Abstract
Objective
To conduct a systematic review and meta-analysis to verify the efficacy of using autologous platelet-rich plasma (PRP) in female pattern alopecia (FPA).
Background
Androgenetic alopecia is the leading cause of hair loss in men andwomen and often impacts self-esteem and quality of life.
Data sources
MEDLINE/PubMed, Cochrane Library, ClinicalTrials.gov, and EMBASE up to May 2021.
Study selection and data extraction
We identified all studies evaluating the effect of PRP in FPA. A narrative synthesis was performed from data on the efficacy of PRP treatment and adverse effects; quantitative results of PRP use compared to control treatment for female androgenetic alopecia (AGA) were synthesized. The outcomes analyzed were terminal density and hair thickness.
Results
Seven articles were selected for this review. Meta-analysis showed that PRP-based interventions were able to increase terminal hair density compared to control (standardized mean difference (SMD)=2.98, 95% confidence intervals (CIs)=1.10, 4.85), with no significant increase in hair thickness (SMD = 1.16, 95% CI= −0.96, 3.28). During and after treatment, no major side effects were reported by patients or researchers.
Conclusions
The use of autologous PRP injections in female AGA seems to be promising, with more consistent results on terminal hair density. However, caution is recommended in the interpretation of these results until they can be replicated in larger and more representative samples. PROSPERO registration number CRD42021257154.
Introduction
Androgenetic alopecia (AGA) is a frequent cause of seeking consultation with a dermatologist (Citation1). This demand can be explained by the fact that this pathology has great esthetic impact on both men and women, as baldness is often associated with aging, something which people want to postpone (Citation2).
The condition in question is characterized by progressive hair loss. The region of this rarefaction varies between men and women. In women, the threads become reduced in diameter and length, and lighter or whiter. This change occurs mainly in the frontal, central, and parietal scalp, with preservation of the frontal line. In men, there is a deep recession of the frontotemporal hair line, and the apex of the scalp is also affected (Citation3).
Tamashunas and Bergfeld (Citation4) emphasize that it is not only in the anatomical pattern that they differentiate between alopecia observed in men and that observed in women, it is believed that the etiology and pathogenesis also differ.
In men, this hair loss occurs because of the effects of dihydrotestosterone (DHT), which is a metabolite of testosterone, on susceptible hair follicles. DHT binds to androgen receptors on hair follicles, resulting in an upregulation of genes responsible for the gradual transformation of hair follicles (Citation2).
In women, some authors believe that a similar process occurs, but there is evidence to the contrary. Those who defend this theory claim that in women with androgenetic disorders, such as micropolycystic ovary syndrome (MPOS), alopecia is a characteristic (Citation4). On the other hand, those who refute this conjecture do so based on studies such as the one by Futterweit et al. (Citation5). These authors tested the androgen levels of 109 women with hair loss, and it was found that only 39% of the participants had laboratory signs of hyperandrogenism.
Regarding genetic relationship, in men this susceptibility is already well established. However, in female alopecia, little is known (Citation2,Citation4,Citation6). Ho et al. (Citation2) conducted a review on the topic and concluded that recently published studies could not clearly identify any alopecia susceptibility locus or gene in women, but they suggest that the etiology differs substantially from male AGA. Thus, while the crucial roles of androgens and genetic susceptibility to male AGA are well accepted, the degree to which these factors contribute to female pattern alopecia (FPA) is less clear.
Tamashunas and Bergfeld (Citation4) emphasize that it was lacking evidence that contributed to the replacement of the terminology ‘androgenetic alopecia’ used for women by the term ‘female pattern hair loss’ (FPHL) or ‘female pattern alopecia’.
Despite this divergence, the essentiality of treatment for both sexes is undeniable since baldness impacts the quality of life of patients. For this, Minoxidil® and alpha-5-reductase inhibitors (finasteride, dutasteride) are often the most adopted therapies, but they still have limits in terms of efficacy, contraindications and side effects, thus, the demand for other measures has grown (Citation2).
Given this scenario, the number of clinical trials evaluating the efficacy of platelet-rich plasma (PRP) in male and female androgenic alopecia has increased exponentially over the last decade and has shown promise (Citation4). It is a centrifuged human blood derivative that contains a high concentration of cytokines, platelet-derived growth factor (PDGF), transforming growth factor (TGF), interleukin-1, VEGF, EGF, and IGF. These growth factors stimulate hair follicle stem cells, promoting neovascularization through interaction with mesenchymal cells of the dermal papilla (Citation7).
With this, researchers have endeavored to conduct systematic literature reviews to ensure that the treatment for AGA with PRP has a high level of scientific evidence (Citation7–9). However, these studies encompass randomized clinical trials (RCTs) that have male and female participants. Thus, given what has been exposed so far, it is possible to assume that the measurement of effectiveness for women can be wrong, since there are differences in the mode of the disease depending on gender.
From this conjecture, the following research question arises: what is the efficacy of using autologous PRP in FPA?
To answer this question, a systematic literature review and meta-analysis were performed to verify the efficacy of using autologous PRP in FPA.
The hypothesis is that the treatment aimed at female patients has a different efficacy from that observed in male individuals.
This theme is relevant for both physicians and patients, as it will allow specialists to base their therapy on quality scientific evidence, and the patient to benefit from a method of proven efficacy and presumed adverse effects, thus ensuring treatment safety.
The justification for carrying out this study is based on the lack of level I scientific evidence that assesses the treatment of alopecia in women through PRP.
Methods
Study design
This is a systematic review and meta-analysis to verify the efficacy and safety of using autologous PRP in the treatment of FPA.
Definition of the clinical issue
The definition of the specific question was carried out under the PICOT acronym, as shown in .
Information sources
To conduct this study, the MEDLINE/PubMed, Cochrane library, Clinicaltrials.gov, and EMBASE databases were used. They were searched without restrictions on dates or languages. In addition, the reference list of included studies was also manually analyzed by the reviewers.
This review was designed and conducted according to PRISMA recommendations (Citation10), and the research protocol (Supplementary material A) was registered in PROSPERO (CRD42021257154).
Search strategy
To retrieve articles, the following search terms were used (the last search date was May 2021) using the Boolean operators OR and AND: (Female Pattern Baldness), (Female Pattern Hair Loss), (Female Androgenic Alopecia), (Androgenic Alopecia in Women) AND (platelet-rich plasma).
On the Cochrane library and Clinicaltrials.gov platforms, the terms described were inserted without modification. However, to carry out the search in the MEDLINE/PubMed and EMBASE databases, MeSH and EMTREE terms were inserted, respectively.
Therefore, to perform the PubMed search, the appropriate MeSH terms were first searched. By inserting the studied condition (Female Pattern Baldness, Female Pattern Hair Loss and Female Androgenic Alopecia) the platform generated the following terms: (Baldness), (Hair Loss), (Hair Losses), (Loss, Hair), (Losses, Hair), (Alopecia, Male Pattern), (Male Pattern Alopecia), (Baldness, Male Pattern), (Male Pattern Baldness), (Female Pattern Baldness), (Baldness, Female Pattern), (Androgenetic Alopecia), (Pattern Baldness), (Baldness, Pattern), (Androgenic Alopecia), (Alopecia, Androgenic), (Alopecias, Androgenic), (Androgenic Alopecias), (Alopecia, Androgenetic), (Pseudopelade), (Alopecia Cicatrisata), (Alopecia Cicatrisatas).
After excluding the terms that retrieved articles referring to male AGA, the flowing search terms remained and were used together with the Boolean operators OR and AND: (Baldness), (Hair Loss), (Hair Losses), (Loss, Hair), (Losses, Hair), (Female Pattern Baldness), (Baldness, Female Pattern), (Androgenetic Alopecia), (Pattern Baldness), (Baldness, Pattern), (Androgenic Alopecia), (Alopecia, Androgenic), (Alopecias, Androgenic).
Regarding the target audience of this review (Women and Females), the platform generated the following terms: (Females), (Girls), (Girl), (Woman), (Women’s Groups), (Women Groups), (Women’s Group).
The following filters for RCTs already standardized for this type of study by Robinson and Dickersin (Citation11) were associated with the MeSH terms already described: (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized controlled trials[mh] OR random allocation[mh] OR double-blind method[mh] OR single-blind method[mh] OR clinical trial[pt] OR clinical trials[mh] OR (‘clinical trial’[tw]) OR ((singl*[tw] OR doubl*[tw] OR trebl*[tw] OR tripl*[tw]) AND (mask*[tw] OR blind*[tw])) OR (‘latin square’[tw]) OR placebos[mh] OR placebo*[tw] OR random*[tw] OR research design[mh:noexp] OR follow-up studies[mh] OR prospective studies[mh] OR cross-over studies[mh] OR control*[tw] OR prospectiv*[tw] OR volunteer*[tw]) NOT (animal[mh] NOT human[mh]).
To search the EMBASE platform, in addition to the terms listed above, EMTREE terms were used together with the Boolean operators OR and AND: ‘platelet-rich plasma cell’/exp, ‘female’/exp, ‘alopecia’/exp.
Furthermore, for the search in this database, filters for RCTs validated by Robinson and Dickersin (Citation11) were also inserted: ‘crossover procedure’/exp AND [embase]/lim OR (‘prospective study’/exp AND [embase]/lim) OR (‘follow up’/exp AND [embase]/lim) OR (‘placebo’/exp AND [embase]/lim) OR (‘clinical trial’/exp AND [embase]/lim) OR (‘single blind procedure’/exp AND [embase]/lim) OR (‘double blind procedure’/exp AND [embase]/lim) OR (‘randomization’/exp AND [embase]/lim) OR (‘controlled clinical trial’/exp AND [embase]/lim) OR (‘randomized controlled trial’/exp AND [embase]/lim).
Study selection
Inclusion criteria were RCTs, non-RCTs and studies assessing treatment with PRP in women with AGA. Studies evaluating treatment with PRP in men with AGA; pre-clinical model (animal studies); in vitro studies; and narrative reviews, case studies/series, hypothetical cases, observational studies were excluded.
Data collection process
The studies were selected and characterized in a table according to the type of study, year of publication, country of origin, number of participants, age of participants and analyzed outcomes.
After that, the articles were analyzed for quality by two researchers (AFQO and MRPR) using the ROB 2.0 tool (Citation12) for RCTs and the ROBINS-I tool (Citation13) for non-RCTs. After the initial analysis, discrepancies between items were discussed and consensus was reached with the help of a third researcher (FPNA).
A narrative synthesis was performed from data on the efficacy of PRP treatment compared to placebo, the efficacy of PRP in different preparations, the efficacy of PRP alone, patient satisfaction, and the adverse effects
Quantitative results of PRP use compared to control for female AGA treatment were synthesized. In the absence of a comparator arm, we carefully selected the most adequate comparator from another study in this review. The outcomes analyzed were terminal density and hair thickness. From this, it was possible to carry out a meta-analysis using standardized mean difference (SMD) with 95% confidence intervals (CIs) within a random effects model. Data were summarized in a forest-plot type graph.
Results
Study selection
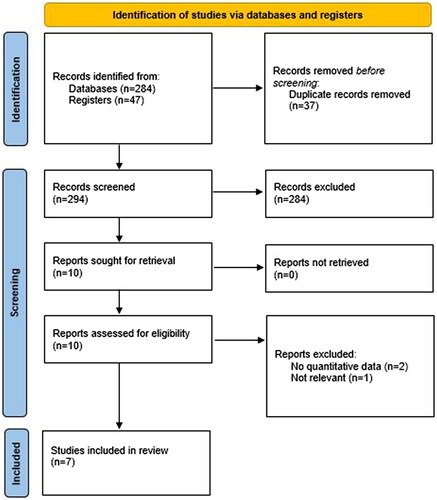
This systematic review aimed to assess the effectiveness of autologous PRP in FPHL/AGA. In our search, we found 331 abstracts (42 in MEDLINE/PubMed; 175 in Cochrane Library; 47 in ClinicalTrials.gov; and 67 in EMBASE). Of these 37 duplicate records were removed. All titles and abstracts were reviewed and then 10 articles were read in full and reviewed for eligibility checking. Seven articles were selected for inclusion in this review ().
Figure 2. Search strategy according to the PRISMA protocol (Citation10).
Study characteristics
The studies included in this review were conducted between 2013 and 2019 (Citation14–17), three studies did not report year of intervention (Citation18–20). Three studies were conducted in the USA (Citation15,Citation16,Citation19), two in Egypt (Citation17,Citation20), one in Italy (Citation14), and one in Korea (Citation18). These studies included a total of 171 female participants, aged ≥18 years, diagnosed with AGA. Five out of the seven studies were reported as randomized, with four being considered as individually randomized, parallel group trials (Citation16,Citation18–20), and one as an individually randomized, crossover trial (Citation15); the study by El-Husseiny et al. (Citation17) was a comparative clinical study and all patients received both treatment regimens in the different halves of the scalp; and the study by Starace et al. (Citation14) was a single group before-and-after study. Further characteristics of the studies included in this review are presented in .
Table 1. Characteristics of studies included in this systematic review.
Risk of bias within studies
The quality of the included studies was independently assessed by two researchers (AFQO and FPNA) using the ROB 2.0 tool (Citation12) for RCTs, and the ROBINS-I tool (Citation13) for non-RCTs. After initial analysis, discrepancies between items were discussed, and consensus was reached.
The results of the quality assessment for the individual randomized, parallel group trials are shown in . For the terminal hair density outcome, the overall rating of one study (Citation16) was low risk, one study (Citation20) was rated as some concerns; and two studies (Citation18,Citation19) were rated as high risk. For the hair thickness/caliber outcome, two studies (Citation16,Citation20) were rated as some concerns; and one study (Citation18) was rated as high risk. Also in this review, there was one individual randomized, crossover trial (Citation15), which was assessed for the terminal hair density outcome and rated as some concerns ().
Table 2. Quality assessment of the individual randomized, parallel group trials.
Table 3. Quality assessment of the individual randomized, crossover trial.
Overall study design raised some concern. Five interventions (Citation15,Citation16,Citation18–20) were described as RCTs; however, only two trials (Citation16,Citation19) had a control group; and in two studies (Citation15,Citation20) the participants represented both the intervention and control arms.
In only four out of seven studies (Citation15–17,Citation20), some baseline characteristics were provided; and in one study baseline data was only provided as figure, with no mean, standard deviation, etc. (Citation18). For the other two studies, no baseline data were informed, data were reported as ‘no improvement’, ‘some improvement’, or ‘substantial improvement’ in percentages between pre- and post-treatment (Citation19); and relative percentage change (%RC) at 9, 12, and 24 weeks after first visit (baseline) (Citation14).
In the trials with different invention arms, only three out of six studies (Citation16,Citation19,Citation20) were considered double-blind; in one trial (Citation18) only the assessors were blinded; information on blinding was not provided in two trials (Citation15,Citation17).
A variety of outcome measures were reported, the most common being hair density (vellus and terminal), hair thickness/diameter, hair growth, hair counts, volume, hair pull test, and patients’ satisfaction/quality of life. The two most common being terminal hair density (Citation15–18,Citation20), and hair thickness (Citation16,Citation18,Citation20).
Data on dropouts were informed for only two studies (Citation15,Citation16). There were two dropouts in each study, in each intervention arm; only one study (Citation15) provided reasons of dropouts, which were personal (1) and medical (1) reasons.
The quality assessment for the non-randomized trials was carried out using the ROBINS-I tool () (Citation13). The overall rating for the two studies was serious (Citation14,Citation17).
Table 4. Quality assessment of non-randomized studies – interventions.
Results of individual studies
Studies comparing PRP with placebo
Of the seven studies, three (Citation16,Citation19,Citation20) compared the use of PRP with placebo. Puig et al. (Citation19) conducted a double-blind, multicenter, placebo-controlled pilot study. This study was conducted in Texas, USA with 26 women (≥18 years) diagnosed with Ludwig II female AGA. Participants were assessed every 4 weeks for possible adverse effects. At week 26, final data were collected for hair count, hair mass index, and patient survey. For the hair count and hair mass index, there was no significant difference between the intervention and placebo groups (p=.503 and .202, respectively); in the patient survey, 26.7% of participants in the intervention group (compared to 18.2% in the placebo group) reported heavier or coarser hair after treatment.
Another double-blind randomized controlled study was performed with 30 females aged 20–45 years, diagnosed with FPHL (Citation20). Patients were submitted to four treatment sessions, with 1-week intervals, with an autologous (double-spin) PRP injection in a randomized area of the scalp and a normal saline injection in the other. The data collection end points, assessed at 6 months after the last treatment, consisted of hair growth, hair density, hair diameter, volume, hair pull test, and patients’ satisfaction. For the PRP-injected areas, there was a significant increase for hair density and thickness when compared to baseline and placebo, p<.005. Mean patient satisfaction was rated at 7.0 on a scale of 1–10 for the PRP-injected sites.
Finally, in a prospective RCT conducted at a single center in the USA, Dubin et al. (Citation16) sought to analyze whether PRP injections were able to improve female AGA. This trial included 30 females diagnosed with AGA, aged ≥18 years. Participants received subdermal scalp injections of Eclipse system PRP at 0, 4, and 8 weeks or saline injection. Outcome measures were analyzed at week 24, and there were two dropouts (one in each intervention arm). Researchers reported a significant improvement in hair density (p<.01), mean hair caliber (p<.01), and blinded photographic assessment (p<.01); regarding adverse effects, the most common was headache (occurring in 50% of those in the PRP group vs. 29% in the placebo group), followed by sensation of scalp tightness (occurring in 50% of those in the PRP group vs. 21% in the placebo group), other adverse effects were only cited by participants in the PRP group, such as swelling, redness, tingling, and post-injection bleeding. Nevertheless, all adverse events were classified as mild and lasted up to 24 h.
Studies comparing different PRP preparations
One clinical study compared the use of different PRP preparations (Citation17). In this comparative clinical study, females aged 23–50 years, diagnosed with FPHL were submitted to three PRP sessions (at 3-week intervals). Participants received an intradermal injection of a single-spin PRP preparation in the left half of the scalp and a double-spin PRP preparation in the right half. Study outcomes were patients’ satisfaction and terminal hair density was measured at baseline and 6 weeks after the last session. For patient satisfaction, on a scale from 1 to 10, mean satisfaction was 6.60 ± 1.59 SD; however, patients did not notice significant difference between the two halves of the scalp. Terminal hair density improved in both treatment arms, but with significant difference (when compared to baseline) in the right half which was treated with double-spin PRP, p=.001.
Studies analyzing the efficacy of PRP treatment alone
One study carried out by Starace et al. (Citation14), in a single group of 10 females aged 33–64 years, diagnosed with AGA, sought to examine the efficacy, tolerability, and clinical improvement of PRP for the treatment of AGA. Participants received an interfollicular injection of PRP preparation in a total of four sessions with 2-week intervals. Relative percentage change in the values of hair density, hair diameter, and vellus density was used to evaluate the potential improvement at 12 and 24 weeks. Median %RC were mostly positive for the parameters of hair density at 24 weeks; mean hair diameter showed a significant increase at 12 and 24 weeks, %RC = 12.5, p<.05; %RC = 14.6, p<.05, respectively. No severe adverse effects were reported by any of the participants.
Studies comparing PRP and/or other treatments
One randomized clinical study was conducted in Korea with 40 Korean females aged 20–60 years with FPHL, randomized to two different interventions, a single session of PRP injection, followed by 12 sessions of PDRN intra-perifollicular injection or 12 sessions of polydeoxyribonucleotide (PDRN) injection only, in the scalp at weekly intervals (Citation18). The primary outcomes of this study were the effects of PRP and PDRN, or PDRN only on mean hair counts, and mean hair thickness. Furthermore, 1/2 of the backs of two male New Zealand rabbits was injected with the PRP preparation, and the other 1/2 was injected with phosphate buffered saline as a control to investigate the effects of PRP injection on the expressions of WNT, PDGF, and fibroblast growth factor 9. Both treatment interventions exhibited clinical improvement in mean hair counts (p<.001) and mean hair thickness (p<.001), when compared to baseline. In the intergroup comparison, the combined therapy induced greater improvement in hair thickness (p=.031), but not in hair counts. For the animal models, the PRP preparation caused significant up-regulation of WNT, PDGF, and fibroblast growth factor expression when compared to saline control.
Finally, the randomized, controlled crossover pilot trial, performed at a single institution in the USA, conducted with 20 consecutive female patients with AGA, aged ≥18 years, investigated PRP compared with topical minoxidil in the treatment of AGA (Citation15). Patients were randomized into two interventions arms, those in arm A received PRP injections every 4 weeks for a total of three treatments, followed by an 8-week washout; at week 20 they received crossover treatment with minoxidil; and those in arm B received the same treatment in reverse sequence. The study outcomes were hair count, vellus hair density, terminal hair density, and cumulative thickness, in addition to a 16-item quality-of-life questionnaire; adverse effects were also assessed. Outcomes were measured at 12 weeks after intervention for both groups. Treatment with PRP resulted in a significant increase in hair count (p=.002) when compared to minoxidil; however, treatment with minoxidil produced significant increases in hair count (p<.001), vellus hair density (p=.03), terminal hair density (p=.004), and cumulative thickness (p=.004). For the quality-of-life analysis, several responses showed improvement at 12 weeks for the PRP group only. At 12 weeks, two participants were lost to follow up (one in each group), one for personal reasons and the other for medical ones. Adverse effects were experienced in 21.1% of participants in the PRP group, these being either pain/discomfort or bruising.
Synthesis of results
For the synthesis of results, four variables were selected due to their analyzability between the studies, (a) terminal hair density, (b) hair thickness, (c) patient satisfaction, and (d) adverse effects.
Terminal hair density
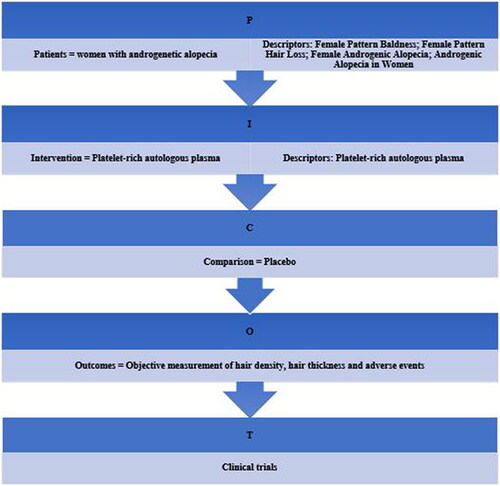
Values for terminal hair density (count per cm2) were extracted and normalized from four out of the seven studies in this review (Citation15–17,Citation20) due to the availability of data. The study by El-Husseiny et al. (Citation17) was included twice, the two different intervention arms in the study were conformed (single spin and double spin). Meta-analysis showed that PRP-based interventions were able to increase terminal hair density compared to control (SMD = 2.98, 95% CI = 1.10, 4.85, p for heterogeneity test <.00001, I2=96%) ().
Hair thickness
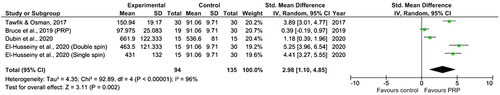
Outcomes for hair thickness (mm) were extracted and normalized from two out of seven studies (Citation16,Citation20), few studies provided comparable data. Meta-analysis showed that PRP-based interventions may possibly increase hair thickness when compared to control, but without statistical significance (SMD = 1.16, 95% CI= −0.96, 3.28, p for heterogeneity test <.0001, I2=95%) ().
Patient satisfaction
This aspect was reported in four studies (Citation15,Citation17,Citation19,Citation20). In the study by Puig et al. (Citation19), by means of a patient survey, 13.3% of participants claimed to have substantial improvement in hair loss, rate of hair loss (shedding), hair thickness, and ease in managing or styling hair in comparison with the placebo group. For El-Husseiny et al. (Citation17), according to a questionnaire completed by patients 6 weeks after the last PRP session, on a scale from 1 to 10, mean patient satisfaction was 6.60 ± 1.59. Patient satisfaction was also measured by Tawfik and Osman (Citation20), and also on a scale from 1 to 10, at the 6 month follow up, mean patient satisfaction was 7.0. A 16-item quality-of-life questionnaire was used by Bruce et al. (Citation15) for patients to assess treatment, at baseline and at week 12; for those in the PRP treatment group, there was a significant improvement in 11 out of the 16 self-reported items.
Adverse effects
During and after PRP treatment, no major side effects were reported by patients or observed by researchers. However, some mild adverse effects were reported such as headache, mild pain, edema and tenderness, scalp tightness, redness, and post-injection bleeding. No meta-analysis was conducted due to the absence of quantitative data. These mild side effects lasted in most cases up to 24 h, and were solved without clinical intervention.
Discussion
To our knowledge, this is the first systematic review with meta-analysis of seven studies involving approximately 170 female patients that provides a comprehensive view on the effect of autologous PRP in the treatment of FPA compared to placebo. In this review, we demonstrated the effects of autologous PRP on terminal hair density, hair thickness, patient satisfaction, adverse effects, and other outcomes.
After analysis of data on terminal hair density, we observed that treatment with PRP favored density, SMD = 2.98 (95% CI = 1.10, 4.85). The study conducted by El-Husseiny et al. (Citation17), showed the most significant results in the double-spin intervention arm; terminal hair density at baseline was 147.5 ± 57.0/cm2 compared to 463.5 ± 121.3/cm2, 6 weeks after the last PRP session. This study describes two different methods for the preparation of PRP – ‘single spin’ and ‘double spin’. The difference being that in the double spin method, the 10 ml of venous autologous whole blood collected into tubes containing tri-sodium citrate was then centrifuged at 112 g units (1000 rpm) for 10 min at room temperature to separate red blood cells at the bottom of the tube, buffy coat (containing white blood cells) in the middle, and plasma above (soft spin). The portion of plasma was then transferred into other plain tubes (free of tri-sodium citrate) and centrifuged further at 448 g units (2000 rpm) for 10 min to obtain a platelet pellet at the bottom of the tube and platelet-poor plasma at the top. This platelet-poor plasma was removed, and calcium gluconate was added (1:9), resulting in a double-spin PRP preparation. This double-spin method was also used by Tawfik and Osman (Citation20), who presented similar results (SMD 3.89, 95% CI = 3.01, 4.77). Nevertheless, adequate interpretation of the results was hindered due to high heterogeneity (I2=95%).
This positive effect of PRP treatment on terminal hair density has also been described in a previous review with predominantly male participants (Citation21); in this review to analyze the effectiveness of PRP injections in the treatment of male AGA, overall SMD in hair density was 0.58 and 0.51, compared to baseline and placebo, respectively. Furthermore, the double-spin method reported by El-Husseiny et al. (Citation17) has also been positively associated with increased hair density in different populations by other researchers (Citation22,Citation23).
In a retrospective observational case-series study, Gentile et al. (Citation24) reported that both patient populations treated with autologous-PRP not activated and autologous activated-PRP a positive response to treatment for hair density measurements when compared to placebo. However, at 3 months, hair density improvement for patients treated with autologous-PRP not activated was greater than those treated with autologous activated-PRP. The authors attributed this performance to the greater efficiency of in vivo thrombin to activate platelets. This result was once again confirmed in another study by Gentile and Garcovich (Citation25) in which 90 patients (63 males and 27 females) with stage I–V AGA treated with autologous-PRP not activated and autologous activated-PRP presented positive results for hair re-growth in the long-term follow-up.
Regarding the hair thickness/caliber outcome, the meta-analysis conducted in this review showed that PRP favored hair thickness, without statistical significance (overall SMD = 1.16, 95% CI= −0.96, 3.28). Again, these results should be analyzed with caution due to high heterogeneity (I2=95%). Furthermore, only two studies were included in this comparison due to the availability of data (Citation16,Citation20). This outcome was analyzed in a third study (Citation18), but due to the nature of the results (without baseline and post-intervention results) we were unable to include this study in the comparison.
The beneficial effect of PRP on hair thickness/caliber has also been demonstrated in other studies. In a pilot study, with male and female participants, Schiavone et al. (Citation26) reported that the clinically visible improvement at 6 months was due to increased hair thickness. Another study with male participants also noted a significant increase in epidermis thickness in PRP-treated hair skin (Citation27).
Other important outcome analyzed in the studies included in the review was patients’ satisfaction. This outcome was assessed in the studies by El-Husseiny et al. (Citation17), Puig et al. (Citation19), Tawfik and Osman (Citation20), and Bruce et al. (Citation15). Overall, there was an increase in patient satisfaction with PRP-injected sites. Patients reported that their hair felt coarser or heavier after treatment, being easier to manage with a positive impact on social well-being. In another review with a mixed sex population by Stevens and Khetarpal (Citation28), the authors reported that both male and female participants experienced an improved quality of life.
Finally, we assessed the safety of autologous PRP treatment. From the results of the studies included in the review, we understand that this treatment may cause some mild adverse effects in some patients, such effects include headache, mild pain, edema and tenderness, scalp tightness, redness, and post-injection bleeding. These effects usually last up to 24 h without the need for clinical intervention. These results are in line with those in another review by Stevens and Khetarpal (Citation28), here the authors reported that the use of PRP for the treatment of AGA presents minimum safety issues and side effects. In a study with 23 patients (five male and 18 female) aged 21–70 years, with male pattern hair loss were treated with interfollicular autologous-PRP (not activated) controlled and mechanical injections at a depth of 5 mm. Three treatment sessions with an interval of 30 days between were performed. The authors reported that with adequate training PRP can be successfully injected into the scalp of the patient with little or no pain in 90% of cases (Citation29). Furthermore, commercial systems for PRP preparations are regulated by European Union safety and quality standards with the mandatory notification of adverse and severe reactions (Citation29,Citation30). Therefore, it may be concluded that this type of intervention is relatively safe. Nevertheless, patients should be informed of potential adverse effects.
This systematic review with meta-analysis has several limitations which should be considered when interpreting the demonstrated results. First, three of the studies included in this review were pilot studies with small sample sizes. Second, on the issue of sample size, none of the studies provided minimal sample size calculations, therefore, it was not possible to detect a clinically relevant treatment effect. Another important limitation to be considered is that each study presented a different treatment protocol, with different PRP preparations, variable number of treatment sessions, follow up times, etc. This issue is important to choose the optimal regimen, and the optimal duration of the regimen. Finally, it appears that there is no standard or methodological consensus to evaluate the results of androgenic alopecia treatment, this aspect made it difficult to make direct comparisons between studies.
Conclusions
Based on the results reported in this review, the use of autologous PRP injections in female AGA seems to be promising, with more consistent results on terminal hair density. Another important consideration is patient satisfaction, which is related to the patient’s expectations and has a subjective character. In this review, participants reported improvements in hair management and styling, general satisfaction with treatment and better social well-being. However, we recommend caution in the interpretation of these results until they can be replicated in larger and more representative samples. Further studies are necessary to determine optimal PRP preparation, concentration, number of sessions, and interval between sessions. In addition, these interventions need to be randomized, double-blind, placebo-controlled, multicenter, clinical trials with minimal sample size calculations and long-term follow-ups to determine the real and sustained effects of PRP treatment.
Author contributions
All authors participated in the collection and analysis of data and in the writing and reviewing of the manuscript and gave their approval for the final manuscript.
Supplemental Material
Download PDF (139.3 KB)Disclosure statement
The authors have no conflicts of interest to declare.
References
- Martinez-Lopez A, Montero-Vilchez T, Sierra-Sánchez Á, et al. Advanced medical therapies in the management of non-scarring alopecia: areata and androgenic alopecia. Int J Mol Sci. 2020;21(21):8390.
- Ho CH, Sood T, Zito PM. Androgenetic alopecia. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021.
- Bhat YJ, Saqib NU, Latif I, et al. Female pattern hair loss—an update. Indian Dermatol Online J. 2020;11(4):493–501.
- Tamashunas NL, Bergfeld WF. Male and female pattern hair loss: treatable and worth treating. Cleve Clin J Med. 2021;88(3):173–182.
- Futterweit W, Dunaif A, Yeh HC, et al. The prevalence of hyperandrogenism in 109 consecutive female patients with diffuse alopecia. J Am Acad Dermatol. 1988;19(5):831–836.
- Redler S, Messenger AG, Betz RC. Genetics and other factors in the aetiology of female pattern hair loss. Exp Dermatol. 2017;26(6):510–517.
- Gentile P, Garcovich S. Systematic review of Platelet-Rich plasma use in androgenetic alopecia compared with Minoxidil®, Finasteride®, and adult stem cell-based therapy. Int J Mol Sci. 2020;21(8):2702.
- Evans AG, Mwangi JM, Pope RW, et al. Platelet-rich plasma as a therapy for androgenic alopecia: a systematic review and meta-analysis. J Dermatolog Treat. 2022;33(1):498–511.
- Roohaninasab M, Goodarzi A, Ghassemi M, et al. Systematic review of platelet-rich plasma in treating alopecia: focusing on efficacy, safety, and therapeutic durability. Dermatol Ther. 2021;34(2):e14768.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. Int J Epidemiol. 2002;31(1):150–153.
- Sterne J, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
- Starace M, Alessandrini A, D'Acunto C, et al. Platelet-rich plasma on female androgenetic alopecia: tested on 10 patients. J Cosmet Dermatol. 2019;18(1):59–64.
- Bruce AJ, Pincelli TP, Heckman MG, et al. A randomized, controlled pilot trial comparing platelet-rich plasma to topical minoxidil foam for treatment of androgenic alopecia in women. Dermatol Surg. 2020;46(6):826–832.
- Dubin DP, Lin MJ, Leight HM, et al. The effect of platelet-rich plasma on female androgenetic alopecia: a randomized controlled trial. J Am Acad Dermatol. 2020;83(5):1294–1297.
- El-Husseiny RM, Saleh HM, Moustafa AA, et al. Comparison between single- versus double-spin prepared platelet-rich plasma injection in treatment of female pattern hair loss: clinical effect and relation to vascular endothelial growth factor. Arch Dermatol Res. 2020;313(7):557–566.
- Lee SH, Zheng Z, Kang JS, et al. Therapeutic efficacy of autologous platelet-rich plasma and polydeoxyribonucleotide on female pattern hair loss. Wound Repair Regen. 2015;23(1):30–36.
- Puig CJ, Reese R, Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42(11):1243–1247.
- Tawfik AA, Osman M. The effect of autologous activated platelet-rich plasma injection on female pattern hair loss: a randomized placebo-controlled study. J Cosmet Dermatol. 2018;17(1):47–53.
- Gupta AK, Cole J, Deutsch DP, et al. Platelet-rich plasma as a treatment for androgenetic alopecia. Dermatol Surg. 2019;45(10):1262–1273.
- Kachhawa D, Vats G, Sonare D, et al. A spilt head study of efficacy of placebo versus platelet-rich plasma injections in the treatment of androgenic alopecia. J Cutan Aesthet Surg. 2017;10(2):86–89.
- Gupta S, Revathi TN, Sacchidanand S, et al. A study of the efficacy of platelet-rich plasma in the treatment of androgenetic alopecia in males. Indian J Dermatol Venereol Leprol. 2017;83(3):412.
- Gentile P, Scioli MG, Bielli A, et al. Platelet-rich plasma and micrografts enriched with autologous human follicle mesenchymal stem cells improve hair re-growth in androgenetic alopecia. Biomolecular pathway analysis and clinical evaluation. Biomedicines. 2019;7(2):27.
- Gentile P, Garcovich S. Autologous activated platelet-rich plasma (AA-PRP) and non-activated (A-PRP) in hair growth: a retrospective, blinded, randomized evaluation in androgenetic alopecia. Expert Opin Biol Ther. 2020;20(3):327–337.
- Schiavone G, Raskovic D, Greco J, et al. Platelet-rich plasma for androgenetic alopecia: a pilot study. Dermatol Surg. 2014;40(9):1010–1019.
- Gentile P, Garcovich S, Bielli A, et al. The effect of platelet-rich plasma in hair regrowth: a randomized placebo-controlled trial. Stem Cells Transl Med. 2015;4(11):1317–1323.
- Stevens J, Khetarpal S. Platelet-rich plasma for androgenetic alopecia: a review of the literature and proposed treatment protocol. Int J Womens Dermatol. 2019;5(1):46–51.
- Gentile P, Garcovich S, Scioli MG, et al. Mechanical and controlled PRP injections in patients affected by androgenetic alopecia. J Vis Exp. 2018;131:56406.
- Gentile P, Calabrese C, De Angelis B, et al. Impact of the different preparation methods to obtain autologous non-activated platelet-rich plasma (A-PRP) and activated platelet-rich plasma (AA-PRP) in plastic surgery: wound healing and hair regrowth evaluation. Int J Mol Sci. 2020;21(2):431.