Abstract
Seborrheic keratoses (SKs) are benign epidermal neoplasms presenting as waxy, brown to black papules and plaques. Patients often seek removal for cosmetic reasons or irritation. The objective of this systematic review is to assess the efficacy and safety of topical treatments for SKs. Studies involving any topical medication indicated for SK removal were retrieved from Embase, Scopus, PubMed, and Cochrane. The final search was conducted on November 9, 2021, and 26 reports met inclusion criteria. A quality rating scheme was utilized to assess evidence quality. Heterogeneity of treatments and outcome measures precluded meta-analysis. Topical treatments that yielded a good-to-excellent response include hydrogen peroxide, Maxacalcitol 25 µg/g, BID Tazarotene 0.1% cream, 5% potassium dobesilate cream, 1% diclofenac sodium solution, urea-based solution, and 65% and 80% trichloroacetic acid. Local skin reactions were often mild and transient. Topical hydrogen peroxide showed the greatest evidence for clinical clearance of SKs, although there are no studies to our knowledge that directly compared hydrogen peroxide to current first-line treatments (e.g. cryotherapy or shave excision). The results of this review suggest viable and safe treatment of SK with topical therapies; however, there remains demand for topical treatments that reliably equate or exceed the efficacy of current first-line therapies.
Key Points
Question: Are safe and efficacious topical treatments for seborrheic keratoses available?
Findings: Topical treatments for seborrheic keratoses yield different responses and may be associated with local skin reactions. Topical hydrogen peroxide shows the greatest evidence for clinical clearance of seborrheic keratoses and may be a viable option for patients requesting noninvasive removal. No studies to our knowledge directly compare hydrogen peroxide to current first-line treatments.
Meaning: There remains demand for topical treatments that reliably equate or exceed the efficacy of current first-line therapies.
1. Introduction
1.1. Seborrheic keratosis
Seborrheic keratoses (SK) are benign epithelial neoplasms with a prevalence of 69% in patients aged 50 and older. They commonly appear on the face, chest, shoulders, or back and present as light tan, brown, or black papules or plaques with a waxy, ‘stuck on’, appearance (Citation1). Treatment is not necessary, although patients often request removal secondary to irritation or pruritus, or for cosmetic reasons. A survey of 594 board-certified dermatologists reported an average of 155 SK diagnoses monthly, with 43% of patients requesting removal (Citation2).
1.2. Current Invasive treatments
First-line treatments include cryotherapy and shave excision. Cryotherapy is generally regarded as the most available and efficacious treatment, with potential side effects including burning, stinging, blistering, and hypo-or-hyperpigmentation. Insurance may cover the procedure for symptomatic lesions or lesions clinically concerning for malignancy; however, cosmetic removal is typically not covered, requiring patients to pay out-of-pocket, with greater lesion number and freeze cycles increasing total cost. Shave excision is effective but requires local anesthesia, with potential side effects of scarring and hyperpigmentation (Citation3). Additional treatment options include laser therapy; ablative and non-ablative lasers have shown efficacy in small studies, although side effects include soreness and pigmentary changes (Citation4). Laser therapy is typically more expensive than other first-line treatments.
1.3. Current Topical treatments
The Food and Drug Administration (FDA) approved the use of topical hydrogen peroxide for the treatment of SK in 2017, although it was subsequently discontinued for financial reasons (Citation5). Researchers have continued studying the efficacy of hydrogen peroxide for SKs, and there remains demand for noninvasive, effective, and safe treatment options.
1.4. Significance of review
Although current standard treatments for SKs, such as cryotherapy, shave excisions, and laser therapy are readily available and efficacious, noninvasive topical treatment may offer a viable treatment option for many patients. Topical alternatives do not require anesthesia or invasive measures and may allow patients to apply treatment from home, reducing the burden on both patients and clinics. However, the potential benefits of topical treatments are limited by their respective efficacies and side effect profiles; thus, it is necessary to evaluate clinical literature regarding topical treatments for SK to better understand how they compare to standard removal.
A prior systematic review of topical treatments for SKs assessed both efficacy and safety of topical therapies (Citation6). However, this review contains an additional sixteen sources published after their last conducted search.
2. Objectives
The objective of this review is to assess the efficacy and safety of topical treatments for SKs.
3. Methods
3.1. Literature Search
As data was retrieved from published literature, this study was exempt from institutional review board approval. The literature search included the following databases: EMBASE, Cochrane Library, Scopus, and PubMed. Results included any topical treatment of SKs and were limited to clinical studies in human subjects. Reports were excluded if treatments included cryotherapy, laser therapy, shave excision, or systemic modalities. The last search was run on November 9, 2021, and the following text exemplifies the search strategy applied to EMBASE and PubMed, respectively:
('topical drug administration’/exp OR topical:ab,ti) AND ('seborrheic keratosis’/exp OR 'seborrheic keratosis’:ab,ti OR 'seborrheic keratoses’:ab,ti OR 'keratosis seborrhoeica’:ab,ti)
((‘Administration, Topical’[Mesh] OR Topical[tiab]) AND (‘Keratosis, Seborrheic’[Mesh] OR ‘seborrheic keratosis’[tiab] OR ‘seborrheic keratoses’[tiab] OR ‘keratosis seborrhoeica’[tiab]))
Due to the relative dearth of clinical studies related to topical treatment of seborrheic keratoses, clinical studies of any design were included. Screening involved title and abstract review; studies were excluded if the subject matter deterred from topical treatment or seborrheic keratoses. The remaining articles were retrieved for a full-text review, although non-English articles were ultimately excluded.
3.2. Data Extraction
Two independent authors screened titles and abstracts to assess inclusion and exclusion criteria and reviewed full texts to extract study characteristics and results. Extracted study characteristics included intervention, frequency, study design, sample size, efficacy, and adverse events. However, many studies utilized different methods to assess efficacy; effect measures included reduction of Physician Lesion Assessment (PLA) grade, reduction of lesion surface area, thickness, and number, histological improvement, and subjective resolution. Studies were grouped based on treatment for synthesis; due to the heterogeneity of treatments and outcomes measured, meta-analysis was not completed. In the event results depicted heterogenous outcomes for similar treatments, study characteristics such as treatment frequency and duration were analyzed. Extracted characteristics and results are included in .
Table 1. Efficacy of topical therapies for treatment of seborrheic keratoses.
3.3. Quality assessment and bias assessment
Two independent authors conducted a quality assessment of each included study using a quality rating scheme depicted in . Questions about quality assessment were brought to A.K. for discussion. In order to minimize reporting bias, omitted information regarding frequency, efficacy, or adverse events is notated accordingly in .
Table 2. Quality rating scheme for studies and other evidence.
4. Results
4.1. Literature Search
The literature search yielded 72 PubMed records, 158 Embase records, 5 Cochrane records, and 160 Scopus records; 200 duplicate records were excluded and abstract screening excluded an additional 156 records. 41 full-text reports were sought for retrieval, of which 15 were excluded for reasons depicted in (Citation7). 26 reports met the inclusion criteria.
Figure 1. PRISMA flow-diagram of the search process utilized in this review (Citation7). The search strategy conducted with four databases yielded 395 records; 200 duplicate records were excluded before screening. Of the remaining 195 records, title and abstract screening excluded an additional 156 records. 41 full-text reports were sought for retrieval and assessed for eligibility, of which 15 were excluded for various reasons. 26 reports met the inclusion criteria; six randomized controlled trials, four non-randomized controlled trials, ten non-randomized open-label studies, and six case reports were included in the systematic review.
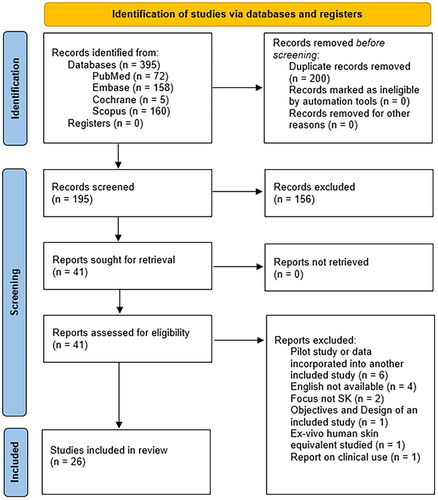
Study characteristics and results were reported in each study, reducing the risk of reporting bias. Missing characteristics are notated accordingly in .
4.2. Efficacy and tolerability
4.2.1. Topical hydrogen peroxide solution
It is hypothesized that hydrogen peroxide achieves clearance of SKs via generation of reactive oxygen species and subsequent death to lesional cells (Citation8). Prescribing information suggests hydrogen peroxide be applied four times during a single in-office session. If complete clearance is not achieved after three weeks, another treatment may be administered (Citation9).
Nine studies evaluated the use of hydrogen peroxide for the treatment of SKs. Two case reports and one safety study were excluded from primary efficacy analysis due to low evidence quality but are included in .
Baumann et al. (2018) demonstrated the efficacy of 40% hydrogen peroxide (HP40) using the Physician’s Lesion Assessment (PLA) (Citation3). A total of 467 patients received HP40 and 470 patients received a vehicle control solution in two identical trials. Four SKs were treated per patient with up to two applications three weeks apart, and response was assessed at day 106 A sub-analysis conducted by Smith et al. reported complete clearance of 43% facial, 26% truncal, and 17% extremity lesions (Citation10). This suggests that lesion clearance may depend on anatomical location, which is further supported by DuBois et al. (2019) (Citation11).
DuBois et al. (2018) assessed the efficacy of HP40 and a less potent treatment group, HP32.5 (Citation12). Lesions were treated with up to two applications three weeks apart. One facial lesion was treated with either HP40(n = 39), HP32.5(n = 39), or placebo(n = 41). At day 106, the researchers reported lesion clearance of 59.5% (HP40) and 46.2% (HP32.5); both concentrations were more effective than control solution (p < .001).
DuBois et al. (2019) included a more potent solution in their analysis: HP45 (Citation11). At day 106, mean lesion clearance was 49.5% HP40(n = 103), 57.9% HP45(n = 100), and 3.7% for vehicle control (n = 50, p < .0001). They separated analysis by anatomic location and found 62.2% HP40 and 67.2% HP45 clearance of facial SKs, and 43.3% HP40 and 55.8% HP45 clearance of truncal SKs. The only significant difference observed between the treatment groups was for lesion clearance of the extremities (p = .02) (Citation11), suggesting a more potent solution may be required for efficacious SK treatment of the extremities.
Lastly, in 2020, DuBois et al. assessed patient satisfaction of HP40 treatment of raised facial SKs (Citation13). Although the primary objective was to determine satisfaction, 90.2% target lesions (n = 41 subjects) depicted clearance. This is notably greater than the 2018 study similarly limiting lesions to the face (90.2% vs. 59.5% (Citation12)). However, DuBois et al. (2020) treated patients with an additional application of HP40 (three total). Furthermore, unlike the previous three studies, Dubois et al. (2020) conducted a non-randomized, open-label study that was not controlled for observer bias, which may have contributed to the drastic difference in observed efficacy.
The authors of each study described local skin reactions (LSRs) following application, including application site pain, burning, stinging, pruritus, hyper and hypopigmentation, erythema, scaling, crusting, and edema, most of which resolved by the end of the studies (Citation3,Citation11–14). Similar rates of LSRs were reported for the HP32.5, HP40, and HP45 treatment groups (Citation11,Citation12,Citation14).
These results suggest topical hydrogen peroxide solution is an effective treatment for SKs. Furthermore, five of the six studies included for primary efficacy analysis were properly conducted randomized controlled trials, representing high evidence quality. In contrast, Dubois et al. (2002) conducted a prospective trial lacking a control group, potentially contributing to observer bias A controlled trial is necessary to directly compare hydrogen peroxide to cryotherapy or shave excision, but the lack of clearance in many patients coupled with the presence of LSRs suggests hydrogen peroxide may fall short compared to first-line, invasive treatments.
4.2.2. Vitamin D-based topical solution
The search strategy yielded four studies performed in 2004–2020 that evaluated the use of vitamin D-based topical solutions for the treatments of SKs, with Calcipotriol (Calcipotriene) most frequently studied. Mitsuhashi et al. analyzed the volume change of lesions treated with Tacalcitol 2 µg/g(n = 45), Calcipotriol 50 µg/g(n = 34), or Maxacalcitol 25 µg/g(n = 37) (Citation15). Subjects applied the topical treatment for 3–12 months (average: 7.3 months). Following treatment, they observed >80% decrease in volume in 17.8%, 35.3%, and 40.5% of lesions treated with Tacalcitol, Calcipotriol, and Maxacalcitol, respectively. These results demonstrated the potential for Calcipotriol and Maxacalcitol to be relatively efficacious in the treatment of SKs, both of which were more effective than Tacalcitol. The authors reported no erythema, swelling, or scarring.
Yousefi et al. similarly noted clinical improvement with Calcipotriol 50 µg/g (Citation16). At 12 weeks, >80% reduction in diameter was observed in 76.7% lesions, with a mean percent improvement of 46 ± 17.66%. The authors stated an intermediate negative correlation existed between tumor size and improvement; because Mitsuhashi et al. did not specify initial lesion sizes, perhaps lesion size may account for the different efficacies observed in each study (76.7% (Citation16) vs. 35.3% (Citation15) lesions depicting >80% diameter reduction).
Herron et al. failed to observe clinical improvement following four-month application of 0.005% calcipotriene, compared to 100% clinical improvement with standard cryotherapy (n = 15, self-controlled) (Citation17). Response was assessed monthly for 6 months. Similarly, Sutedja et al. noted only a mild decrease in lesion size with three-month calcipotriol treatment (6.18%) compared to a 100% decrease with cryotherapy (n = 18, self-controlled) (Citation18). Evidence quality for vitamin D-based topical solutions was relatively high, with three properly conducted randomized controlled trials and one open-label prospective study. Despite the relative success observed by Yousefi and Mitsuhashi, vitamin D-based topical treatments fail to achieve the same reliability and efficacy as standard invasive treatments for SKs.
4.2.3. Vitamin A-based topical solution
Two studies performed in 2004–2020 evaluated the use of vitamin A-based topical solutions for the treatment of SKs. Herron et al. additionally assessed the efficacy of Tazarotene 0.1% cream, a retinoid derived from vitamin A, on area and clearance of SKs (n = 15, self-controlled) (Citation17). BID application of tazarotene 0.1% for four months resulted in significant clinical and histological improvement in 7/15 patients; however, one treatment with cryosurgery led to histological improvement for all 15 patients. Furthermore, 10/15 patients reported initial burning, pruritus, and redness subsequent to treatment (Citation17). None of the subjects discontinued treatment due to side effects; however, the authors state the effectiveness of treatment was limited by its irritation.
Wijayanti et al. described a case in which remission was not obtained following four-week use of Tretinoin 0.05% cream (Citation19). However, this case was atypical in that the multiple lesions mimicked porokeratosis, which may have impacted treatment efficacy. In addition, the lesions were only treated for four weeks, in comparison to four months as described by Herron et al. It is also necessary to note that the evidence reported by Wijayanti et al. is of the lowest quality as a single case report. Given the only moderate success and resulting irritation, it remains unlikely that vitamin A-based topical treatments would become accepted as a preferred alternative to current invasive approaches.
4.2.4. 5-Fluorouracil (5-FU)
5-FU inhibits the formation of thymidylate from uracil, resulting in inhibition of DNA synthesis and subsequent cell death (Citation20). In 1995, Tsuji and Morita published a report detailing the successful clearance of a large SK of the frontal scalp using 5-FU cream and polyethylene glycol (Citation21). 5-FU was applied daily for three weeks followed by topical polyethylene glycol for six months to prevent secondary infection and induce epithelization. Response was assessed at 6 months. Erythema, edema, and erosion initially appeared within the treated lesion, although full clearance was observed within six months with long hairs covering the area, suggesting the absence of scarring.
Sutedia et al. additionally included topical 5-FU in their self-controlled trial (n = 18) (Citation18). 5-FU was applied twice daily for three months. After twelve weeks, there was a 24.06% mean decrease in lesion size, compared to a 100% mean decrease in lesion size with cryosurgery (Citation18). Safety and LSRs were not discussed. As a randomized controlled trial, the study conducted by Suteja et al. is of higher quality than the case report presented by Tsuji and Morita.
4.2.5. Urea-based topical solution
Burkhart and Burkhart evaluated a solution consisting of 50% urea, vitamin D, lactic acid, and zinc (Citation22). To determine patient satisfaction, subjects responded to a six-week follow-up survey. There was reasonable agreement that some reduction of thickness could be achieved, although the lack of clinical observation hinders the ability to determine efficacy. Furthermore, as the study was conducted open-label, observer bias may impact the reported patient satisfaction. Yet, all participants reported no side effects, and the average rating of preference to continue using the product after trial completion was 8.3/10 (Citation22).
Campione et al. evaluated a solution containing 42% urea (n = 20) (Citation23). A significant reduction in lesion thickness and number was confirmed by epiluminescence microscopy following BID application for thirty days. As 19/20 patients reported excellent tolerability at day 30, these results suggest that urea-based topical solutions may be an effective treatment for the clearance of SKs with low risk to patients, although a controlled trial with a standardized solution is necessary to compare efficacy to standard treatment. Although both study designs were open-label and prospective, the lack of clinical observation in the study published by Burkhart and Burkhart increases observer bias and reduces the validity of results.
4.2.6. Trichloroacetic acid
Chun et al. assessed a 65% focal TCA peel on lesion clearance (Citation24). The peel was applied every 1–2 months, with an average 1.5 treatment courses(n = 23), and response was assessed 6 months following treatment completion.A 57% excellent, 26% good, 13% fair, and 4% poor response was observed. No significant complications were observed, although minor stinging upon application was reported.
Komalasari et al. described a case in which a large, 1–3 cm SK of the scalp was successfully treated with a single application of 80% TCA solution (Citation25). The authors noted significant reduction in size and thickness; at the four-month follow-up visit, the lesion was almost completely resolved, leaving minimal hyperpigmentation macules without alopecia. The presence of hair growth on the treated lesion suggests the absence of scarring.
These results suggest the potential efficacy of topical TCA solution. However, as only 57% of patients in the open-label study depicted excellent lesion improvement (Citation24), the results suggest that perhaps a stronger concentration of TCA solution is required. In addition, the open-label study was not controlled for observer bias. Lastly, as the case report conducted by Komalasari et al. is of the lowest evidence quality, we are unable to generalize the efficacious results depicted. A randomized, controlled study would be necessary to determine the topical TCA regimen that yields the greatest lesion clearance and its efficacy compared to first-line treatments.
4.2.7. Other acid-based solutions
Lacarrubba et al. evaluated a 30–50% nitric-zinc oxide solution with organic acids on the clearance of 50 lesions from fifteen patients (Citation26). At eight weeks, 74% of lesions achieved complete clinical and dermoscopic clearance. None of the cleared lesions relapsed at 6-months, although some cases depicted minimal hypopigmentation. The authors reported no adverse events (AEs) but noted slight, transient post-treatment erythema.
In 2019, Tribó et al. assessed two monthly applications of a Nitrizinc Complex solution (NZCS) (organic acids, nitric acid, copper, and zinc salts) on clinical clearance of 59 lesions from 32 patients (Citation27). At month six, complete clearance was observed in 80% of lesions, with 93.3% of lesions demonstrating >50% diameter reduction. Erythema occurred in 24.1% of subjects and three subjects had persistent hypopigmentation at month twelve. After the first and second applications of the NZCS application, patients reported pain scores of 1.70 ± 2.22 and 0.94 ± 2.05. Burn-itching scores were slightly higher at 3.81 ± 2.69 after the first application and 3.44 ± 3.27 after the second application. Still, the average subjective subject rating of the treatment process was 8.66/10 (Citation27).
Feuerman et al. observed lesion clearance with a Solcoderm solution (copper with acetic, lactic, nitric, and oxalic acid) (Citation28). Subjects applied the solution every two weeks until completely healed, with an average of 1.3 treatments. At regular follow-up, 62.3% of lesions completely cleared; 13.0% completely healed with erythema, and 24.6% partially healed with the persistence of crust (n = 36). Patients reported a slight burning sensation during and immediately after Solcoderm application. All three open-label prospective studies are of comparable evidence quality and are at risk of observer bias. These results suggest acidic solutions may be relatively efficacious in the clearance of SKs, although mild pain, burning, and erythema may be expected.
4.2.8. Other
The search strategy yielded five studies or reports performed in 1990–2020 that included a treatment group(s) that did not correspond with the above categories. Klaus et al. assessed the effect of 12% ammonium lactate (Lac-Hydrin) on lesion characteristics(n = 58) (Citation29). After BID application for sixteen weeks, a significant reduction in SK elevation was observed, with no difference in width or number. The unchanging size and minimal texture/color improvement suggest Lac-Hydrin is ineffective compared to first-line treatments and other topicals in the treatment of SKs.
Herron et al. included Imiquimod 5% cream as a final treatment group in their self-controlled trial (Citation17). Imiquimod is an acid-containing solution that stimulates immune responses and induces apoptosis of tissue (Citation30,Citation31), although four-month application failed to result in clinical improvement of SKs (Citation17). Furthermore, 5/15 subjects experienced redness, burning, and ulceration following BID application. The AEs coupled with the lack of clearance suggest imiquimod is not a viable treatment for SKs.
Cuevas et al. published a report detailing the successful clearance of two facial SKs following daily application of 5% potassium dobesilate cream (Citation32). Complete clearance was observed at the end of the six-month treatment with no LSRs, although a larger study including multiple patients is necessary to confirm efficacy.
Rosen et al. assessed the efficacy of Ingenol Mebutate 0.05% gel on SK clearance (Citation33). They enrolled 24 patients (80 lesions) and instructed patients to apply the gel once daily for three days. A modest 15% lesion clearance was observed, and LSRs included erythema (93%), flaking or scaling (46%), swelling (60%), and vesiculation/pustulation (30%).
Lastly, Afify and Hana assessed the efficacy of 1% Diclofenac sodium solution(n = 30) (Citation34). Following BID treatment for eight weeks, a significant decrease in lesion surface area (p = .001) was observed. The mean change in lesion surface area was 61.7 ± 17.06%, and there were no reported AEs. The quality of evidence was greatest in the randomized, controlled studies conducted by Klaus et al., Herron et al., and Afify and Hana. Case reports are of the lowest evidence quality due to low sample size and reduced generalizability of results; thus, the efficacy of 5% potassium dobesilate cream cannot be determined from this data alone.
5. Discussion
Poor response to topical treatment was found following application of Tacalcitol 2 µg/g, once-daily application of Tazarotene 0.1% cream, Tretinoin 0.05% cream, 5-FU, 12% ammonium lactate lotion (Lac-Hydrin), Imiquimod 5% cream, and Ingenol Mebutate 0.05% cream. A good to excellent response to topical treatment was found following application of hydrogen peroxide, Maxacalcitol, BID Tazarotene cream, 5% potassium dobesilate, 1% diclofenac sodium, urea-based topical solution, TCA, and other acid-based solutions. Calcipotriol yielded mixed results.
Hydrogen peroxide was the most frequently studied and demonstrated efficacy in the treatment of SKs. The authors observed clinical clearance ranging from 30% to 90.2%, with studies differing in the anatomical location of lesions treated and the number of treatment applications (Citation3,Citation11–13). LSRs following application were common, with similar incidences described following application of different potencies.
Studies comparing topical treatment to a control solution observed statistically significant differences in outcome measure, except for once-daily application of calcipotriene 0.005% and tazarotene 0.1% cream, imiquimod 5% (Citation17), and Lac-Hydrin (Citation29). All studies directly comparing topical treatment to cryotherapy found cryotherapy to be more effective. Topical hydrogen peroxide, urea-containing solutions, and acid-based solutions were not directly compared to standard therapy.
5.1. Limitations
The prevalence of small-scale studies lacking control groups limits the generalizability of results; only eight studies were properly conducted randomized trials, each rated 1 for the quality assessment. Furthermore, the included studies differed in designs, populations, and outcomes measured, hindering the ability to directly compare efficacy and safety via meta-analysis. Lastly, most studies failed to include standard treatment, such as cryotherapy, as a treatment group, hindering the ability to directly assess the efficacy of topical treatments of SKs compared to conventional treatment.
5.2. Future research
There remains demand for randomized clinical trials directly comparing topical treatments, such as HP40, to first-line treatments such as cryotherapy. Furthermore, future research could seek to determine specific patient or lesion characteristics that predict treatment efficacy with topical treatments. It is possible that certain histopathologic variants, such as acanthotic, reticulated, hyperkeratotic, etc. are more receptive to topical treatment than others.
Conclusion
This review of clinical studies outlines the potential safety and efficacy of topical treatments for SKs. Most topical treatments elicited a good to excellent response, and local skin reactions were often mild and transient. Topical hydrogen peroxide depicted the greatest evidence for clinical clearance of SKs, although no studies directly compared hydrogen peroxide to current first-line treatments such as cryotherapy or shave excision. The results of this review suggest topical treatment may be a viable and safe method of SK removal, but there remains demand for topical treatments that reliably equate or exceed the efficacy of current first-line therapies.
IRB approval status
Exempt.
| Abbreviations | ||
| AEs | = | Adverse events |
| BID | = | Twice daily |
| CCR | = | Clinical clearance rate |
| CR | = | Case report |
| CT | = | Controlled trial |
| HP | = | Hydrogen peroxide |
| HP32.5 | = | 32.5% hydrogen peroxide |
| HP40 | = | 40% hydrogen peroxide |
| HP45 | = | 45% hydrogen peroxide |
| LSR | = | Local Skin Reaction |
| NZCS | = | Nitrizinc Complex topical solution |
| OL | = | Open label study |
| PLA | = | Physician’s Lesion Assessment |
| RCT | = | Randomized controlled trial |
| SCT | = | Self-controlled trial |
| SK | = | Seborrheic Keratosis |
| SSA | = | Subject Satisfaction Assessment |
| TCA | = | Trichloroacetic acid |
| 5-FU | = | 5-Fluorouracil |
Disclosure statement
James Grichnik, MD, PhD is a Consultant to Galileo and Canfield Scientific. The remaining authors have no relationships to disclose.
References
- Seborrheic keratosis. Mayo Clinic. 2022. https://www.mayoclinic.org/diseases-conditions/seborrheic-keratosis/symptoms-causes/syc-20353878
- Jackson JM, Alexis A, Berman B, et al. Current understanding of seborrheic keratosis: prevalence, etiology, clinical presentation, diagnosis, and management. J Drugs Dermatol. 2015;14(10):1119–1125.
- Baumann LS, Blauvelt A, Draelos ZD, et al. Safety and efficacy of hydrogen peroxide topical solution, 40% (w/w), in patients with seborrheic keratoses: Results from 2 identical, randomized, double-blind, placebo-controlled, phase 3 studies (A-101-SEBK-301/302). J Am Acad Dermatol. 2018;79(5):869–877. Article
- Greco MJ, Bhutta BS. Seborrheic Keratosis. StatPearls. StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Aclaris Therapeutics, Inc. Aclaris Therapeutics reports second quarter 2019 financial results, provides business strategy update and provides update on clinical and commercial developments. GlobeNewswire News Room. 2019. https://www.globenewswire.com/news-release/2019/08/08/1899526/0/en/Aclaris-Therapeutics-Reports-Second-Quarter-2019-Financial-Results-Provides-Business-Strategy-Update-and-Provides-Update-on-Clinical-and-Commercial-Developments.html
- Gonzalez M, Ramos V, Tan C. Topical treatments for seborrheic keratosis: a systematic review. J Dermatol Nurses’ Assoc. 2020;12(2):305–312.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89.
- Balasubramaniam D, Burkhart CG. Hydrogen peroxide use for chemical destruction in seborrheic keratosis: a review. TODJ. 2019;13(1):68–70.
- Highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209305s000lbl.pdf
- Smith SR, Xu S, Estes E, et al. Anatomic site–specific treatment response with 40% hydrogen peroxide (W/W) topical formulation for raised seborrheic keratoses: Pooled analysis of data from two phase 3 studies. J Drugs Dermatol. 2018;17(10):1092–1098.
- DuBois J, Grande K, Bradshaw M, et al. Effectiveness of hydrogen peroxide topical solution 40% and 45% (w/w) in patients with seborrheic keratoses on the trunk, extremities, and face: Results of a phase 2, randomized, double-blind, vehicle-controlled, parallel group study. J Am Acad Dermatol. 2019;81(4):AB197.
- DuBois JC, Jarratt M, Beger BB, et al. A-101, a proprietary topical formulation of High-Concentration hydrogen peroxide solution: a randomized, Double-Blind, Vehicle-Controlled, parallel group study of the Dose-Response profile in subjects with seborrheic keratosis of the face. Dermatol Surg. 2018;44(3):330–340.
- DuBois J, Grande K, Rhodes T, et al. Assessing patients’ satisfaction with hydrogen peroxide topical solution, 40% for treatment of raised seborrheic keratoses. J Drugs Dermatol. 2020;19(12):1184–1191.
- DuBois J, Grande K, Bradshaw M, et al. Safety of hydrogen peroxide topical solution 40% and 45% (w/w) in patients with seborrheic keratoses on the trunk, extremities, and face: Results of a phase 2, randomized, double-blind, vehicle-controlled, parallel-group study. J Am Acad Dermatol. 2019;81(4):AB271.
- Mitsuhashi Y, Kawaguchi M, Hozumi Y, et al. Topical vitamin D3 is effective in treating senile warts possibly by inducing apoptosis. J Dermatol. 2005;32(6):420–423.
- Yousefi M, Nabaei L, Ghasemnia H, et al. Eficacy of calcipotriol in the treatment of seborrheic keratosis: a pilot study. Iran J Dermatol. 2013;16(66):132–136.
- Herron MD, Bowen AR, Krueger GG. Seborrheic keratoses: a study comparing the standard cryosurgery with topical calcipotriene, topical tazarotene, and topical imiquimod. Int J Dermatol. 2004;43(4):300–302.
- Sutedja EVA, Suwarsa OKI, Febrina DIA. Comparison of the effectiveness of topical 5-fluorouracil, topical calcipotriol, and cryosurgery in seborrheic keratosis. J Dermatol Nurses’ Assoc. 2020;12(2):85–88.
- Wijayanti W, Nurdin AR, Widita W, et al. A case of atypical seborrheic keratoses mimicking porokeratosis. Forum Nordic Dermato Venerol. 2020;25(3):1–3.
- Fluorouracil. Uses, Interactions, Mechanism of Action | DrugBank Online. Updated October 17, 2022. https://go.drugbank.com/drugs/DB00544%C2%A0
- Tsuji T, Morita A. Giant seborrheic keratosis on the frontal scalp treated with topical fluorouracil. J Dermatol. 1995;22(1):74–75.
- Burkhart CG, Burkhart CN. Use of a keratolytic agent with occlusion for topical treatment of hyperkeratotic seborrheic keratoses. Skinmed. 2008;7(1):15–18.
- Campione E, Cosio T, Di Prete M, et al. Effectiveness of a cosmetic device containing a combination of alpha- and beta-hydroxy acids, urea, and thuja for the treatment of seborrheic keratoses. J Cosmetic Dermatol. 2022;21(5):2113–2119.
- Chun EY, Lee JB, Lee KH, et al. Focal trichloroacetic acid peel method for benign pigmented lesions in Dark-Skinned patients. Dermatol Surg. 2004;30(4 Pt 1):512–516; discussion 516.
- Komalasari DN, Djawad K, Nurdin AR, et al. Unusual clinical manifestation of seborrheic keratosis on the scalp successfully treated with topical trichloroacetic acid: an atypical case report. Pan Afr Med J. 2020;37:1–6.
- Lacarrubba F, Rita Nasca M, Elisa Verzì A, et al. A novel topical agent in the treatment of seborrheic keratoses: an open-label pilot study with clinical and dermoscopic evaluation. J Am Acad Dermatol. 2017;76(6):AB19.
- Tribó MJ, Aladren S, Garre A, et al. A novel nitrizinc complex® solution for topical treatment of seborrhoeic keratosis: an interventional clinical study. Eur J Dermatol. 2019;29(2):203–208.
- Feuerman EJ, Katzenelson V, Halevy S. Solcoderm in the treatment of solar and seborrheic keratoses. Dermatologica. 1984;168(SUPPL. 1):33–35.
- Klaus MV, Wehr RF, Rogers Iii RS, et al. Evaluation of ammonium lactate in the treatment of seborrheic keratoses. J Am Acad Dermatol. 1990;22(2 Pt 1):199–203.
- Food and Drug Administration. February 25, 2010. https://www.accessdata.fda.gov/drugsatfda_docs/anda/2010/078548Orig1s000.pdf
- Imiquimod. Uses, Interactions, Mechanism of Action | DrugBank Online. Updated October 17, 2022. https://go.drugbank.com/drugs/DB00724
- Cuevas P, Angulo J, Salgüero I, et al. Clearance of seborrhoeic keratoses with topical dobesilate. BMJ Case Reports. 2012;2012(jun21 1):bcr0120125628.
- Rosen R, Freeman M, Zibert JR, et al. Ingenol mebutate 0 05% gel reduces cancer cells in squamous cell carcinoma in situ and shows marginal effect in seborrhoeic keratosis. Br J Dermatol. 2014;171:50.
- Afify AA, Hana MR. Comparative evaluation of topical diclofenac sodium versus topical ibuprofen in the treatment of seborrheic keratosis. Dermatol Ther. 2020;33(6):e14370.
- Callender VD, Frankel EH, Weiss JS, et al. An open-label study of the safety of A-101, a 40% hydrogen peroxide topical solution, in patients with seborrheic keratoses. J Am Acad Dermatol. 2018;79(3):AB39.
- DuBois J, Grande K, Schnyder J, et al. Assessing patient satisfaction with hydrogen peroxide topical solution, 40% (w/w) treatment of seborrheic keratoses on the face, neck, and décolletage: Objectives and design of the phase IV, openlabel SK-FAN study. J Clin Aesthetic Dermatol. 2019;12(5):S32.
- Peredo M, Murphy E, Karibayeva D. Clinical experience with 40% hydrogen peroxide topical solution for the treatment of seborrheic keratosis. J Drugs Dermatol. 2019;18(7):s173–s177.
- Schlesinger TE, Favre C. Enhancing outcomes in seborrheic keratosis: using a novel treatment solution. J Drugs Dermatol. 2019;18(7):s178–s182.

