Abstract
Background
CJM112 is a potent anti-IL-17A monoclonal antibody, whose clinical efficacy in psoriasis was recently documented. This study aimed to assess the effect of IL-17A blockade, using CJM112, in patients with moderate to severe acne.
Methods
A randomized, placebo-controlled, double-blind, parallel-group, proof-of-concept study was conducted on patients with moderate to severe acne. Patients received CJM112 300 mg, 75 mg, or placebo subcutaneously during Treatment Period 1 (0–12 weeks). Patients receiving placebo were re-randomized to receive CJM112 300 mg or 75 mg during Treatment Period 2 (12–24 weeks). The primary endpoint was the number of inflammatory facial lesions at Week 12.
Results
As the futility criterion was met during the interim analysis, only 52/75 (69.3%) patients were recruited. In total, 48/52 (92.3%) and 26/41 (63.4%) completed Treatment Periods 1 and 2, respectively. All groups exhibited a reduction in facial inflammatory lesions, with no difference observed between CJM112 and placebo (CJM112 300 mg 27.6 ± 20.7; CJM112 75 mg 30.4 ± 34.8; placebo 23.6 ± 13.6; primary endpoint). Additionally, no differences were observed between groups in other secondary and exploratory endpoints at Week 12.
Conclusions
Anti-IL-17A therapy was not significantly different compared to the placebo in reducing inflammatory lesions in patients with moderate to severe acne.
Introduction
Moderate to severe acne vulgaris is a psychologically debilitating disease developing in the pilosebaceous follicles, that manifests in visible inflammatory lesions, most often on the face, and carries a risk of permanent and disfiguring scarring (Citation1,Citation2). The standard of care for acne is a combination of several topical treatments (topical retinoids and antibacterials) with oral antibiotics, and/or hormonal treatment, with oral isotretinoin used as a last resort therapy (Citation3). However, oral antibiotics and hormonal treatments are often only moderately effective, and antibiotics may introduce bacterial resistance. Further, isotretinoin, while effective, is associated with severe side effects including teratogenicity and a possible link to suicidal ideation (Citation4).
Acne is a chronic relapsing inflammatory skin disease, whereby Cutibacterium acnes (C. acnes; a commensal skin bacterium found in the pilosebaceous follicles) and the innate immune system play a critical role (Citation3,Citation5,Citation6). Infiltrating CD4+ T cells have been observed in the perifollicular space of early acne lesions and suggest that helper T (Th) cells may be involved in immune responses during the development of acne (Citation7). These data are further supported by the presence of IL-17+CD3+ T cells in acne lesions (Citation8). Recently, IL-17A was detected in early acne lesions and was predominantly produced by mast cells which were co-localized with CD4+ T helper cells (Citation9). Furthermore, C. acnes promotes Th17 and Th17/Th1 responses (Citation10), and in vitro studies have shown that C. acnes induced a Th17 response through increased secretion of IL-17A and expression of Th17-related genes, retinoic acid-related orphan receptor alpha (RORα), protein Turandot C (TOTc) and IL-17 receptor antagonist (IL-17RA), an effect which was inhibited by retinoids (Citation11). In addition, increased serum IL-17A may serve as a biomarker of disease severity in acne patients, whose levels were reduced due to effective treatment with isotretinoin (Citation12–14). Another study based on transcriptomic analyses, however, demonstrated that although IL-17A was clearly upregulated in acne lesional skin, its expression was not reduced following successful treatment with isotretinoin (Citation15). These data, coupled with case reports that indicate anti-IL-17A therapy may work in certain subforms of acne (Citation16), suggest that targeting the IL-17A pathway may be a rational approach to the treatment of acne (Citation17,Citation18).
CJM112 is a novel fully human anti-IL-17A IgG1/κ monoclonal antibody that targets a different epitope compared to other IL-17A inhibitors, such as secukinumab. CJM112 exhibits favorable physicochemical properties and has demonstrated up to 50–100-fold higher affinity to IL-17A in vitro compared to secukinumab. CJM112 was developed for the potential treatment of autoimmune and inflammatory conditions. The preliminary clinical safety and efficacy of CJM112 were investigated in a first-in-human study in psoriasis patients (NCT02998671) which showed a positive outcome, with patients receiving 150 mg or 450 mg CJM112 achieving high reductions in Psoriasis Area and Severity Index (PASI) with a single subcutaneous (s.c.) dose, lasting for several months (Citation19).
This study was designed to assess the preliminary efficacy and safety of IL-17A blockade with CJM112 in reducing inflammatory lesions in patients with moderate to severe acne.
Methods
Study design
This study (NCT02998671) was a randomized, placebo-controlled, double-blind, parallel-group, exploratory proof-of-concept study in patients with moderate to severe acne. This study was intended to be carried out in two consecutive treatment periods; Treatment Period 1 (0–12 weeks) and Treatment Period 2 (12–24 weeks). A total number of 75 patients were planned to be included with a 1:1:1 randomization ratio, providing approximately 21 patients in each treatment group with complete 12-week data, assuming a dropout rate of approximately 15%.
Patients were randomized to one of three treatment groups: CJM112 300 mg s.c., CJM112 75 mg s.c. or placebo administered at Week 0, 4, and 8. At the end ofTreatment Period 1 (Week 12), patients were offered the opportunity to remain in the study for Treatment Period 2. Placebo patients were re-randomized to receive monthly s.c. doses of either CJM112 300 mg or 75 mg from Week 12 to 24. Patients who received CJM112 300 mg or 75 mg in Treatment Period 1 continued to receive the same treatment in Treatment Period 2. Dosing was carried out on Weeks 12, 16, 20, and 24. All patients completed a 13-week safety follow-up following the last treatment dose. The study design is summarized in Figure S1.
Patients
Patients aged between 18 and 45 years and weighing 50–120 kg with moderate to severe acne who had failed other systemic therapies were included in this study. Inclusion criteria included a facial inflammatory lesion count between 25 and 100 but with ≤5 facial inflammatory nodules at baseline, and an Investigator’s Global Assessment (IGA) score of at least moderate acne severity of the face (IGA of ≥3). The use of any investigational drug, any oral/systemic treatment or immunomodulator, and previous surgical, physical, light, or laser therapy was prohibited and required a washout period of 4 weeks. Topical prescription and over-the-counter anti-acne treatment also required a 2- and 1-week washout period, respectively, but were allowed as needed in Treatment Period 2. Patients previously treated with either IL-17 or IL-17 receptor blocking agents were excluded. Other forms of acne such as severe nodulocystic acne, secondary acne, acne inversa (hidradenitis suppurativa), or acne associated with a hormonal imbalance were excluded.
Study outcomes and analysis
The primary objective of the study was to determine whether CJM112 was statistically significantly better than placebo in the reduction of the total facial inflammatory lesion (sum of facial papules, pustules, nodules counted on the forehead, right cheek, left cheek, the chin, and nose) at Week 12. A Bayesian model for repeated measurements was used to analyze the log-transformed inflammatory facial lesion count. Non-informative prior distributions were utilized to obtain the posterior estimates and an unstructured covariance matrix was assumed. The log-transformed baseline was centered and standardized before inclusion in the model. To assess preliminary efficacy and futility, an interim analysis was carried out once 50% of the planned number of patients completed Treatment Period 1. Efficacy was defined as at least a 30% difference in inflammatory lesion count at Week 12, based on a placebo-controlled study with an oral antibiotic (Citation20). Futility was defined as at least a 60% probability that the treatment effect of CJM112 at Week 12 was worse than the placebo.
The key secondary objectives of this study were to assess the safety, pharmacokinetics, and immunogenicity of CJM112. Further exploratory study endpoints included IGA and Comprehensive Acne Severity Scale (CASS) responses (used here to assess facial and non-facial acne, respectively (Citation21)), the Dermatology Life Quality Index (DLQI) score, and the facial sebum excretion rate (SER) at Week 12. Non-inflammatory lesion counts (sum of open and closed comedones on the face only, including the forehead, right cheek, left cheek, the chin, and nose) were also obtained.
Ethics statement
The study protocol was reviewed by the Independent Ethics Committee or Institutional Review Board for each study center. This study was conducted according to the ethical principles of the Declaration of Helsinki. Written informed consent was obtained from each patient before the screening.
Results
Study population
In total, 52 patients out of the initially planned 75 patients with moderate to severe facial acne were randomized in Treatment Period 1 (CJM112 300 mg, N = 21; CJM112 75 mg, N = 13; placebo, N = 18). The majority of patients (N = 48, 92.3%) completed 12 weeks of treatment. Of these 52 acne patients, 41 (78.8%) continued to Treatment Period 2 (CJM112 300 mg, N = 17; CJM112 75 mg, N = 10; placebo/CJM112 300 mg, N = 6; placebo/CJM112 75 mg, N = 8) and 11 (21.2%) patients directly entered the follow-up period. The disposition of patients is described in the CONSORT diagram in . Based on the outcome of the interim analysis, this study was terminated early as the predefined criteria for futility were met (≥60% probability that the treatment effect of CJM112 at Week 12 was worse than placebo); further recruitment was stopped from this point in time. Of the 41 patients that entered Treatment Period 2, 26 (63.4%) patients completed the study, and 15 (36.6%) discontinued. The primary reason for discontinuation was study termination by the sponsor (N = 12, 29.3%). The baseline characteristics of all patients (N = 52) entering the study are described in .
Figure 1. CONSORT diagram. Flowchart demonstrating patient disposition in Treatment Period 1 (Weeks 0–12) and Treatment Period 2 (Weeks 12–24). Overall, 48/52 (92.3%) completed Treatment Period 1 and 41/52 (78.8%) entered Treatment Period 2.
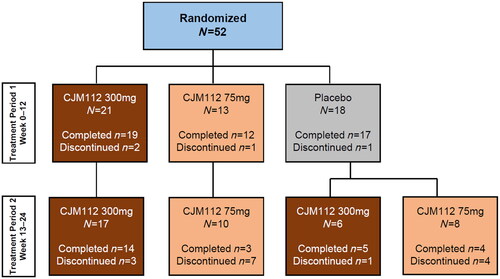
Table 1. Baseline characteristics.
Patients had a high burden of disease at baseline, with a total inflammatory lesion count of 41.5 across groups, 71% (37/52) having an IGA score of 3 (moderate) and 29% (15/52) with an IGA score of 4 (severe). In addition to facial acne, 46% (24/52) of the total study population reported moderate to severe non-facial involvement (CASS score of 3–4). In general, baseline demographics and disease characteristics were balanced between the treatment groups. Of note, there was an imbalance between placebo and treatment groups regarding the mean nodule count at baseline (CJM112 300 mg, 3.5 ± 7.0; CJM112 75 mg, 3.8 ± 3.1; placebo, 0.9 ± 1.6).
Efficacy
The study was terminated early since the futility criterion was met at the time of the interim analysis. At the time of interim analysis, 14 patients in CJM112 300 mg, three patients in the CJM112 75 mg group, five patients in the placebo/CJM112 300 mg group, and four patients in the placebo/CJM112 75 mg groups completed Week 24.
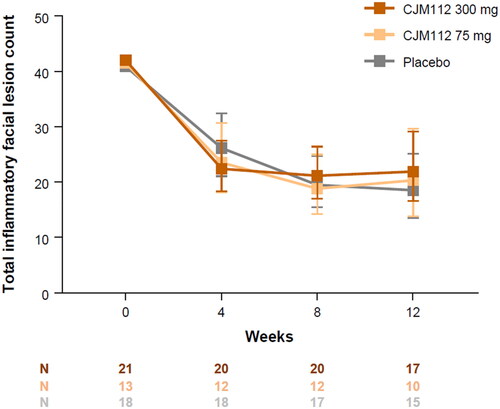
There was a reduction in the total facial inflammatory lesion count (papules, pustules, and nodules) in all treatment groups at Week 12, with no significant difference between groups (geometric mean total lesion count and 90% confidence intervals at Week 12: CJM112 300 mg, 21.9 (16.58, 29.14); CJM112 75 mg, 20.3 (13.76, 29.66); placebo, 18.5 (13.51, 25.13)) ().
Figure 2. Total inflammatory facial lesion counts up to Week 12. Line graph demonstrating the effect of CJM112 300 mg, CJM112 75 mg, and placebo on the total inflammatory facial lesion count in patients with moderate to severe inflammatory acne from baseline to Week 12. The log-transformed inflammatory facial lesion count was analyzed using a Bayesian model for repeated measurements. Data are demonstrated as geometric mean and 90% confidence intervals. Numbers, and the number of patients included in the analysis at each time point.
An overview of primary and secondary endpoints from baseline to Week 12 is described in . In the first 12 weeks of treatment, there was no clear reduction in non-inflammatory lesion counts observed in the CJM112 treatment groups. The mean percentage change from baseline to Week 12 in non-inflammatory lesion counts was 2%, 26%, and −32% in the CJM112 300 mg, CJM112 75 mg, and placebo groups, respectively. The total nodule count (facial and non-facial) however, was reduced in CJM112-treated patients compared to placebo. At Week 12, the mean absolute change from baseline was −3.5, −2.5, and 0.1 for CJM112 300 mg, CJM112 50 mg, and placebo patients, respectively.
Table 2. Key efficacy endpoint results at Week 12.
At Week 12, the observed number of IGA responders (defined as having a score of IGA 0/1 indicating clear or almost clear skin of the face) at Week 12 was 0% (0/17), 30% (3/10), and 6.7% (1/15) in patients treated with CJM112 300 mg, 75 mg or placebo, respectively. The observed number of CASS responders (defined as a two-grade improvement or achieving clear/almost clear the skin of the trunk) at Week 12 was 36% (5/14), 50% (5/10), and 39% (5/13) in CJM112 300 mg, 75 mg or placebo groups, respectively.
Similar to efficacy outcomes, DLQI scores improved in all groups, with no differences observed between CJM112 and placebo. DLQI at baseline vs. Week 12 was 7.0 ± 4.9 vs. 4.3 ± 5.3 for CJM112 300 mg, 8.8 ± 5.7 vs. 7.3 ± 7.1 for CJM112 75 mg, and 7.8 ± 7.1 vs. 5.7 ± 5.5 for placebo, respectively.
At Week 12, a reduction in mean SER was also observed in all treatment groups. The mean percentage change in SER from baseline was −15%, −36%, and −21% in CJM112 300 mg, CJM112 75 mg, and placebo groups, respectively. Most of the patients had baseline hsCRP values within normal ranges (0–5 mg/L); however, there were no major changes observed across groups up to Week 12.
The number of patients completing Treatment Period 2 was even lower and varied across groups, with just 26 patients completing the Week 24 visit (see for a number of patients completing Treatment Period 2). Even at this later time point, there were no clear patterns suggesting clinically meaningful reductions above the natural variability of inflammatory lesions up to 6 months of treatment with either dose of CJM112. None of the other efficacy parameters mentioned above showed meaningful results in Treatment Period 2 (data on file).
Pharmacokinetics and immunogenicity
Serum trough concentrations of CJM112 300 mg and CJM112 75 mg increased progressively from Week 0 to 12. The mean trough concentration at Week 12 was 17,000 ± 8080 ng/mL and 2040 ± 1570 ng/mL in the CJM112 300 mg and CJM112 75 mg groups, indicating a tendency for an over-proportional increase of exposure with dose. Mean trough concentrations of CJM112 remained relatively constant from Week 12 to 24, in both the CJM112 300 mg (19,400 ± 9650 ng/mL at Week 24) and CJM112 75 mg groups (3890 ± 1890 ng/mL at Week 24), with CJM112 75 mg showing greater variability. Trough concentrations were in the expected range. For placebo patients who switched to CJM112 300 mg, the mean trough concentrations increased progressively from Weeks 12 to 24. A similar pattern was observed in patients who switched from placebo to CJM112 75 mg.
Two of 27 (7.4%) patients exposed at least once to CJM112 300 mg treatment were considered anti-drug-antibody (ADA) positive with low titers; however, pharmacokinetic profiles of ADA-positive patients were not affected. No patients exposed to CJM112 75 mg were ADA-positive.
Safety
Of the 52 randomized patients, 48 patients were exposed to at least one dose of CJM112 throughout the study. Overall, 37/52 (71.2%) patients experienced at least one adverse event (AE) in Treatment Period 1 (a total of 94 AEs reported), with no serious AEs reported. In Treatment Period 1, the incidence of AEs was higher in the pooled CJM112 groups (27/34, 79.4%) compared to placebo (10/18, 55.6%), though no dose-response was observed. The most frequently observed AE among patients treated with CJM112 was infections (MedDRA system organ class term: ‘infections and infestations’) (15/34, 44.1%), which were higher in the CJM112 pooled group compared to placebo (44.1% vs. 16.7%). The most common infections by preferred term were nasopharyngitis, upper respiratory tract infection, and urinary tract infection. The incidence of AEs occurring in at least two patients in any given group is described in . Two patients (one in CJM112 75 mg and one in the placebo group) discontinued study treatment due to an AE. One patient in the CJM112 75 mg reported acne worsening on Day 64, and the study treatment was permanently discontinued at Week 12 because of this event. A second patient in the placebo group reported a moderate AE of depression on Day 15, and treatment was permanently discontinued. Overall, 29/41 (70.7%) patients experienced at least one AE in Treatment Period 2, although the incidence of AEs did not reveal any additional clear data due to small numbers. One serious AE was reported in Treatment Period 2; a patient in the CJM112 300 mg group reported cholelithiasis on Day 123 and underwent a cholecystectomy on Day 162. This event was deemed unrelated to study treatment and the study dosing was not changed or interrupted.
Table 3. Incidence of adverse events in Treatment Period 1.
Discussion
The pathophysiology of inflammation in acne has not yet been fully elucidated. Several immune-mediated inflammatory processes (in particular from the innate immune system) have been described, including upregulated cytokines such as IL-6, IL-8, or IL-1 (Citation22,Citation23) or pro-inflammatory leukotriene (such as leukotriene B4) (Citation23,Citation24). Monoclonal antibodies targeting IL-1 have been previously tested in acne patients, such as anti-IL-1α (bermekimab, also known as MABp1) or anti-IL1β antibodies (gevokizumab) with only minor beneficial effects observed in reducing inflammatory lesions (Citation25,Citation26). A Phase 2 trial with imsidolimab, which blocks the IL-36 receptor, has been initiated in adolescent and adult patients with acne (clinicaltrials.gov identifier NCT04856917).
In recent years, several studies have alluded to a potential pro-inflammatory role of IL-17A in acne (Citation7–15). Kelhälä et al. demonstrated significant elevation of IL-17A and other Th17 signature cytokines, Th17 lineage differentiation cytokines, and IL-17-related microbial peptides in acne lesions. IL-17A+T cells were also identified in immunohistochemical analysis of early acne lesions that localized to the perilesional infiltrate and epidermis (Citation8,Citation27). Furthermore, T cells stimulated with C. acnes induced IL-17A production in vitro, an effect which was reversed with all-trans-retinoid acid (ATRA) therapy. Clinically, IL-17A was increased in the serum of acne patients and was decreased following isotretinoin treatment, suggesting a potential role for IL-17A as a biomarker of disease in acne (Citation12,Citation13,Citation28). Finally, increased levels of antimicrobial peptides associated with the IL-17A-pathway (e.g. β defensin 2 and calprotectin) (Citation29), have been shown to be upregulated in acne lesions and respond to treatment (Citation30,Citation31). Taken together, these data suggest a potential role for IL-17A in the pathophysiology of acne.
To the best of our knowledge, this prospective randomized placebo-controlled clinical study was the first to test the clinical utility of an IL-17A inhibitor in patients with moderate to severe acne. Unexpectedly, this study was terminated early following a planned interim analysis, having met the predefined futility criterion. At Week 12, no difference was observed between either of the two CJM112 dose groups and placebo in the reduction of total inflammatory facial lesion count from baseline (primary endpoint). The reduction of facial inflammatory lesions was between 24 and 43% across CJM112 and placebo groups; these results are comparable to larger studies which demonstrated a reduction of 38% and 35% in placebo-treated patients (Citation32,Citation33). No significant differences or trends were observed in CJM112-treated patients compared to placebo in other clinical endpoints at Week 12 or even later endpoints up to 24 weeks, although only a limited number of patients completed the 24-week study treatment (n = 26). Of note, facial and non-facial nodules decreased more from baseline to Week 12 in CJM112-treated patients. However, due to the imbalance in the mean number of nodules at baseline, these results should be interpreted with caution.
CJM112, a classical IgG1 selective and potent anti-IL-17A antibody, has previously shown significant efficacy in patients with psoriasis. A 75% reduction in PASI (PASI75) was achieved with single or multiple doses either lower or similar to the levels in the current study, and considered to be an effective anti-IL-17A agent in the treatment of psoriasis (Citation19). Monthly s.c. administration of CJM112 300 mg and CJM112 75 mg in the present study resulted in the expected increase of CJM112 trough concentrations over time, approaching a steady state after approximately four doses. A comparison of the steady-state serum levels of the 300 mg and CJM112 75 mg dose groups indicated that exposure increased in a more than dose-proportional manner. CJM112 was also safe and well-tolerated. There were no deaths and only one serious AE was reported (not considered related to the study drug). Most reported AEs were considered mild in intensity, regardless of the treatment group. As expected with an anti-IL-17A antibody, slightly more infections were observed in the CJM112 groups compared to placebo; however, these AEs remained non-serious and did not lead to treatment discontinuation. The safety findings are in line with previous observations with anti-IL-17A antibodies (Citation1).
Despite its obvious limitations, in particular, the small sample size and unexpected early termination, this exploratory study was the first to access the clinical utility of IL-17A blockade in moderate to severe acne. Under these study conditions and within this patient population, CJM112 therapy, despite having demonstrated clear efficacy in psoriasis, did not demonstrate significant efficacy in reducing inflammatory facial lesions in patients with moderate to severe acne compared to placebo.
One recent case series demonstrated the clinical utility of secukinumab in treating acne fulminans, a less common subtype of severe inflammatory acne. Five patients treated with secukinumab achieved partial (n = 2) or complete (n = 3) response at 6 months (Citation16). This data indicate that anti-IL-17A therapy may be effective in a more inflamed acne phenotype. Further clinical studies should be undertaken to delineate the underlying role of IL-17A in driving inflammation in acne, leading to the clinical applicability of anti-IL-17 agents in the treatment of this skin disorder.
Supplemental Material
Download PDF (64.4 KB)Acknowledgements
The authors thank Trudy McGarry, PhD for providing medical writing support/editorial support, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Disclosure statement
The authors of this manuscript have the following competing interests: JLJ, EG, and CL are employed by Novartis. MS is currently employed with Priothera SAS but was employed by Novartis at the time the study was conducted. RR and NC have no financial disclosures to declare. DMT has served as a consultant to Novartis.
Data availability statement
Novartis is committed to sharing with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations.
Additional information
Funding
References
- Harper JC, Thiboutot DM. Pathogenesis of acne: recent research advances. Adv Dermatol. 2003;19:1–10.
- Chernyshov PV, Zouboulis CC, Tomas-Aragones L, et al. Quality of life measurement in acne. Position Paper of the European Academy of Dermatology and Venereology Task Forces on Quality of Life and Patient Oriented Outcomes and Acne, Rosacea and Hidradenitis Suppurativa. J Eur Acad Dermatol Venereol. 2018;32(2):194–208.
- Dreno B, Gollnick HP, Kang S, et al. Understanding innate immunity and inflammation in acne: implications for management. J Eur Acad Dermatol Venereol. 2015;29(Suppl. 4):3–11.
- Das S, Reynolds RV. Recent advances in acne pathogenesis: implications for therapy. Am J Clin Dermatol. 2014;15(6):479–488.
- Dreno B, Pecastaings S, Corvec S, et al. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: a brief look at the latest updates. J Eur Acad Dermatol Venereol. 2018;32(Suppl. 2):5–14.
- Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet. 2012;379(9813):361–372.
- Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121(1):20–27.
- Kelhala HL, Palatsi R, Fyhrquist N, et al. IL-17/Th17 pathway is activated in acne lesions. PLOS One. 2014;9(8):e105238.
- Eliasse Y, Leveque E, Garidou L, et al. IL-17(+) mast cell/T helper cell axis in the early stages of acne. Front Immunol. 2021;12:740540.
- Kistowska M, Meier B, Proust T, et al. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J Invest Dermatol. 2015;135(1):110–118.
- Agak GW, Qin M, Nobe J, et al. Propionibacterium acnes induces an IL-17 response in acne vulgaris that is regulated by vitamin A and vitamin D. J Invest Dermatol. 2014;134(2):366–373.
- Karadag AS, Ertugrul DT, Bilgili SG, et al. Immunoregulatory effects of isotretinoin in patients with acne. Br J Dermatol. 2012;167(2):433–435.
- Ebrahim AA, Mustafa AI, El-Abd AM. Serum interleukin-17 as a novel biomarker in patients with acne vulgaris. J Cosmet Dermatol. 2019;18(6):1975–1979.
- Singh A, Khurana A, Sardana K, et al. Correlation of serum 25-hydroxy vitamin D and interleukin-17 levels with disease severity in acne vulgaris. Indian J Dermatol. 2021;66(3):291–296.
- Kelhala HL, Fyhrquist N, Palatsi R, et al. Isotretinoin treatment reduces acne lesions but not directly lesional acne inflammation. Exp Dermatol. 2016;25(6):477–478.
- Bocquet-Tremoureux S, Corvec S, Khammari A, et al. Acne fulminans and Cutibacterium acnes phylotypes. J Eur Acad Dermatol Venereol. 2020;34(4):827–833.
- Kurokawa I, Layton AM, Ogawa R. Updated treatment for acne: targeted therapy based on pathogenesis. Dermatol Ther. 2021;11(4):1129–1139.
- Thiboutot DM, Layton AM, Anne Eady E. IL-17: a key player in the P. acnes inflammatory cascade? J Invest Dermatol. 2014;134(2):307–310.
- Kaul M, Jarvis P, Rozenberg I, et al. First-in-human study demonstrating the safety and clinical efficacy of novel anti-IL-17A monoclonal antibody CJM112 in moderate to severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2021;35(5):1143–1151.
- Fleischer AB Jr., Dinehart S, Stough D, et al. Safety and efficacy of a new extended-release formulation of minocycline. Cutis. 2006;78(4 Suppl.):21–31.
- Tan JK, Tang J, Fung K, et al. Development and validation of a Comprehensive Acne Severity Scale. J Cutan Med Surg. 2007;11(6):211–216.
- Kurokawa I, Danby FW, Ju Q, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18(10):821–832.
- Dagnelie MA, Corvec S, Saint-Jean M, et al. Cutibacterium acnes phylotypes diversity loss: a trigger for skin inflammatory process. J Eur Acad Dermatol Venereol. 2019;33(12):2340–2348.
- Alestas T, Ganceviciene R, Fimmel S, et al. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med. 2006;84(1):75–87.
- Carrasco D, Stecher M, Lefebvre GC, et al. An open label, phase 2 study of MABp1 monotherapy for the treatment of acne vulgaris and psychiatric comorbidity. J Drugs Dermatol. 2015;14(6):560–564.
- Announces encouraging interim results from gevokizumab phase 2 study for moderate to severe acne vulgaris; 2013 [Internet]. Available from: https://www.servier.ch/de/aktuell/news-detail/2/xoma-announces-encouraging-interim-results-from-gevokizumab-phase-2-study-for-moderate-to-severe-acne-vulgaris/
- Farag AGA, Maraee AH, Rifaat Al-Sharaky D, et al. Tissue expression of IL-17A and FOXP3 in acne vulgaris patients. J Cosmet Dermatol. 2021;20(1):330–337.
- Abd-Elmaged WM, Nada EA, Hassan MH, et al. Lesional and circulating levels of interleukin-17 and 25-hydroxycholecalciferol in active acne vulgaris: correlation to disease severity. J Cosmet Dermatol. 2019;18(2):671–676.
- Chronnell CM, Ghali LR, Ali RS, et al. Human beta defensin-1 and -2 expression in human pilosebaceous units: upregulation in acne vulgaris lesions. J Invest Dermatol. 2001;117(5):1120–1125.
- Aksoy G, Adisen E, Erdem O, et al. Comparison of efficacy of doxycycline and isotretinoin on cutaneous human beta-defensin-1 and -2 levels in acne vulgaris. Indian J Dermatol. 2018;63(5):380–385.
- Borovaya A, Dombrowski Y, Zwicker S, et al. Isotretinoin therapy changes the expression of antimicrobial peptides in acne vulgaris. Arch Dermatol Res. 2014;306(8):689–700.
- Leyden JJ, Sniukiene V, Berk DR, et al. Efficacy and safety of sarecycline, a novel, once-daily, narrow spectrum antibiotic for the treatment of moderate to severe facial acne vulgaris: results of a phase 2, dose-ranging study. J Drugs Dermatol. 2018;17(3):333–338.
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17(9):987–996.

