9). Moreover, the device delivers less energy into the skin than typical laser and electrocautery treatments (Citation14) which typically involve vaporization, destruction and necrosis of the tissue. There is no risk of lateral spread/damage with microwaves, as they penetrate to a pre-determined depth into the targeted area.
2.1.4. Microwave therapy versus conventional treatments
Sterilization of the applicator tips is not required, since a new one is used for each patient (Citation9). As a result of the short microwave treatment time (2–5 s), this technology offers clinical advantages over current wart treatments such as cryotherapy and electrosurgery. In contrast to ablative lasers and electro-surgery, microwave treatment does not produce vapor or smoke, eliminating the need for air extraction systems to contain the spread of viral particles (Citation15).
Besides a light debridement of the warty lesion, generally, no other pre-treatment preparation or anesthesia is required. The noninvasive nature of the procedure requires no post-treatment dressing (Citation9). Although this procedure involves some pain, patients do not generally experience pain after the procedure (Citation9).
2.2. Corns
Corns are concentrated areas of hyperkeratosis on the plantar foot surface, usually located on weight-bearing areas of the foot and thought to originate due to locomotive mechanical stress on these areas (Citation16). Corns are associated with significant pain. Incidence is estimated at 10–48% of adults. Cases tend to be chronic, with removal by scalpel providing temporary relief from pain between recurrences. As corns present with similar features to plantar warts, pilot use of the Swift® microwave system was performed in two patients presenting with recalcitrant corns () (Citation16).
2.2.1. Microwave treatment protocol
Routine dosing has not been established. Treatment of Patient 1 used 10 W applied to the corn lesion area for 2 s, repeated at monthly intervals for up to 4 months. The second patient was provided with 8 W for 2 s, repeated 5 times; treatment was repeated at Week 2 and Week 6.
2.2.2. Clinical evidence
Evaluation of the two pilot patients 6 months after the last treatment showed that corns remained in both patients. However, both subjects reported having no pain from the corns at the 6-month follow-up.
2.2.3. Adverse events
No adverse events were reported in these two patients.
2.2.4. Microwave therapy versus conventional treatments
Standard treatments for corns consist chiefly of debridement through mechanical and/or chemical methods (Citation16). The microwave treatments here also used debridement prior to treatment, but the thermal mechanisms of the microwave treatment provided long-term pain relief post-treatment in contrast to the standard treatment. Pain relief was hypothesized to come from thermal alterations of local pain systems, such as local nerve denervation, increased nitric oxide release and/or decreased cytokine IL-1β (Citation16). Podiatric interventions to reduce frictional hyperkeratosis, such as better-fitting and more-padded footwear, may be needed to improve outcomes and reduce recurrence.
2.3. Fungal infection
Treatment of dermatophyte infections primarily involves oral and/or topical formulations of azoles or allylamines, particularly itraconazole and terbinafine. Topical medications applied once or twice daily are the primary treatment for tinea corporis/cruris and tinea pedis/manuum, while daily treatments need to be used for 12 months or more for onychomycosis. Oral antifungals are associated with numerous drug interactions and have the potential for significant risk. Sometimes oral/topical antifungals are not effective, and there are increasing reports of dermatophyte resistance, particularly to terbinafine therapy (Citation17–19).
2.3.1. In vitro data
In a preliminary in vitro study, the effects of a range of continuous and pulsed microwave exposures were examined on cultures of T. rubrum (10 days on agar or 72 h in liquid broth) [Unpublished data, Emblation Ltd]. The microwave applicator was applied to the bottom of the petri dish vertically from the outside to mimic the nail plate and skin. The doses delivered were specific to in vitro use only. The following settings were tested (Citation1): H-50 (50 °C): 15 W for 20 s followed by 5 W for 30 s (Citation2) M-44 (44 °C): 15 W for 13 s followed by 5 W for 20 s (Citation3) L-41 (41 °C): 20 W for 10 s followed by 5 W for 20 s (Citation4) pulsed dose P-50 (50 °C): 20 W for 3 s repeated 3 times followed by 20 W for 5 s repeated 3 times. Halos of inhibition were measured on the agar each day after microwave ablation, and inhibition of growth in the liquid broth was examined. Inhibition was clear and optimal for regimens P-50 (mean area of clearance 87.625 mm2) and H-50 (mean area of clearance 46.75 mm2) for T. rubrum in agar plates. Similar results were seen in liquid broth with H-50 (least optical density after 50 h; however, there was no complete growth inhibition). H50 (50 °C) regimen was optimal for inhibition of T. rubrum in agar and broth. This study suggests that sustained 8 GHz microwave ablation treatment can inhibit the growth of T. rubrum.
Budihardja et al. investigated the effect of microwave radiation on dermatophyte-infected (Trichophyton rubrum, T. rubrum var. nigricans, Trichophyton interdigitale and Microsporum canis) cork and polyethylene sponge shoe insoles (Citation20). The microwave used for this experiment was a 2450 MHz (2.45 GHz) microwave oven (SHARP type R-24W, 800 W). In polyethylene sponge insoles, there was complete growth inhibition of T. rubrum when irradiated at 240 W for 20 s, T. rubrum var. nigricans at 240 W for 40 s, M. canis at 240 W for 70 s, and T. interdigitale at 560 W for 30 s. Similarly, in cork insoles, there was complete growth inhibition of T. rubrum when irradiated at 240 W for 30 s, T. rubrum var. nigricans at 240 W for 40 s, M. canis at 240 W for 40 s, T. interdigitale at 480 W for 50 s. The maximum temperature reached was 35 °C, 50 °C, 50 °C, and 60 °C for T. rubrum, T. rubrum var. nigricans, M. canis, and T. interdigitale, respectively. To achieve complete growth inhibition in T. interdigitale, higher intensities and longer irradiation durations were required (Citation20).
Earlier studies of microwave irradiation of micro-organisms (e.g. Escherichia coli, Staphylococcus aureus) utilized the thermal effects (Citation21,Citation22). However, disinfection of shoes may involve the non-thermal effect of microwaves (Citation20). This microwave irradiation requires only a short duration, protects the material, and has no adverse effect on the skin, as the insoles remained undamaged, validating the non-thermal effect of microwaves (Citation20).
2.3.2. Onychomycosis
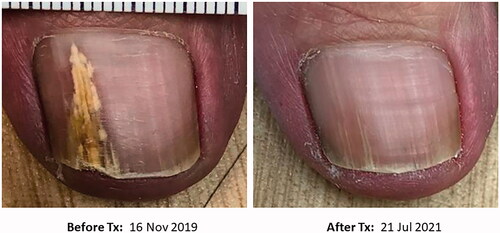
Microwave therapy was used to treat a 78-year-old female patient with toenail dermatophytoma. A positive finding with the Diafactory dermatophyte test strip suggested a dermatophyte infection, but a specific organism identification was not confirmed by culture (Citation23). The patient received 7–9 W of energy for 3 s, repeated 3 times in each location of the infected area. The nails were pretreated by soaking in warm water for 5 min. The patient received a total of 6 treatments with an interval of 6–32 weeks between each treatment session due to COVID-19 restrictions interfering with the intended 4-week interval. shows the successful response of the patient to microwave therapy. The preliminary data suggest that microwave treatment is a consideration for the treatment of onychomycosis. Further studies are underway to evaluate this treatment modality for managing onychomycosis.
2.4. Actinic keratosis
Actinic keratoses (AKs) are a premalignant skin condition that can progress to cutaneous squamous cell carcinoma (cSCC) (Citation24). Cryotherapy is widely used to treat AKs. Topical agents include 5-fluorouracil and imiquimod. Other treatments with devices include laser and photodynamic therapy (Citation25). Many AK therapies necessitate weeks of dedicated application that may cause severe inflammation. Furthermore, many AK patients are in the older cohort, and a lesion-directed treatment would make compliance easier (Citation26,Citation27).
2.4.1. Microwave treatment protocol
The Swift® microwave device is used for the treatment of AKs. The applicator delivers microwave energy to the skin at a diameter of up to 6 mm and a depth of 2–6 mm depending on the treatment protocol (Citation28).
2.4.2. Clinical evidence
Jackson et al. (Citation19) investigated the feasibility and efficacy of microwaves to treat AKs on the forehead, bald scalp, and dorsal hand. This was the first-in-human two-stage study on microwave therapy for AKs. The first stage used specialist instrumentation to determine the dielectric properties of the lesion(s) thus deriving an optimized dose for the microwave device to deliver targeted non-ablative energy (below 50 °C) (Citation29). The second stage was the randomized, controlled trial evaluating the safety and efficacy of the optimized microwave treatment in resolving AKs.
Patients with a minimum of six AK lesions on both the right and left sides of the forehead/scalp/dorsal hand were included in the study. The dielectric properties of the lesion (n = 7 patients), such as the relative permittivity, conductivity, and loss tangent, were measured in stage I. These measurements were used to compute the energy required to raise the tissue temperature into the non-ablative range (Citation29). Thus, the optimized computed microwave treatment was calculated as 5 W for 3 s and repeated delivery three times with a 20-s gap.
In Stage II, patients (n = 11 patients) were randomized to receive treatment (n = 93 AKs) on one side of the forehead/scalp/dorsal hand, and on the other side was the untreated control (n = 86 AKs) (Citation24). The applicator was placed in the center of the lesion for each delivery. Lesions larger than the probe were also treated to evaluate the effectiveness of this treatment. However, this energy caused a lot of discomfort, pain, and scabbing in patients (n = 2). Based on modeling information the study protocol was amended to use 4 W for 3 s for hyperkeratotic ‘thick’ (Olsen scale grade 3) and 3 W for 3 s for nonhyperkeratotic ‘thin’ (grade 1 or 2) lesions. Ten of 11 patients underwent a second treatment session after 28 days. Patients were followed up at 1, 2, 4, 6, 8- and 16-weeks post-treatment.
A significantly larger proportion of treated AKs (78% on day 8 and 90% on day 120 post-treatment) resolved partially or completely with microwave therapy compared to the untreated control AKs (2% on day 8 and 15% on day 120 post-treatment) (p < 0.001) (Citation24). Lesions larger than the applicator tip showed complete resolution of the area under the tip, but there was some persisting AK outside of this treatment area; hence these lesions were reported as a partial response. Thin lesions exhibited a higher response rate than thick lesions (p < 0.001). During treatment, the majority of individuals felt 'moderate' or 'severe' pain, lasting 5 min or less. No patients reported pain after 30 min (Citation24). Microwave therapy appears to be a potential treatment for AK, with 90% resolution of lesions at 120 days post-treatment.
2.4.3. Adverse events
Adverse events were mild, such as erythema (n = 6 patients), flaking (n = 3 patients), and itch (n = 3 patients) (Citation24).
2.4.4. Microwave therapy versus conventional treatments
Microwaves could be a potential lesion-directed therapy used in a physician’s office. It is important to assess the advantages and disadvantages of each available therapeutic option and patient adherence.
2.5. Axillary hyperhidrosis and osmidrosis
Axillary hyperhidrosis (AH, excessive underarm sweating) affects 1.4% of the US population and originates in axillary eccrine glands which are the targets for treatment (Citation30). Axillary osmidrosis is an excessive foul odor arising from bacterial decomposition of excessive axillary apocrine gland hypersecretion, with our without associated AH (Citation31). Axillary hyperhidrosis and osmidrosis can be a significant burden, impairing personal interactions, emotional well-being, and self-esteem. Treatment is similar for both conditions, with lifestyle and behavioral modifications as an initial recommendation. The primary treatment is the use of antiperspirants containing aluminum chloride (Citation32–34). Botox may be an effective treatment with minimal side effects. However, the procedure warrants a qualified health care provider to administer injections (Citation35). Although laser treatment is effective, there may be adverse effects such as hyperpigmentation, hypopigmentation, erythema, edema, pain, blistering, and scarring (Citation36,Citation37). The use of oral anticholinergics such as glycopyrrolate may be considered in more severe, generalized axillary hyperhidrosis. Surgical procedures such as excision, curettage and liposuction, or endoscopic thoracic sympathectomy are used as a last resort in North American treatment due to the risk of scarring and pain, though earlier, more frequent use in Asian populations is noted (Citation32–34).
Due to their non-invasive nature, microwave-based devices are becoming more popular for the treatment of axillary hyperhidrosis. The miraDry microwave device was US FDA cleared in 2011 and is CE-marked in Europe to treat axillary hyperhidrosis (Citation38). The miraDry System is indicated for the treatment of primary axillary hyperhidrosis, and unwanted and permanent axillary hair loss in all colors of Fitzpatrick skin types I-IV (Citation39). The device provides a constant frequency of 5.8 GHz, with energy level controlled by the duration of treatment application from level 1 (shortest period) to level 5 (longest period) (Citation40). An integrated vacuum and cooling system deliver precisely controlled electromagnetic energy through the handheld applicator to the axillary sweat glands while superficial layers of the skin are simultaneously cooled and protected from thermolysis (Citation41). Through suction, the device brings the sweat glands closer to the surface and the energy selectively heats the water-rich dermis and sweat glands using microwaves. After treatment, the sweat glands are not expected to regenerate, so the effects are noticeable almost immediately and long-lasting.
2.5.1. Microwave treatment protocol
The miraDry unit provides a microwave output frequency of 5.8 GHz, and the user can adjust the energy level settings ranging from 1 to 5. There are no approved regimens established to date. Two to three treatments using setting level 3 or higher have typically provided the best outcome rates (). Treatment intervals have varied from 2 weeks, 1 month and 3 months; the 2-week interval may be less desirable, as longer intervals provide a better assessment of the extent of post-operative fibrosis and undertreated areas (Citation42). Pre-treatment with local anesthesia is used to minimize patient discomfort during microwave administration.
Table 2. Clinical outcomes of microwave therapy for axillary hyperhidrosis and osmidrosis.
2.5.2. Clinical evidence
The severity of axillary hyperhidrosis is generally assessed using the subjective patient-rated 4-point Hyperhidrosis Disease Severity Scale (HDSS), where a grade of 1 represents “Underarm sweating is not noticeable and does not interfere with daily activities”, and goes up to grade 4: “Underarm sweating is intolerable and always obstructs daily activities” (Citation30,Citation42,Citation43). Gravimetric sweat assessment was also used to provide a more-objective efficacy outcome (Citation44). A pre-weighed gauze or cotton pad is held in the axilla for 1–5 min, then reweighed, with the difference in weight equally sweat production within the time period elapsed.
Two to three microwave treatments provided efficacy in 89%–90.3% of treated patients with axillary hyperhidrosis, based on achievement of an HDSS score of 1 or 2 at 30 days post-treatment (). A majority of patients found efficacy extended for 6–12 months, and there is some evidence of efficacy continuing to 24 months beyond the end of treatment (Citation45). High efficacy rates were also seen in osmidrosis patients receiving 1 or 2 treatments, as reflected in reduction in odor scores (). Isolated histological exams showed evidence of apocrine gland destruction post-treatment (Citation43,Citation46).
2.5.3. Adverse events
Underarm swelling, redness, and tenderness are common and minor side effects of therapy, typically resolving in 1–4 weeks post-treatment () (Citation30,Citation42,Citation43). Some patients reported prolonged hypotrichosis in the treatment areas (Citation40,Citation46). Rarely, do patients experience numbness or tingling sensation in the upper limbs that persisted for 1–3 months or longer (Citation30,Citation43,Citation46).
2.5.4. Microwave therapy versus conventional treatments
For both conditions, microwave therapy has offered a noninvasive, tolerable, low-risk therapy that may lead to a permanent reduction in symptoms. Effects of microwave therapy may be longer-lasting than Botox injection which can require ongoing injections, and microwaves also avoid the systemic adverse effects of oral anticholinergics (Citation30,Citation32). Cosmetic outcomes of microwave therapy may be more acceptable to patients than surgical treatments which are associated with scarring (Citation31).
2.6. Hidradenitis suppurativa
Hidradenitis suppurativa (HS) is an inflammatory disorder marked by recurrent painful erythematous nodules that usually appear in the axilla or inguinal region (Citation47). Patients experience a significant psychological and functional burden because of the painful lesions (Citation47). Treatments include topical therapy (e.g. clindamycin), systemic medications (e.g. tetracycline), biological agents, surgery, and light-based therapies, with mixed outcomes (Citation48). Immunomodulatory treatments such as tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1) inhibitors, and selective phosphodiesterase-4 inhibitors are among the more recent therapy options (PDE-4) (Citation49). The only biologic approved by the US Food and Drug Administration (FDA) for moderate-to-severe HS is adalimumab (Citation48). The clinical endpoints pertain to pain severity (based on visual analogue scale (VAS)), clinical response (based on hidradenitis suppurativa clinical response score (HiSCR)), and quality of life (based on Dermatology Life Quality Index (DLQI)), and abscess count (Citation50).
2.6.1. Microwave treatment protocol
MiraDry (Miramar Labs Incorporated, Santa Clara, CA) microwave device is a potential treatment for targeting HS lesions, due to thermolysis of hair follicles, eccrine, and apocrine glands in the (hypo)dermis through the heat generated by the microwaves (Citation42,Citation51).
2.6.2. Clinical evidence
Vossen et al. investigated the efficacy and safety of this microwave treatment for mild axillary HS in a randomized intrapatient-controlled trial with 9 mild HS patients, each with a total of 3–5 abscesses or nodules (AN) per axilla with less than one abscess or draining sinus (Trial registered at https://www.clinicaltrials.gov (identifier NCT03238469)) (Citation51). Patients received a single microwave session (5.8 GHz, energy level 5, manufacturer-recommended settings) of one axilla (other axilla acted as control), following the administration of tumescent anesthesia, and followed up for 3 months. The primary outcome measure was to compare the axillary areas from left to the right using the HiSCR. The definition of responders was: (i) reduction in ANs, (ii) no increase in the number of abscesses, and (iii) no increase in the number of draining fistulas from baseline (identifier NCT03238469). Secondary outcomes assessed include pain score per axilla, treatment satisfaction, and hair follicle count (average number of hair-containing follicles in 3 fields of 1 cm2 assessed by dermoscopy).
The authors hypothesized that ablative therapy could improve the clinical symptoms of HS by lowering the number of hair follicles and reducing the inflammatory infiltrate and have a long-term effect due to the permanent removal of hairs and sweat glands (Citation51). However, the interim analysis observed negative clinical results.
The nonselective targeting of the dermal zone, instead of a specific structure, may have contributed to the unsatisfactory trial results. While microwaves can eliminate HS lesions, the treatment triggers an intense inflammatory response that extends beyond the initially diagnosed lesions.
2.6.3. Adverse events
The study reported a serious adverse event, where one patient developed cellulitis of the upper arm, requiring antibiotic treatment (Citation51).
2.6.4. Microwave therapy versus conventional treatments
The risks have outweighed the benefits, and clinicians feel that microwaves cause more harm in HS patients (Citation51). Based on similar reports from other clinics, the value of microwave ablative therapy for HS needs to be further evaluated.
3. The future of microwave-based therapy in dermatology
Although microwave treatment provides a new therapeutic option with favorable effects for patients suffering from different dermatological conditions, it is necessary to weigh the benefits against the risks. It is shown to be effective for cutaneous warts, actinic keratosis, and axillary hyperhidrosis. However, patients with hidradenitis suppurativa had symptoms worsened with microwaves, and the trial was terminated. Currently, the two main microwave devices provide alternate mechanisms of action: specifically, Miradry operates at 5 GHz and penetrates deeper to provide ablative therapy, while the Swift® system uses 8 GHz for sub-ablative hyperthermic stimulation of apoptosis and immune response. More work is needed to better understand the mechanisms of action of microwave ablative and non-ablative therapies, and to determine other possible indications for use.
Preliminary in vitro studies suggest that microwaves may be effective for treating fungal infections; furthermore, we have reported the first human case of onychomycosis to be effectively treated using microwave therapy. Further studies are in progress to evaluate microwaves for managing onychomycosis and condyloma acuminatum (anogenital warts) where the lesions share an HPV origin. More randomized, well-conducted, high-quality clinical trials with larger patient groups and longer follow-up periods are required to evaluate the clinical benefits of microwaves in treating dermatological conditions.
Pharmacoeconomic analysis will be required once data becomes available from Phase II/III trials, as cost-effectiveness will be of interest to physicians, patients and insurance companies. As a new therapy, microwaves do not yet have coverage in national health programs. Microwave treatment costs will include device costs and provider fee costs. As an ‘off-formulary’ treatment, microwave treatment appears to provide a feasible option for patients who are intolerant of cryotherapy/salicylic acid. For recalcitrant wart treatment, a significant number of visits/treatments may be required with cryotherapy to provide a resolution; based on the data presented here, a high proportion of recalcitrant warts are cleared with approximately 3 treatments. In contrast to cryotherapy and microwaves, salicylic acid has few, low costs associated with use but requires high patient compliance for success, which could give microwave therapy an advantage in clearance and patient satisfaction. These arguments suggest a cost analysis of microwave use versus standard therapies is warranted for warts treatments and could be expanded to actinic keratosis. These considerations will feature in determining the extent to which microwave therapy becomes a mainstream therapy for recalcitrant warts and actinic keratosis.
Acknowledgements
We thank Dr. Lovleen Tina Joshi and Dr. Lee P Hutt for the preliminary in vitro data on the use of microwaves to inhibit dermatophytes. We also extend our thanks and acknowledge Dr. Ivan Robert Bristow (Private practice, Lymington, UK) and Dr. Christopher Webb of the Podiatry Centre, Portsmouth, UK, for the preliminary patient data on the successful use of microwaves to treat toenail dermatophytoma.
Disclosure statement
AKG, MV, LTJ, and EAC have no conflicts of interest to declare.
References
- Tang J. Unlocking potentials of microwaves for food safety and quality. J Food Sci. 2015;80(8):E1776–1793.
- Gartshore A, Kidd M, Joshi LT. Applications of microwave energy in medicine. Biosensors. 2021;11(4):96.
- Vrba J. Medical applications of microwaves. Electromagn Biol Med. 2005;24(3):441–448.
- Frey B, Weiss EM, Rubner Y, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia. 2012;28(6):528–542.
- Gibbs S, Harvey I. Topical treatments for cutaneous warts. Cochrane Database Syst Rev. 2006;3:CD001781.
- Ringin SA. The effectiveness of cutaneous wart resolution with current treatment modalities. J Cutan Aesthetic Surg. 2020;13(1):24–30.
- Sterling JC, Gibbs S, Haque Hussain SS, et al. British association of dermatologists’ guidelines for the management of cutaneous warts 2014. Br J Dermatol. 2014;171(4):696–712.
- Kwok CS, Holland R, Gibbs S. Efficacy of topical treatments for cutaneous warts: a meta-analysis and pooled analysis of randomized controlled trials. Br J Dermatol. 2011;165(2):233–246.
- Hochstein A. Microwaves: a painless, efficient new treatment for plantar warts. Podiatry Manag. 2020;39(2):63–64,66.
- Epifano I, Conley M, Stevenson A, et al. Short Oral Presentation. Research and Education for HPV Elimination Conference presented at: International Papillomavirus Conference & Basic Science, Clinical and Public Health; 2021. Nov 15.
- Bristow I, Lim WC, Lee A, et al. Microwave therapy for cutaneous human papilloma virus infection. Eur J Dermatol. 2017;27(5):511–518.
- Bristow IR, Webb C, Ardern-Jones MR. The successful use of a novel microwave device in the treatment of a plantar wart. Case Rep Dermatol. 2017;9(2):102–107.
- Bristow I, Joshi S, Williamson J, et al. Post marketing surveillance for microwave treatment of plantar and common warts in adults. medRxiv. 2022. DOI:10.1101/2022.02.08.22270290
- How Safe Are Microwaves? – Swift. [cited 2022 Mar 8]. Available from: https://www.treatwithswift.com/global/for-patients/duplicate-of-how-safe-are-microwaves.html.
- Karsai S, Däschlein G. Smoking guns”: hazards generated by laser and electrocautery smoke. J Dtsch Dermatol Ges. 2012;10(9):633–636.
- Bristow IR, Webb CJ. Successful treatment of hard corns in two patients using microwave energy. Case Rep Dermatol. 2020;12(3):213–218.
- Gaurav V, Bhattacharya SN, Sharma N, et al. Terbinafine resistance in dermatophytes: time to revisit alternate antifungal therapy. J Mycol Med. 2021;31(1):101087.
- Saunte DML, Hare RK, Jørgensen KM, et al. Emerging terbinafine resistance in trichophyton: clinical characteristics, squalene epoxidase gene mutations, and a reliable EUCAST method for detection. Antimicrob Agents Chemother. 2019;63(10):e01126–19.
- Singh A, Masih A, Khurana A, et al. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses. 2018;61(7):477–484.
- Budihardja D, Mayser P. The effect of microwave irradiation on the vitality of various dermatophytes. Mycoses. 2014;57(4):209–213.
- Fitzpatrick JA, Kwao-Paul J, Massey J. Sterilization of bacteria by means of microwave heating. J Clin Eng. 1978;3(1):44–47.
- Yeo CB, Watson IA, Stewart-Tull DE, et al. Heat transfer analysis of Staphylococcus aureus on stainless steel with microwave radiation. J Appl Microbiol. 1999;87(3):396–401.
- JNC Corporation. Diafactory Tinea Unguium (Dermatophyte Test Strip Package Insert). [cited 2022 May 31]. Available from: https://www.fiveminutefungus.com/_files/ugd/421f69_f2b1048a8f5f491db3e5b46ac4e14af9.pdf.
- Jackson DN, Hogarth FJ, Sutherland D, et al. A feasibility study of microwave therapy for precancerous actinic keratosis. Br J Dermatol. 2020;183(2):222–230.
- Foley K, Gupta AK, Martin G, et al. Topical treatments and photodynamic therapy for actinic keratosis of the face and scalp. Cochrane Database Syst Rev. 2019;10:CD013452.
- Poggi G, Tosoratti N, Montagna B, et al. Microwave ablation of hepatocellular carcinoma. World J Hepatol. 2015;7(25):2578–2589.
- Wang T, Lu XJ, Chi JC, et al. Microwave ablation of hepatocellular carcinoma as first-line treatment: long term outcomes and prognostic factors in 221 patients. Sci Rep. 2016;6:32728.
- Ogura Y, Naito H, Tsurukawa T, et al. Microwave hyperthermia treatment increases heat shock proteins in human skeletal muscle. Br J Sports Med. 2007;41(7):453–455.
- Mikhail AS, Negussie AH, Graham C, et al. Evaluation of a tissue-mimicking thermochromic phantom for radiofrequency ablation. Med Phys. 2016;43(7):4304–4311.
- Hong HC-h, Lupin M, O'Shaughnessy KF. Clinical evaluation of a microwave device for treating axillary hyperhidrosis. Dermatol Surg. 2012;38(5):728–735.
- Chen SQ, Wang TT, Zhou Y, et al. Comparison of long-term effectiveness and safety of microwave and surgery in the treatment of axillary osmidrosis: a single-center retrospective study. Dermatol Surg. 2022;48(1):126–130.
- Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669–680.
- Solish N, Bertucci V, Dansereau A, et al. A comprehensive approach to the recognition, diagnosis, and severity-based treatment of focal hyperhidrosis: recommendations of the Canadian Hyperhidrosis Advisory Committee. Dermatol Surg. 2007;33(8):908–923.
- Morioka D, Nomura M, Lan L, et al. Axillary osmidrosis: past, present, and future. Ann Plast Surg. 2020;84(6):722–728.
- Singh S, Davis H, Wilson P. Axillary hyperhidrosis: a review of the extent of the problem and treatment modalities. Surgeon. 2015;13(5):279–285.
- Goldman A, Wollina U. Subdermal Nd-YAG laser for axillary hyperhidrosis. Dermatol Surg. 2008;34(6):756–762.
- Aydin F, Pancar GS, Senturk N, et al. Axillary hair removal with 1064-nm Nd:YAG laser increases sweat production. Clin Exp Dermatol. 2009;35(6):588–592.
- US FDA. miraDry System – K103014 510(K) Summary. 2011. [cited 2022 May 31]. Available from: https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K103014.
- US FDA. miraDry System – K180396 510(K) Summary. 2018. [cited 2022 May 31]. Available from: https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K180396.
- Scuderi S, Manoharan P, Lim D, et al. A survey of patient satisfaction with use of microwave device for axillary hyperhidrosis. Australas J Dermatol. 2017;58(2):126–129.
- Wade R, Rice S, Llewellyn A, et al. Interventions for hyperhidrosis in secondary care: a systematic review and value-of-information analysis. Health Technol Assess. 2017;21(80):1–280.
- Glaser DA, Coleman WP, Fan LK, et al. A randomized, blinded clinical evaluation of a novel microwave device for treating axillary hyperhidrosis: the dermatologic reduction in underarm perspiration study. Dermatol Surg. 2012;38(2):185–191.
- Lee SJ, Chang KY, Suh DH, et al. The efficacy of a microwave device for treating axillary hyperhidrosis and osmidrosis in Asians: a preliminary study. J Cosmet Laser Ther. 2013;15(5):255–259.
- Hund M, Kinkelin I, Naumann M, et al. Definition of axillary hyperhidrosis by gravimetric assessment. Arch Dermatol. 2002;138(4):539–541.
- Lupin M, Hong HCH, OʼShaughnessy KF. Long-term efficacy and quality of life assessment for treatment of axillary hyperhidrosis with a microwave device. Dermatol Surg. 2014;40(7):805–807.
- Chang YY, Chen CH, Hui RCY, et al. A prospective clinical and histologic study of axillary osmidrosis treated with the microwave-based device. Dermatol Sin. 2015;33(3):134–141.
- Aleisa A, Feingold DS. Development of inflammatory nodules and scarring mimicking hidradenitis suppurativa after treatment of axillary hyperhidrosis using a microwave-based energy device. JAAD Case Rep. 2020;6(10):999–1000.
- Amat-Samaranch V, Agut-Busquet E, Vilarrasa E, et al. New perspectives on the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis. 2021;12:20406223211055920.
- Thorlacius L. Severity staging of hidradenitis suppurativa: is hurley classification the answer? Br J Dermatol. 2019;181(2):243–244.
- Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa: a cochrane systematic review incorporating GRADE assessment of evidence quality. Br J Dermatol. 2016;174(5):970–978.
- Vossen ARJV, van Huijkelom MAPC, Nijsten TEC, et al. Aggravation of mild axillary hidradenitis suppurativa by microwave ablation: results of a randomized intrapatient-controlled trial. J Am Acad Dermatol. 2019;80(3):777–779.