Abstract
Hyperhidrosis can significantly curtail patient quality of life, from debilitating physical symptoms to social stigmatization and reduced life opportunities. Current treatments often prove unsatisfactory, especially in sufferers of generalized hyperhidrosis. In this open trial, we present the case of a refractory generalized hyperhidrosis treated with cannabinoids. We found a remarkable reduction in the volume of sweat and an improvement to the patient’s quality of life using this novel low-cost and low-impact approach.
Introduction
Hyperhidrosis describes sweating in excess of that required for physiological thermoregulation. Over 90% of hyperhidrosis occurs idiopathically as primary hyperhidrosis or due to underlying diseases as secondary hyperhidrosis (Citation1,Citation2). Two types exist, a focal type affecting primarily the palmoplantar or axillary regions, and a less frequent generalized type presenting as diffuse sweating of the entire integument. The prevalence of primary hyperhidrosis varies between 3% and 5% in the USA and reaches up to 16% in other Western countries, such as 16.3% in Germany, with over 80% showing a multifocal pattern, though, cases are likely underreported (Citation3–5). Therapies include topical aluminum chloride, oral anticholinergics, iontophoresis, botulinum toxin injection, sweat gland excision, and thoracic sympathectomy (Citation1). Unfortunately, especially in generalized hyperhidrosis, there is often no adequate therapy available, with detrimental consequences for patient’s quality of life (Citation6).
In our literature search for alternative treatments, we identified multiple unscientific and anecdotal sources claiming that cannabis can inhibit sweating. Our search of the medical literature revealed no evidence of a treatment attempt using cannabinoids, and thus, we initiated our study of one case with refractory generalized hyperhidrosis treated with cannabinoids from March to May 2021. We observed a marked reduction in measured sweat and a significant improvement in the patient’s psychological well-being. We conclude that, potentially, cannabinoids represent an effective therapeutic agent for hyperhidrosis and are worthy of further high-quality clinical investigation.
Case report
We report of a 28-year-old male patient without other preexisting conditions who suffered from generalized hyperhidrosis since adolescence. Symptoms focused on the forehead, chest, and neck. All prior therapies as topical aluminum chloride, oral anticholinergics, iontophoresis, and botulinum toxin injection failed to produce improvements. The disease caused a significant reduction in quality of life, leading to a depressive state. Surgery was ruled out due to the significant integumental involvement.
Methods
We searched the databases PubMed, Cochrane Library, Embase, and Web of Science to retrieve all relevant articles with the search terms ‘hyperhidrosis’’, ‘cannabinoids,’ ‘tetrahydrocannabinol,’ and ‘sweat’ on 30 June 2021. There were no restrictions on language or publication date. There was a lack of literature, so we developed a new study protocol for this case. This included a weekly assessment of the health-related quality of life (HRQoL) using an assortment of questionnaires, as well as three separate sweat measurements at four-week intervals each to quantify transpiration. To detect body temperature, we used a thermographic camera.
Evaluation of life quality
Prior to initiation of the cannabinoid therapy, the patient performed a subjective assessment of distress using the Dermatology Life Quality Index (DLQI), EQ-5D-3L, EQ-VAS, Hyperhidrosis Disease Severity Scale (HDSS), and Hyperhidrosis Quality of Life Index (HidroQoL). The patient answered these each week.
Measurement of the amount of sweat
To quantify the amount of sweat, we performed three standardized examinations at 4-week intervals. First, the patient consumed hot tea; immediately, we collected the sweat on the forehead, the cheeks, the chest, and the neck using weighed cellulose round filters (Qualitative filter paper 413, Ø 5.5 cm, VWR® International bvba, Leuven, Belgium). Then we re-weighed the filters at room temperature. We repeated this measurement after the patient climbed seven floors. All weight measurements were taken by a precision balance (Sartorius analytical balance BP221S, Sartorius AG, Göttingen, Germany). The first measurement took place before cannabinoid consumption, and the second and third measurements under the same conditions, at four and eight weeks of treatment, respectively.
Thermoregulation
Changes in skin temperature during the examination were measured by a thermographic camera (Flir C3-X, Teledyne FLIR LLC, Wilsonville, OR). We took the first measurement prior to stimulation, then 5 minutes after drinking the hot beverage or exercising.
Cannabinoid intervention
In the first month, the patient received dronabinol (tetrahydrocannabinol [THC]) drops 25 mg/ml in increasing doses up to six drops (5 mg) three times daily. In the second month, the patient inhaled an average of 0.5 g medical cannabis buds (Pedanios 8% THC, 8% CBD) for two weeks and for two weeks an average of 0.5 g medical cannabis buds (Pedanios 20% THC, 1% CBD) daily using a vaporizer.
Results
The patient’s subjective and objective well-being improved throughout our trial. The DLQI decreased from eighteen to eight points (55.6% decrease), representing an improvement in the perceived discomfort from severe to moderate (). The EQ-5D-3L improved by 2 points – a 25% increase. Similarly, the EQ VAS increased by 20 points (40% increase). The HDSS decreased from four to two – an 80% reduction in distress. The HidroQoL also reflected this, dropping by seventeen points (63% decrease), a reduction of hyperhidrosis-mediated impairment on daily life from a large to a small effect. The amount of measured sweat also decreased during the course of therapy (). Forehead transpiration nearly ceased, and other areas showed an 80 to 90% reduction in the amount of sweat post-stimulation (). Interestingly, chest transpiration increased post-stimulation and decreased by a smaller margin compared to the other areas investigated.
Figure 1. Change in sweat volume in a monthly interval after drinking tea (a) and after exercise (b).
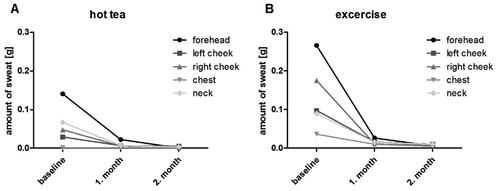
Table 1. Assessment of the quality of life by using different questionnaires in a monthly interval.
Table 2. Measurement of the amount of sweat in grams and percent over the course of three months.
Discussion
Based on the variable prevalence of primary hyperhidrosis in western countries, we assume many cases go unreported. There is a need for effective low-impact hyperhidrosis therapy, especially in the generalized and more therapy-resistant variants (Citation6,Citation7).
Within recent years, cannabinoids have gained acceptance as effective treatments in the medical armamentarium, although research is limited by legal regulations despite increasing legalization and social acceptance (Citation8,Citation9). Although numerous sources outside of evidence-based medicine report on the treatment of excessive sweating with cannabinoids (Citation10), there is an acute lack of clinical studies corroborating this effect, as we have found. In some palliative patients, cannabinoids, such as nabilone and dronabinol, were effective in reducing paraneoplastic night sweats and improving patient quality of life (Citation11,Citation12). To consolidate the literature, we conducted this scientific investigation of cannabinoids therapy in a case of therapy-refractive primary hyperhidrosis.
Our patient reported a reduction in symptoms shortly after initiation of therapy, reflected by improvements in quality-of-life-scoring systems over a two-month course. We measured the amount of sweat before and during therapy, using the effect of hot tea and exercise as transpiratory stimuli. Our results show a significant decrease in sweat recovered from the investigated skin areas. Moreover, this effect appears to be dose-dependent, as the effect enhanced with the gradual increase of the daily dose during the course of therapy.
Although cannabinoid therapy indications are steadily increasing, our understanding of the underlying pharmacodynamics is still incomplete. Cannabinoids bind to G protein-coupled cannabinoid receptors CB1 and CB2. CB1 receptors are primarily distributed in the peripheral and central nervous systems, and among other effects, inhibit presynaptic neurotransmission. The CB2 receptors, on the other hand, are localized predominantly in the peripheral immune and nervous system and have an inhibitory effect. We speculate one effect of cannabinoids in primary hyperhidrosis is presynaptic inhibition of acetylcholine release, and thus, diminished sweat secretion. The pathophysiology of hyperhidrosis does not seem to involve eccrine glands directly, as evidenced by a lack of histopathological changes. Instead, data points to neuronal overexcitations in the context of a more involved autonomic dysfunction (Citation1,Citation13). These hypotheses require validation.
In summary, we report a case of precisely analyzed effects of cannabinoid therapy in generalized hyperhidrosis. We believe cannabinoids hold potential as a low side-effect and well-tolerated therapy, especially in refractory cases of hyperhidrosis. This reflects not only in the reduced perspiration, but also in the significant improvement in the participant’s quality of life. We hope this informative case will provide the basis for larger randomized studies to substantiate the observed value of this novel therapeutic approach beyond our brief report.
Author contributions
Till Kaemmerer, Benjamin Maximilian Clanner-Engelshofen: Conceptualization, Methodology.
Till Kaemmerer: Data curation, Writing- Original draft preparation.
Till Kaemmerer, Benjamin Maximilian Clanner-Engelshofen: Visualization, Investigation.
Till Kaemmerer, Benjamin Maximilian Clanner-Engelshofen, Tony Lesmeister, Markus Reinholz, Lars Einar French: Supervision, Critical review and Editing.
Till Kaemmerer, Tony Lesmeister: Writing- Reviewing and Editing.
Acknowledgments
The patient in this manuscript has given written informed consent to publication of the case details.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: etiology and clinical work-up. J Am Acad Dermatol. 2019;81(3):657–666.
- Lenefsky M, Rice ZP. Hyperhidrosis and its impact on those living with it. Am J Manag Care. 2018;24(23):S491–S495.
- Doolittle J, Walker P, Mills T, et al. Hyperhidrosis: an update on prevalence and severity in the United States. Arch Dermatol Res. 2016;308(10):743–749.
- Grabell DA, Hebert AA. Current and emerging medical therapies for primary hyperhidrosis. Dermatol Ther (Heidelb). 2017;7(1):25–36.
- Augustin M, Radtke MA, Herberger K, et al. Prevalence and disease burden of hyperhidrosis in the adult population. Dermatology. 2013;227(1):10–13.
- McConaghy JR, Fosselman D. Hyperhidrosis: management options. Am Fam Physician. 2018;97(11):729–734.
- Schollhammer M, Brenaut E, Menard-Andivot N, et al. Oxybutynin as a treatment for generalized hyperhidrosis: a randomized, placebo-controlled trial. Br J Dermatol. 2015;173(5):1163–1168.
- Zarrabi AJ, Frediani JK, Levy JM. The state of cannabis research legislation in 2020. N Engl J Med. 2020;382(20):1876–1877.
- Friedman D, French JA, Maccarrone M. Safety, efficacy, and mechanisms of action of cannabinoids in neurological disorders. Lancet Neurol. 2019;18(5):504–512.
- Chaboya-Hembree J. Palmar hyperhydrosis and medical marijuana. 2021. [cited 06 September 2021]. Available from: https://www.medicalmarijuana.com/medical-marijuana-treatments-cannabis-uses/palmar-hyperhydrosis-and-medical-mrijuana/
- Maida V. Nabilone for the treatment of paraneoplastic night sweats: a report of four cases. J Palliat Med. 2008;11(6):929–934.
- Carr C, Vertelney H, Fronk J, et al. Dronabinol for the treatment of paraneoplastic night sweats in cancer patients: a report of five cases. J Palliat Med. 2019;22(10):1221–1223.
- Bovell DL, Clunes MT, Elder HY, et al. Ultrastructure of the hyperhidrotic eccrine sweat gland. Br J Dermatol. 2001;145(2):298–301.

