Abstract
Background
Clinical trials have shown promising results for interleukin-23 inhibitors in the treatment of psoriasis. The drugs have been used in clinical practice since 2017.
Objective
To investigate the drug survival and effectiveness of interleukin-23 inhibitors in the treatment of psoriasis and psoriatic arthritis (PsA) in a real-world setting.
Methods
The study was a retrospective analysis of patients treated with either guselkumab, tildrakizumab, or risankizumab at the Department of Dermatology, Aarhus University Hospital, during the period from June 11 2018, to July 14 2021.
Results
A total of 80 patients were included. During the study, 19 patients discontinued treatment with an interleukin-23 inhibitor, and mean treatment duration (SD) was 61.4 weeks (43.7). Seventy-six patients (95%) had previous use of ≥1 biologic. One-year drug survival was 81.0%. Among patients, 64.3% achieved a Psoriasis Area and Severity Index (PASI) ≤ 2 at weeks 12–17; 61.3%, at weeks 40–60. There was no statistically significant difference between the drugs regarding the chance of achieving PASI ≤ 2 (p>.05). Twenty-two patients (27.5%) had PsA. Among these, 40.9% and 36.4% achieved complete remission and partial remission, respectively.
Conclusions
Interleukin-23 inhibitors appear to have high and similar drug survival and effectiveness in patients with difficult-to-treat psoriasis and PsA.
Introduction
Knowledge of the complex pathogenesis of psoriasis has advanced much over the past decade. Unearthing the pivotal roles of interleukin (IL)-17 and IL-23 in the pathogenesis of both psoriasis and psoriatic arthritis (PsA) has sparked development of biological drugs targeted against them (Citation1,Citation2). IL-23 is composed of the subunits p19 and p40 (Citation3). The p40 subunit is also a subunit of IL-12, whereas p19 is exclusive to IL-23. The first drug targeting IL-23 was ustekinumab which inhibits both the IL-23-driven Th17-pathway and the IL-12-driven Th1-pathway by binding to p40 (Citation4). IL-23 is believed to play a bigger role than IL-12 in the pathogenesis, increasing the focus on drugs targeted specifically at the p19 subunit and consequently only blocking IL-23 signaling (Citation5–7).
Since 2017, three IL-23 inhibitors have been approved for treatment of moderate to severe psoriasis: guselkumab (2017), tildrakizumab (2018), and risankizumab (2019) (Citation8–13). Guselkumab was also approved for treatment of PsA in 2020, while risankizumab has just recently been approved for this indication (2021/2022). Data from clinical trials show promising results for these three IL-23-inhibitors; however, efficacy and safety profiles in clinical trials are known to differ from real world experiences, and especially the effectiveness toward PsA needs to be further evaluated (Citation14–21). The drugs have now been used by the Department of Dermatology at Aarhus University Hospital since guselkumab became available for clinical use in Denmark in 2018. This study aims to investigate the drug survival and effectiveness of IL-23 inhibitors in the treatment of psoriasis and PsA in a real-world setting.
Methods
Study design, study population, and data collection
This is a retrospective, observational study. All patients with dermatologically confirmed psoriasis treated with either guselkumab, tildrakizumab, or risankizumab at the Department of Dermatology, Aarhus University Hospital, Denmark, in the period from June 11 2018, to July 14 2021, were enrolled in the study. Patients were treated according to national treatment guidelines issued by the Danish Medicines Council governing the choice of biologic according to a prioritized list. Patients who had received an IL-23 inhibitor as part of a clinical trial were excluded (n = 2). Patients who received treatment with two different IL-23 inhibitors during the study period were included as two separate treatment series.
Data were collected through electronic patient journals and DERMBIO, a database of patients undergoing biological treatment for psoriasis in Denmark (Citation22). The last day of data collection was September 10 2021. We retrieved data on demographics, medical history, previous treatments, Dermatology Life Quality Index (DLQI), and Psoriasis Area and Severity Index (PASI). Furthermore, reasons for discontinuing treatment, start date, and last day of anti-IL-23 treatment or the last registered day of continuous treatment were noted.
Outcome measures
Primary endpoints
The primary endpoints were (i) drug survival, (ii) reasons for discontinuation, (iii) clinical effectiveness on psoriasis, as assessed by the percentage of patients achieving an absolute PASI ≤ 2, and (iv) clinical effectiveness against PsA. Drug survival was defined as the time from drug initiation to discontinuation due to side effects; lack or loss of effect on either skin, joints, or both skin and joints; or discontinuation due to other reasons. If treatment was still ongoing on the last day of data collection, the patients were censored in the survival analysis on that date.
Clinical effectiveness against PsA was assessed through the patients’ electronic health records. PsA response was categorized as: (1) unknown; (2) remission, defined as no inflammatory joint pain and no clinically active inflammation; (3) partial remission, defined as limited inflammatory activity with a need for non-steroidal anti-inflammatory drugs (NSAIDs) and/or sporadic use of steroid injections; and (4) failure, defined as disease activity with joint swelling and/or disease progression on X-ray or magnetic resonance imaging (MRI).
Secondary endpoints
The secondary endpoints were the percentage of patients achieving a 100%, ≥90%, or ≥ 75% reduction in PASI from baseline (PASI100, PASI90, and PASI75). Additionally, the proportion of patients achieving an end DLQI of 0 or 1 was assessed (DLQI 0/1). Patients with a baseline PASI of 0 (n = 7) were excluded from the calculations of percentage PASI change as they would only be able to experience worsening of their skin disease.
Besides the evaluation at the end of follow-up, PASI ≤ 2 and ΔPASI90/100 response rates were also evaluated at weeks 12–17 and 40–60.
Statistical analysis
Descriptive statistics were used to summarize data. Categorical variables were described as absolute numbers and percentage proportions. Continuous variables were described with means ± standard deviation (SD). Only patients with available data were included for analysis. Fisher’s exact test was used to test the association between selected endpoints (PASI ≤2 and PASI90 and 100 response rates) and drug type, and the association with potential predictors of treatment response (previous use of > 2 biologics, body mass index (BMI)≥ 30, and baseline PASI ≥ 10). Drug survival was estimated with Kaplan–Meier survival curves. Log-rank tests were used to test the association of the above-mentioned parameters with drug survival. p Values<.05 were considered significant. Statistical analyses were made with Stata/MP 17.0 (StataCorp LLC, College Station, TX).
Ethical considerations
The project was reviewed and approved by Aarhus University Hospital’s administration which allowed access to patient health records. DERMBIO is considered to be part of the clinical registrations for patients receiving treatment with biologics at Aarhus University Hospital. Register studies require no ethics committee approval in Denmark. Personal data were protected according to the Data Protection Act and the General Data Protection Regulation. The study was conducted in accordance with the Declaration of Helsinki.
The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines (Citation23).
Results
Patient characteristics
Study population characteristics are summarized in . Eighty patients were included in the study of whom 62.5% were male. Age varied between 18 and 80 years (mean 48.1 ± 13.6). Most patients were overweight with a mean BMI of 31.2 ± 6.5 kg/m2. Seventy-six patients (95%) had previous inadequate response or intolerance to ≥ 1 biologic, the most common being an anti- tumor necrosis factor alpha (TNF-α) (86.3%). Prior to initiating anti-IL-23 treatment, 92.5% had received methotrexate. Many patients had psoriasis-related joint problems, as 27.5% had PsA and 22.5% had psoriatic arthropathy. Both diagnoses were verified by rheumatologists. At baseline, PASI was mean 8.0 (6.4); DLQI, mean 8.3 (7.7). Baseline characteristics were comparable between the drugs on most parameters. However, a lower percentage of patients receiving tildrakizumab were female (14.3%); guselkumab (41.4%), risankizumab (43.2%). More patients treated with guselkumab had PsA (44.8%); tildrakizumab (14.3%), risankizumab (18.8%). Furthermore, the drugs differed in baseline PASI, which was lower for risankizumab-treated patients (5.8) than for those treated with tildrakizumab and guselkumab (both 9.7).
Table 1. Characteristics of the Study Populationa.
Drug survival
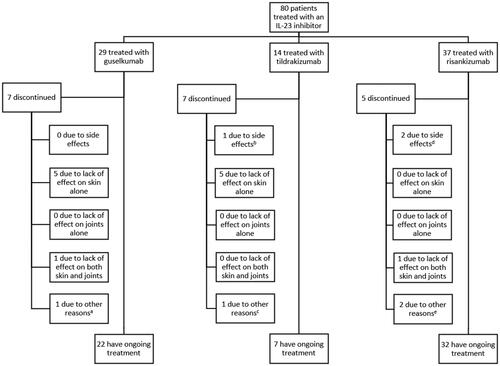
presents the patient distribution according to drug type. Of the 80 included patients, 29 received treatment with guselkumab; 14, tildrakizumab; 37, risankizumab. Mean (SD) treatment duration was 61.4 weeks (43.7) for the drugs combined. For risankizumab, it was 30.3 weeks (17.1); for tildrakizumab, 64.3 weeks (23.8); for guselkumab, 99.8 weeks (44.2). Nineteen patients (23.8%) stopped treatment with an IL-23 inhibitor during the study. Seven patients (24.1%) discontinued treatment with guselkumab, seven (50.0%) stopped treatment with tildrakizumab, while five (13.5%) had discontinued treatment with risankizumab at the end of follow-up.
Figure 1. Distribution of patients according to drug type and reason for discontinuation. IL-23 indicates interleukin-23. aTreatment was stopped as rheumatologists suspected psoriatic arthritis and treatment with ixekizumab was initiated instead. bThe patient stopped treatment due to an injection site reaction, headaches, and abdominal pain. cThe patient stopped treatment despite showing a good response and was switched to 150 mg risankizumab as the patient was receiving tildrakizumab in double dosage due to incomplete clearance at 100 mg. dOne patient had an injection site reaction and another experienced exanthema on the trunk and extremities following the 2nd injection and later also developed a petechial rash on arms and thorax. eOne stopped due to a desire for pregnancy and one stopped due to reevaluation of the psoriasis diagnosis.
shows the overall drug survival rate during the study period. After 31 weeks, drug survival fell below 90% (89.9%). At 52 weeks, overall drug survival was 80.96%. The patient with the longest available survival time received an IL-23 inhibitor for 165 weeks; at this point, overall survival was estimated to be 62.8%.
Figure 2. Kaplan–Meier estimates of (A) overall drug survival and (B) drug survival for each IL-23 Inhibitor. Patients who were still receiving treatment on the last day of follow-up were censored (illustrated with a tick mark). aThe curve for risankizumab drops to 0% survival at 69 weeks. At 69 weeks, all but one patient had either been censored or stopped treatment. The remaining patient discontinued treatment after 69 weeks. Risankizumab is the newest of the drugs, and therefore many patients in this group have been censored.
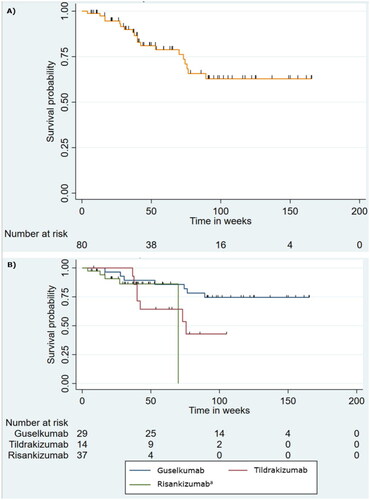
Separate Kaplan–Meier survival curves for the three drugs are presented in . One-year survival was 89.3% (guselkumab), 64.3% (tildrakizumab), and 86.2% (risankizumab). A log-rank test found no statistically significant difference between the drugs (p=.11). Median survival was reached for tildrakizumab at 74.4 weeks, while median drug survival was not reached for guselkumab and risankizumab.
Overall survival primarily dropped until week 72, while it remained almost constant from week 72 onwards (). The drop is mainly due to the drop in the survival for tildrakizumab () as the curve for guselkumab ran a more constant course. Likewise, survival for risankizumab remained constant throughout follow-up until week 69 (see footnote to ).
Three possible predictors of drug survival were analyzed; however, neither baseline PASI ≥ 10 (p= .64), BMI ≥ 30 (p= .62) nor previous treatment with >2 biologics (p= .46) was significantly associated with survival.
The drugs were generally well-tolerated as adverse events led to discontinuation in only three patients.
PASI response
Throughout the study period, mean (SD) PASI decreased from 8.0 (6.4) at baseline to 2.2 (3.4) at the end of follow-up. Among patients with available follow-up data, 71.6% achieved an absolute PASI ≤ 2 (). The corresponding percentages were 76.9% for patients treated with risankizumab, 71.4% for patients treated with guselkumab, and 61.5% for patients treated with tildrakizumab. However, the difference between the drugs did not reach significance (p=.64). Overall, 41.8% achieved a PASI100 response and 50.9% reached PASI90 (). Similar effectiveness was seen when calculating PASI90 and PASI100 responses for each of the three drugs. PASI90 response rates were 48.0%, 50.0%, and 55.6% for guselkumab, tildrakizumab and risankizumab, respectively. PASI100 response rates were 44.0%, 41.7%, and 38.9%, respectively.
Table 2. Effectiveness overall and for each drug at the end of follow-up and weeks 12–17 and 40–60a.
No association was found between attaining PASI ≤ 2, PASI90, or PASI100, and having previously received >2 biologics, having a BMI ≥ 30, or baseline PASI ≥ 10 (p>.05).
To estimate the effectiveness of the drugs after similar treatment duration, the endpoints were calculated for patients with data available at weeks 12–17 and 40–60 (). At weeks 12–17, overall PASI90 and 100 response rates were 45.7% and 25.7%, respectively, while both response rates were 44.4% at weeks 40–60. PASI ≤ 2 was achieved by 64.3% at weeks 12–17; by 61.3% at weeks 40–60. No statistical significance was seen between the drugs in achieving PASI ≤ 2 either at weeks 12–17 or at weeks 40–60 (p>.51 and .79).
PsA response
Twenty-two patients (27.5%) had PsA. Among these, 40.9% reached remission of their PsA; 36.4%, partial remission; 18.2%, exacerbation or no improvement. Among patients treated with guselkumab, 38.5% achieved remission; 46.1%, partial remission. Only 15.4% of guselkumab-treated patients experienced treatment failure. Only two patients treated with tildrakizumab had PsA; however, both patients achieved remission. Among patients treated with risankizumab, an equal number achieved remission, partial remission, and failure.
DLQI response
The mean (SD) DLQI at baseline was 8.3 (7.7). DLQI decreased to a mean of 1.9 (3.4) throughout the study period. Furthermore, 72.7% of the patients achieved DLQI 0/1 regardless of drug type.
Discussion
To our knowledge, this is the first study to evaluate the drug survival and effectiveness of all clinically available IL-23 inhibitors on psoriasis and PsA in a real-world setting.
In this study, overall drug survival after one year was 81.0%, which is similar to results from other real-world studies (Citation24,Citation25). Some have reported higher one-year drug survival rates of 88.0–96.4% (Citation26–30). A Danish study investigating drug survival of guselkumab in 50 patients found a lower one-year drug survival rate of approximately 67% (Citation31). The differences in drug survival between the studies may be due to the varying complexity of the patient populations. Schwensen et al. reported a baseline PASI of 4, which is lower than both the PASI reported in our study (8.0) and in the other mentioned studies (8.6–14.6). Furthermore, the percentage of patients who had previous failure or intolrance to biologic therapy differed between the studies (54.4–100%) (Citation24–31).
Randomized controlled trials (RCTs) have demonstrated the efficacy of all three IL-23 inhibitors. However, no direct head-to-head comparisons of the drugs have been conducted. Some reports suggest that tildrakizumab is inferior to guselkumab and risankizumab (Citation32,Citation33). We found no statistically significant differences between the drugs regarding drug survival, chance of reaching PASI ≤ 2 or PASI90/100. These results must be interpreted with caution as some baseline characteristics differed between the drugs. Psoriasis treatment with biologics in Denmark is based on treatment guidelines issued by the Danish Medicines Council. The guidelines ensure homogenous use of biologics across hospitals according to a prioritized list based on a combined scientific assessment and a cost analysis with a subsequent tendering procedure (Citation34). It is, therefore, unlikely that any differences in clinical effectiveness could be attributed to the treating physicians’ individual clinical assessments and biologic drug preferences.
In general, the response rates found in this study fall below what RCTs have reported. For guselkumab, we found a PASI90 response rate of 46.7% at weeks 12–17, which is lower than results from the VOYAGE-1/2 and ECLIPSE trials (73.3%, 70.0%, and 69% respectively) (Citation16,Citation18,Citation19). By weeks 40–60, 50% had achieved both PASI90 and PASI100 in our study. Trials have reported higher PASI90 (76.3–84%) but similar PASI100 response rates (47.4–58%) at week 48 (Citation16,Citation18). Our results for tildrakizumab are comparable to the PASI90 response rates reported in the ReSURFACE-1/2 trials (40% vs. 35% and 39%) but lower for PASI100 (40% vs. 12% and 14%) at weeks 12–17 (Citation17). However, only data from five patients treated with tildrakizumab were available for analysis at weeks 12–17. For risankizumab, both the PASI90 and PASI100 response rates in our study are lower than the response rates reported in the five RCTs investigating its efficacy (Citation14,Citation15,Citation20,Citation21).
It is important to emphasize the much larger sample sizes of RCTs and differences in baseline characteristics which may explain some of the observed divergences between the results. RCTs are designed to study efficacy under ideal circumstances, while our study reflects effectiveness in complex real-life patients. In this study, 95% of patients had previously been treated with biologics. In RCTs, previous biologics-treatment ranged from 13.0% to 56.5% (Citation14–21). Another factor that may explain the lower effectiveness shown in this study is the lower baseline PASI compared to trials (8.0 vs. 17.2–22.1), as baseline PASI ≥ 10 has previously been shown to be a predictor for achieving PASI90 (Citation35). Furthermore, differences in the handling of missing data and imputation methods also hamper any direct comparison of RCTs and real-world evidence.
In the last couple of years, studies have evaluated the effectiveness of IL-23 inhibitors in real-world settings. These studies have reported PASI90 responses ranging from 36% to 86.4% and PASI100 ranging from 18% to 67% at week 12 (Citation28,Citation29,Citation31,Citation36–39). At week 16, PASI90 responses ranged from 50.6% to 88.6%; PASI 100, from 32.1% to 49.1% (Citation39–44). In this study, results from weeks 12–17 for all IL-23 inhibitors collectively showed that PASI90 and PASI100 response rates were achieved by 45.7% and 25.7%, respectively. Considering the longer time span in our study, the results are in the lower end, but they are still in line with the results of those studies.
While PASI75, PASI90, and PASI100 response rates are often used as endpoints, it has gradually become favorable to use absolute PASI ≤ 2 or 3 as clinical endpoints (Citation45). Evaluating relative responses in real-world circumstances is difficult compared to RCTs, as there often is no washout period between discontinuing one treatment and starting another. Thus, our primary endpoint for effectiveness was absolute PASI ≤ 2.
When assessing the three drugs overall, PASI ≤ 2 was achieved by 64.3% at weeks 12–17 and 61.3% at weeks 40–60. The results are comparable to those by Schwensen et al., where 68.6% and 31.4% achieved PASI < 3 and < 1, respectively, at 12 weeks (Citation31). Higher percentages have been reported, especially for week 52, where two studies found that 84.2% and 88.2% of patients treated with guselkumab and risankizumab, respectively, achieved PASI ≤ 3 (Citation37,Citation42). Additionally, 79.4% achieved PASI ≤1 in the study of risankizumab (Citation37). Importantly, a risk of selection bias exists in both studies. The studies also had a higher baseline PASI (16.7–20.0) and a higher portion of biologically naïve patients (38.3–42.3%) than our study. Thus, the higher effectiveness reported in those studies could be a reflection of the higher complexity of the patients in our study.
We found that previous treatment with >2 biologics neither significantly affected drug survival nor the possibility of achieving PASI ≤ 2. These real-world results are important as patients with psoriasis have often tried multiple systemic biologics and non-biological therapies before initiating IL-23 inhibitors. Furthermore, the fact that the IL-23 inhibitors remained effective in obese patients is of importance as many patients with psoriasis are overweight, and these patients often have a poorer response to other antipsoriatic treatments, including various biologics such as TNF-α inhibitors, ustekinumab and IL-17-inhibitors (Citation46–52).
While the results regarding the effectiveness of the IL-23 inhibitors against PsA are preliminary, the data for guselkumab and the drugs collectively indicate that more patients experienced improvement (either partial or complete remission) than failure. Even though guselkumab and recently also risankizumab are approved for the treatment of PsA, they are still not routinely used for this indication in Denmark. These results from the real world might encourage increased awareness of this treatment option.
One of the strengths of this study is the collection of data on survival, quality of life, PASI, and PsA response. This broad spectrum of clinical measures allowed thorough assessment of the effectiveness of anti-IL-23 drugs on different parameters in a uniform patient population. The long follow-up (up to 165 weeks) for some patients allowed us to evaluate long-term drug survival and discontinuation due to late treatment failure and side effects.
A limitation is that PsA response was mainly based on qualitative measures. The data and retrospective nature of the study limit the evaluation of effectiveness against PsA, but the results presented in this study are promising and warrant future studies. In this study, we estimated both the drug survival of all anti-IL-23 inhibitors together and the drug survival for each drug. The drug-specific survival rates must be interpreted with caution, as 10 or more events should generally be present in a survival curve for it to be valid (Citation53). We included these results as we believe this is an early opportunity to cautiously compare the drugs in a uniform patient population. Another limitation is that it was possible to assess effectiveness only within broad time periods, i.e. after 12–17 and 40–60 weeks of treatment. Due to the COVID-19 pandemic, many in-person consultations were canceled or postponed; thus, the time of registration of PASI varied. The sample sizes in the analysis at 12–17 and 40–60 weeks were quite small for each drug. This also leaves a risk of survival bias as the analysis was performed only on individuals with available data. In the comparison of individual drug effectiveness, we consider the risk of bias due to missing data to be minimal, as data were missing by random and there were no systematic differences between missing and observed values. Future studies with a prospective method could secure more complete datasets.
Conclusion
IL-23 inhibitors are well-tolerated by and effective for patients with difficult-to-treat psoriatic disease in the long term in a real-world setting. The study has confirmed the high overall drug survival reported by other studies. Significant differences between the drugs in terms of drug survival or chance of achieving a PASI ≤ 2 were not detected. Although data on the effectiveness on PsA were limited, the study shows somewhat promising results as more patients achieved improvement than failure. More studies with larger sample sizes are needed to draw clear conclusions on the effectiveness of anti-IL-23 on PsA in real-world settings.
Author contributions
Concept and design: KFH. Acquisition, analysis, or interpretation of data: CDBE, KFH. Drafting of the manuscript: CDBE. Critical revision of the manuscript for important intellectual content: CDBE, LI, KFH. Statistical analysis: CDBE. Administrative, technical, or material support: LI. Supervision: KFH.
| Abbreviations | ||
| BMI | = | Body mass index |
| DLQI | = | Dermatology Life Quality Index |
| IL | = | Interleukin |
| MRI | = | Magnetic resonance imaging |
| NSAID | = | Non-steroidal anti-inflammatory drug |
| PASI | = | Psoriasis Area and Severity Index |
| PASI100 | = | 100% reduction in Psoriasis Area and Severity Index score from baseline |
| PASI90 | = | 90% or greater reduction in Psoriasis Area and Severity Index score from baseline |
| PASI75 | = | 75% or greater reduction in Psoriasis Area and Severity Index score from baseline |
| PsA | = | Psoriatic arthritis |
| RCT | = | Randomized controlled trial |
| TNF-α | = | Tumor necrosis factor alpha |
Disclosure statement
Cathrine Elgaard has received a personal scholarship from Novo Nordisk Foundation outside of the submitted work. Dr Iversen has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Astra Zeneca, BMS, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, Janssen Cilag, Kyowa, Leo Pharma, MSD, Novartis, Pfizer, Regranion, Samsung and Union Therapeutics UCB. Dr Hjuler has served as a consultant and advisor for the following companies: AbbVie, Bristol Myers Squibb (BMS), Janssen, LEO Pharma, UCB and Novartis, and has received speaking fees or grants from AbbVie, Eli Lilly, LEO Pharma, Novartis, and Janssen.
References
- Hawkes JE, Yan BY, Chan TC, et al. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201(6):1605–1613.
- Suzuki E, Mellins ED, Gershwin ME, et al. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev. 2014;13(4–5):496–502.
- Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13(5):715–725.
- Ben Abdallah H, Johansen C, Iversen L. Key signaling pathways in psoriasis: recent insights from antipsoriatic therapeutics. Psoriasis (Auckl). 2021;11:83–97.
- Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199(1):125–130.
- Gooderham MJ, Papp KA, Lynde CW. Shifting the focus - the primary role of IL-23 in psoriasis and other inflammatory disorders. J Eur Acad Dermatol Venereol. 2018;32(7):1111–1119.
- Fotiadou C, Lazaridou E, Sotiriou E, et al. Targeting IL-23 in psoriasis: current perspectives. Psoriasis (Auckl). 2018;8:1–5.
- Tremfya: EPAR - product information [Internet]. European Medicines Agency (EMA); 2017. [cited 2021 August 18]. Available from: https://www.ema.europa.eu/en/documents/product-information/tremfya-epar-product-information_en.pdf
- Ilumetri: EPAR - product information [Internet]. European Medicines Agency (EMA); 2018. [cited 2021 August 18]. Available from: https://www.ema.europa.eu/en/documents/product-information/ilumetri-epar-product-information_en.pdf
- Skyrizi: EPAR - product information [Internet]. European Medicines Agency (EMA); 2022. [cited 2022 March 24]. Available from: https://www.ema.europa.eu/en/documents/product-information/skyrizi-epar-product-information_en.pdf
- Tremfya prescribing information [Internet]. US Food and Drug Administration; 2020. [cited 2022 March 16]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761061s007lbl.pdf
- Skyrizi prescribing information [Internet]. US Food and Drug Administration; 2022. [cited 2022 March 24]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761105s014lbl.pdf
- Ilumya prescribing information [Internet]. US Food and Drug Administration; 2018. [cited 2022 March 17]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761067s000lbl.pdf
- Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184(1):50–59.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650–661.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831–839.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390(10091):276–288.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–417.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418–431.
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients With moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(6):649–658.
- Reich K, Gooderham M, Thaçi D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394(10198):576–586.
- Biological treatment in Danish dermatology [Internet]. Copenhagen: Copenhagen Healthtech Cluster; 2022. Available from: https://www.danishhealthdata.com/find-health-data/National-kvalitetsdatabase-for-psoriasispatienter-i-biologisk-behandling
- von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349.
- Lytvyn Y, Zaaroura H, Mufti A, et al. Drug survival of guselkumab in patients with plaque psoriasis: a 2 year retrospective, multicenter study. JAAD Int. 2021;4:49–51.
- Egeberg A, Rosenø NAL, Aagaard D, et al. Drug survival of biologics and novel immunomodulators for rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, and psoriasis - A nationwide cohort study from the DANBIO and DERMBIO registries. Semin Arthritis Rheum. 2022;53:151979.
- Torres T, Puig L, Vender R, et al. Drug survival of IL-12/23, IL-17 and IL-23 inhibitors for psoriasis treatment: a retrospective Multi-Country, multicentric cohort study. Am J Clin Dermatol. 2021;22(4):567–579.
- Iznardo H, Vilarrasa E, López-Ferrer A, et al. Real-world drug survival of guselkumab, ixekizumab and secukinumab for psoriasis. Br J Dermatol. 2021;185(3):660–662.
- Ruiz-Villaverde R, Rodriguez-Fernandez-Freire L, Armario-Hita JC, et al. Guselkumab: mid-term effectiveness, drug survival, and safety in real clinical practice. Dermatol Ther. 2021;34(2):e14798.
- Dapavo P, Siliquini N, Mastorino L, et al. Efficacy, safety, and drug survival of IL-23, IL-17, and TNF-alpha inhibitors for psoriasis treatment: a retrospective study. J Dermatolog Treat. 2021;13:1–6.
- Yiu ZZN, Becher G, Kirby B, et al. Drug survival associated With effectiveness and safety of treatment With guselkumab, ixekizumab, secukinumab, ustekinumab, and adalimumab in patients With psoriasis. JAMA Dermatol. 2022;e222909.
- Schwensen JFB, Nielsen VW, Nissen CV, et al. Effectiveness and safety of guselkumab in 50 patients with moderate to severe plaque psoriasis who had previously been treated with other biologics: a retrospective real-world evidence study. J Eur Acad Dermatol Venereol. 2021;35(5):e341–e343.
- Sawyer LM, Malottki K, Sabry-Grant C, et al. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: a systematic review and network Meta-analysis of PASI response. PLoS One. 2019;14(8):e0220868.
- Yang K, Oak ASW, Elewski BE. Use of IL-23 inhibitors for the treatment of plaque psoriasis and psoriatic arthritis: a comprehensive review. Am J Clin Dermatol. 2021;22(2):173–192.
- Danish Medicines Council [Internet]. Copenhagen: Danish medicines council. [cited 2022 Jul 14]. Available from: https://medicinraadet.dk/om-os/in-english
- Zweegers J, Roosenboom B, van de Kerkhof PC, et al. Frequency and predictors of a high clinical response in patients with psoriasis on biological therapy in daily practice: results from the prospective, multicenter BioCAPTURE cohort. Br J Dermatol. 2017;176(3):786–793.
- Burlando M, Castelli R, Cozzani E, et al. Treatment of moderate-to-severe plaque psoriasis with tildrakizumab in the real-life setting. Drugs Context. 2021;10:1–4.
- Galluzzo M, Tofani L, Lombardo P, et al. Use of guselkumab for the treatment of moderate-to-severe plaque psoriasis: a 1 year real-life study. J Clin Med. 2020;9(7):2170.
- Megna M, Fabbrocini G, Cinelli E, et al. Guselkumab in moderate to severe psoriasis in routine clinical care: an italian 44-week real-life experience. J Dermatolog Treat. 2020;4:1–5.
- Zhuang JY, Li JS, Zhong YQ, et al. Evaluation of short-term (16-week) effectiveness and safety of guselkumab in patients with psoriasis: a prospective real-life study on the Chinese population. Dermatol Ther. 2021;34(5):e15054.
- Hansel K, Zangrilli A, Bianchi L, et al. A multicenter study on effectiveness and safety of risankizumab in psoriasis: an Italian 16-week real-life experience during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2021;35(3):e169–e170.
- Benhadou F, Ghislain PD, Guiot F, et al. Real-life effectiveness and short-term (16-week) tolerance of guselkumab for psoriasis: a Belgian retrospective multicentre study. J Eur Acad Dermatol Venereol. 2020;34(12):e837–e839. Dec
- Gkalpakiotis S, Cetkovska P, Arenberger P, et al. Risankizumab for the treatment of moderate-to-Severe psoriasis: real-life multicenter experience from the Czech Republic. Dermatol Ther (Heidelb). 2021;11(4):1345–1355.
- Fougerousse AC, Ghislain PD, Reguiai Z, et al. Effectiveness and short-term (16-week) tolerance of guselkumab for psoriasis under real-life conditions: a retrospective multicenter study. J Eur Acad Dermatol Venereol. 2020;34(10):e644–e646. Oct
- Megna M, Cinelli E, Gallo L, et al. Risankizumab in real life: preliminary results of efficacy and safety in psoriasis during a 16-week period. Arch Dermatol Res. 2022;314(6):619–623.
- Puig L, Dossenbach M, Berggren L, et al. Absolute and relative psoriasis area and severity indices (PASI) for comparison of the efficacy of ixekizumab to etanercept and placebo in patients with moderate-to-severe plaque psoriasis: an integrated analysis of UNCOVER-2 and UNCOVER-3 outcomes. Acta Derm Venereol. 2019;99(11):971–977.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232(6):633–639.
- Lebwohl M, Yeilding N, Szapary P, et al. Impact of weight on the efficacy and safety of ustekinumab in patients with moderate to severe psoriasis: rationale for dosing recommendations. J Am Acad Dermatol. 2010;63(4):571–579.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019;80(1):27–40.
- Singh S, Facciorusso A, Singh AG, et al. Obesity and response to anti-tumor necrosis factor-α agents in patients with select immune-mediated inflammatory diseases: a systematic review and Meta-analysis. PLoS One. 2018;13(5):e0195123.
- Puig L. Obesity and psoriasis: body weight and body mass index influence the response to biological treatment. J Eur Acad Dermatol Venereol. 2011;25(9):1007–1011.
- Kyntheum: EPAR - assessment report [Internet]: European Medicines Agency (EMA); 2017. [cited 2022 September]. Available from: https://www.ema.europa.eu/en/documents/assessment-report/kyntheum-epar-public-assessment-report_en.pdf
- Augustin M, Reich K, Yamauchi P, et al. Secukinumab dosing every 2 weeks demonstrated superior efficacy compared with dosing every 4 weeks in patients with psoriasis weighing 90 kg or more: results of a randomized controlled trial. Br J Dermatol. 2022;186(6):942–954.
- van den Reek J, Kievit W, Gniadecki R, et al. Drug survival studies in dermatology: principles, purposes, and pitfalls. J Invest Dermatol. 2015;135(7):1–5.

