Abstract
Objectives
The objective of this systematic review was to evaluate the efficacies of different biologic therapies in treating tumor necrosis factor-alpha (TNFα)-induced paradoxical psoriasis (PXP) and controlling inflammatory bowel disease (IBD) symptoms.
Methods
We conducted a literature search of the Ovid EMBASE, Ovid Medline, Web of Science Core Collection, and Cochrane Central Register of Controlled Trials databases from their inception to October 3, 2021. We considered all peer-reviewed, randomized controlled trials, chart reviews, and observational studies that discussed the TNFα-induced PXP treatment outcomes in IBD patients of switching to different biologic therapies.
Results
Switching to ustekinumab (UST) resulted in complete or partial resolution of TNFα-induced PXP in 83.1% of patients (74 out of 89 patients), while switching to either vedolizumab (VDZ) or secukinumab led to complete resolution in 100% of patients (eight out of eight patients). Approximately 75.4% of patients who were switched to UST remained in IBD remission, 4.6% in partial remission, and 20.0% in the flare of IBD.
Conclusions
UST has sufficient data to demonstrate the efficacy in treating TNFα-induced PXP and controlling IBD symptoms concurrently. More data is needed to validate the efficacies of VDZ and SEC in treating TNFα-induced PXP in IBD patients.
Introduction
Introduction of biologic therapies has transformed the way we manage inflammation in the gut and the skin. Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gastrointestinal (GI) tract. Over the past several decades, anti-tumor necrosis factor (anti-TNFα), such as infliximab (IFX) and adalimumab (ADA), has been the mainstay biologic therapy for these patients. However, with increasing utilization of anti-TNFα agents, paradoxical inflammation of the skin, specifically paradoxical psoriasis (PXP), has been reported in IBD patients (Citation1). For these patients, switching to a different class of biologic therapies has been shown to successfully treat PXP as well as control GI symptoms (Citation2). Here, we performed a systematic review to evaluate the efficacies of different biologic therapies in treating PXP and controlling symptoms of IBD.
Methods
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and using pre-defined search terms, we conducted electronic literature searches to identify relevant studies (RR, SW, and JSM) (). We searched the following databases from their inception to the date of search on October 3, 2021: Ovid EMBASE, Ovid Medline, Web of Science Core Collection, and Cochrane Central Register of Controlled Trials. Key search terms included ‘inflammatory bowel disease,’ ‘Crohn’s disease,’ ‘ulcerative colitis,’ ‘tumor necrosis factor inhibitor,’ ‘psoriasis,’ ‘adverse reaction,’ and specific biologics therapies (e.g. ustekinumab, vedolizumab, secukinumab, and others). The reference section of each eligible study was manually screened for potentially relevant studies. Our inclusion criteria were peer-reviewed, English-language articles that discussed the TNFα-induced PXP treatment outcomes in IBD patients of switching to alternative biologic therapies. Randomized controlled trials (RCTs), chart reviews, and observational studies (including case reports and case series) were considered. Review articles, basic research studies, and articles that did not meet the inclusion criteria were excluded. Three investigators (RR, HP, and JSM) initially assessed study eligibility by screening titles and abstracts, followed by three investigators (RR, MR, and JSM) reviewing full text for final study inclusion and data extraction. Any disagreement or data inconsistency were resolved by discussion. Included studies were assessed for methodological quality using the modified criteria for systematic reviews involving case reports () (Citation1,Citation3–19).
Figure 1. PRISMA flow diagram for systematic review. PRISMA: preferred reporting items for systematic reviews and meta-analyses.
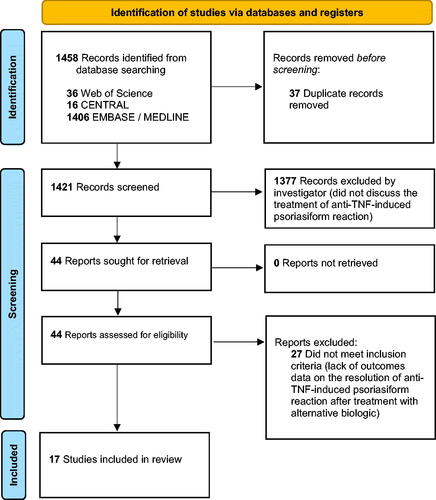
Table 1. Quality appraisal of included studies.a
Results
At the end of our screening process, we identified and included 17 studies in our final analysis (3 prospective cohort studies, 1 retrospective cohort study, 5 retrospective chart reviews, 1 case-control study, 3 case reports, and 4 case series) (). Across all studies, there were 380 IBD patients who experienced PXP after initiation of anti-TNFα agents with 58.4% (n = 222) and 39.7% (n = 151), 1.6% (n = 6), 0.3% (n = 1), and 0% (n = 0) reporting IFX, ADA, CZP, ETA, and GOL as culprits, respectively (). Sixty-four out of 380 patients reported therapy duration with anti-TNFα agents before developing PXP with an average duration of 18.75 months. 56 out of 380 patients were subsequently switched to a different anti-TNFα agent, and only 39.2% of patients (22 out of 56) reported improvement in the skin (). When switching to a different class of biologic therapy, ustekinumab (UST) was the most common choice (89 out of 97 patients) () (Citation4). 83.1% of patients (74 out of 89) who were switched to UST experienced partial (10 out of 89) or complete resolution (64 out of 89) of PXP (). A much smaller subset of patients was switched to vedolizumab (VDZ) (6 out of 97) and secukinumab (SEC) (2 out of 97), but 100% of these patients experienced complete resolution (). 56 out of 97 patients reported time to resolution of PXP upon switching to a new biologic therapy (i.e. UST, VDZ, and SEC) with an average treatment duration of 7.29 months (). At the same time, disease status of IBD was reported in 70 out of 97 patients with 65 of 89 and 5 out of 6 for UST and VDZ, respectively (). After being switched to UST, 75.4% of patients remained in remission (49 out of 65), 4.6% in partial remission (3 out of 65), and 20.0% in flare of IBD (13 out of 65). After being switched to VDZ, 80.0% of patients remained in remission (4 out of 5) and 20.0% in flare of IBD (1 out of 5) ().
Table 2. Anti-TNF treatments most likely to lead to a psoriasiform reaction.
Table 3. Resolution of anti-TNF-induced psoriasiform reaction and status of IBD upon treatment with alternative biologic therapy.
Discussion
In summary, our systematic review demonstrates that IFX and ADA are responsible for the majority of PXP reported in IBD patients treated with anti-TNFα agents, albeit CZP and GOL are without sufficient treatment data. Switching to UST results in complete or partial resolution of PXP in 83.1% of patients (n = 89), while switching to either VDZ or SEC led to complete resolution in 100% of patients (n = 8). However, more treatment data with VDZ and SEC is needed to determine if they are superior to UST. Additionally, switching to a different biologic therapy for the treatment of PXP should be considered in the context of IBD status. Switching to UST results in IBD flare in 20.0% of patients (n = 65). While switching to VDZ also results in IBD flare in 20.0% of patients (n = 6), treatment data is insufficient at this time to draw any conclusion. Our study was limited by lack of RCTs. However, it highlighted the need for continued investigation in this multidisciplinary field to evaluate the efficacies of available biologic therapies to develop evidence-based treatment algorithms for IBD patients suffering from TNFα-induced PXP.
Author contributions
JSM: concept of the study. RR, SW, and JSM: design of the study. RR, HP, MR, SW, and JSM: acquisition and analysis of data. RR and JSM: interpretation of data. RR, HP, and JSM: drafted the manuscript. RR and JSM: revised the manuscript. All authors approved the version to be published.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Tillack C, Ehmann LM, Friedrich M, et al. Anti-TNF antibody-induced psoriasiform skin lesions in patients with inflammatory bowel disease are characterised by interferon-γexpressing Th1 cells and IL-17A/IL-22-expressing Th17 cells and respond to anti-IL-12/IL-23 antibody treatment. Gut. 2014;63(4):567–577.
- Egeberg A, Thyssen JP, Burisch J, et al. Incidence and risk of inflammatory bowel disease in patients with psoriasis—a nationwide 20-Year cohort study. J Invest Dermatol. 2019;139(2):316–323.
- Abdel-Wahab N, Lopez-Olivo MA, Pinto-Patarroyo GP, et al. Systematic review of case reports of antiphospholipid syndrome following infection. Lupus. 2016;25(14):1520–1531.
- Ezzedine K, Visseaux L, Cadiot G, et al. Ustekinumab for skin reactions associated with antitumor necrosis factor-α agents in patients with inflammatory bowel diseases: a single-center retrospective study. J Dermatol. 2019;46(4):322–327.
- Boggs J, Ramsay B, Lynch M. Paradoxical psoriasis caused by tumour necrosis factor inhibitor therapy. Clin Exp Dermatol. 2021;46(3):580–582.
- Dolinger MT, Rolfes P, Spencer E, et al. Outcomes of children with inflammatory bowel disease who develop anti-Tumor necrosis factor induced skin reactions. J Crohns Colitis. 2022;16(9):1420–1427.
- Eickstaedt JB, Killpack L, Tung J, et al. Psoriasis and psoriasiform eruptions in pediatric patients with inflammatory bowel disease treated with anti-Tumor necrosis factor alpha agents. Pediatr Dermatol. 2017;34(3):253–260.
- George LA, Gadani A, Cross RK, et al. Psoriasiform skin lesions are caused by anti-TNF agents used for the treatment of inflammatory bowel disease. Dig Dis Sci. 2015;60(11):3424–3430.
- Gold SL, Cohen-Mekelburg S, Schneider Y, et al. Management of severe anti-tumor necrosis factor (TNF) induced psoriasis in patients with inflammatory bowel disease. American Journal of Gastroenterology. 2016;111:S827.
- Guerra I, Pérez-Jeldres T, Iborra M, Spanish GETECCU group (ENEIDA project), et al. …. Incidence, clinical characteristics, and management of psoriasis induced by anti-TNF therapy in patients with inflammatory bowel disease: a nationwide cohort study. Inflamm Bowel Dis. 2016;22(4):894–901.
- Hirsch A, Colman RJ, Lang GD, et al. Successful treatment of ulcerative colitis with vedolizumab in a patient with an Infliximab-Associated psoriasiform rash. ACG Case Rep J. 2015;2(4):236–238.
- Kodama S, Gupta D, Sullivan E, et al. Prevalence of paradoxical psoriasis after exposure to tumor necrosis factor inhibitors (TNFI) in children - results from a single tertiary center. Research Square Preprints. 2022. DOI:10.21203/rs.3.rs-133378/v1
- Olbjørn C, Rove JB, Jahnsen J. Combination of biological agents in moderate to severe pediatric inflammatory bowel disease: a case series and review of the literature. Paediatr Drugs. 2020;22(4):409–416.
- Pijls PA, Gilissen LP. Vedolizumab is an effective alternative in inflammatory bowel disease patients with anti-TNF-alpha therapy-induced dermatological side effects. Dig Liver Dis. 2016;48(11):1391–1393.
- Prieto V, Fernandez A, Velasco A, et al. P368 rare skin disorders in IBD patients treated with anti-tnfs. Journal of Crohn’s and Colitis. 2014;8:S219.
- Pugliese D, Guidi L, Ferraro PM, et al. Paradoxical psoriasis in a large cohort of patients with inflammatory bowel disease receiving treatment with anti-TNF alpha: 5-year follow-up study. Aliment Pharmacol Ther. 2015;42(7):880–888.
- Sahuquillo-Torralba A. Paradoxical psoriasiform reactions by anti-TNF treated with ustekinumab in patients with inflammatory bowel disease. Journal of the American Academy of Dermatology. 2019;81(4):AB250.
- Santos S, Gamelas V, Carvalho D, et al. P578 therapeutic strategies in the approach of paradoxical psoriasis in IBD: Experience of a Centre. Journal of Crohn’s and Colitis. 2019;13(Supplement_1):S403–S404.
- White B, Holderman W. Vedolizumab resulted in resolution of anti-tumor necrosis factor psoriasis and sustained clinical and endoscopic remission of Crohnʼs colitis. Inflammatory Bowel Diseases. 2016;22:S79.

