Introduction
Non-melanoma is the most common skin cancer worldwide particularly affecting Caucasians (Citation1). This tumor is characterized by uncontrolled growth of abnormal keratinocytes and includes squamous cell carcinoma (SCC) and basal cell carcinoma (BCC). BCC is a relatively benign tumor, while SCC shows higher mortality rates due to the increased risk of metastasis although significantly lower on skin unlike other SCCs in systemic organs. Consequently, lesion removal is the treatment of choice whereas chemotherapy has an important role in advanced/metastatic disease (Citation2–4).
Survivin belongs to the inhibitor of apoptosis (IAP) protein family and in addition to the apoptotic functions it also regulates the cell cycle. Survivin has been an exciting field of research mainly because of its highest expression in human cancers (Citation5). In contrast to normal adult tissues where it is poorly or not expressed, results from human transcriptome analysis showed that survivin is the fourth most highly expressed protein in cancer tissue compared with normal counterparts, hence pointing to as a putative ‘tumor specific antigen’ (Citation6,Citation7). Under cancer background, survivin enhances antigen presentation and supports production of autoantibodies (Citation5).
Dallaglio et al. analyzed intracellular localization of survivin and its correlation with keratinocytes differentiation and SCC (Citation5). They found notable elevation of this nuclear protein expression in SCC in situ strikingly higher in poorly differentiated SCC. In skin, it is quite established that genetic alterations in keratinocyte stem cells (KSC) give rise to SCC-derived stem-like cells (SCC-SC). Survivin over-expression is a key factor in the transformation of KSC to SCC-SC and tumor-producing KSC (Citation8,Citation9).
In contrast, in human BCC survivin is weakly expressed suggesting the presence of a human-specific expression pattern of survivin in the skin (Citation10–12). Taking in account the above, in this study, we measured the preoperative and postoperative survivin peripheral blood levels in patients with BCC and SCC, in an effort to identify its potential role as a diagnostic and prognostic biomarker.
Materials and methods
The study design
This study was conducted between January 2018 and May 2019 in the Outpatient Dermatological Clinic at Tzaneio General Hospital of Piraeus, in Greece, including 20 patients with non-melanoma skin cancer. The population was divided in two subgroups: (a) 10 patients suffering from BCC (mean age ± standard deviation [SD] = 70.7 ± 8.3 years; 7 males, 3 females; min age: 59 years, max age: 84 years) and (b) 10 patients suffering from SCC (mean age ± SD = 81.8 ± 6.3 years; 8 males, 2 females; min age: 70 years, max age: 88 years). All patients underwent surgical removal under local anesthesia with xylocaine for their malignancy. All patients underwent measurement in their serum of protein survivin and C-reactive protein (CRP), preoperatively and 15 d after surgery when the sutures were removed (). Written informed consent was obtained from all participants of the study, approved by the Institutional Ethics Committee of Tzaneio General Hospital of Piraeus, in accordance to the Declaration of Helsinki.
Table 1. Demographics and Laboratory results of patients.
Measurement of serum BIRC-5 (survivin) levels and CRP
Blood samples were collected in serum separator tubes (SST) and allowed to separate for about 2 h, then centrifuged for 15 min at approximately 1000 ×g to collect sera and, either proceeded immediately for measuring CRP and survivin levels or stored at −20 °C until measurement. The serum survivin was stable at −20 °C for 6 months.
Serum CRP was measured by Turbidimetric Immuno-Assay (TIA) in an Advia 1800 machine (Siemens), immediately after collection. Normal values are less than 0.5 mg/dL for both sexes equally.
Serum survivin levels were measured using the Human Survivin/BIRC-5 ELISA, kit Catalogue number KBB-06E6A2, BioSite, Sweden. Briefly, the kit consists of a solid phase immunoassay specially designed to measure Human BIRC5 with a 96-well strip plate that is pre-coated with antibody specific for BIRC-5. The capture and the detection antibody are mouse monoclonal and goat polyclonal, respectively. Standard is composed of a recombinant human survivin protein of 34 kD molecular mass. Standard, control, and samples are tested in duplicate according to the manufacturer and the result is read on an ELISA reader at 450 nm. Sensitivity or minimum detectable dose (MDD) is the lower limit of target protein that can be detected by the kit, and is <2 pg/ml while the range is 62.5 − 4000 pg/ml.
Statistical analysis
All analyses were conducted using SPSS Statistics for Windows version 26.0 (IBM Corp. Released 2018. IBM SPSS Statistics for Windows version 26.0. Armonk, NY: IBM Corp.). The Statistical Significance level was set at p < 0 .05. All survivin levels across all groups were found to violate the assumption of normal distribution. Both the Shapiro–Wilk as well as the Kolmogorov–Smirnov tests of normality were able to reject the null Hypothesis (p < 0.0001) in all cases, regardless of whether the sample was divided into the two groups. Since normal distribution cannot be assumed, a non-parametric test such as the Wilcoxon test would be more fitting as an alternative. The same did not apply to the CRP levels. In this case, although the data do not follow the normal distribution (p < 0 .0001) with very few exceptions, they are clearly distributed exponentially. Therefore, apart from the non-parametric method of the Wilcoxon test that will be performed, it is also worth to try a logarithmic transformation in order to achieve normality, thus allowing us to perform paired samples t-test for each group separately. In addition to this, the use of eta and eta2 as well as Lambda are deemed appropriate for the measurement of association between the variables of age and group, and gender and group, respectively, since gender and group are nominal variables while Age is on a continuous level.
Results
Evaluation of serum survivin levels
The survivin levels before (mean ± SD = 37.67 ± 77.5; min: 9.5, max: 257.8; median 10.3) and after (mean ± SD = 50.6 ± 115.78; min: 7.6, max: 379.7; median 14.29) the operation did not appear to have a statistically significant difference for the BCC group (split by group, Wilcoxon test, variables: survivin pre, survivin post, p = 0.386).
Similar results were observed in the case of the survivin levels before (mean ± SD =35.8 ± 42.8; min: 8.4, max: 152.3; median 24.4) and after (mean ± SD = 37.49 ± 72.48; min: 8.2, max: 242.8; median 12.75) operation, where yet again the results could not satisfy the criteria in order to reject the null Hypothesis (H0) for the SCC Group (split by group, Wilcoxon test, variables: survivin pre, survivin post, p = 0.241).
Lastly, a final test was conducted, which disregarded the two groups and solely compared the levels of the survivin before (mean ± SD = 36.74 ± 60.95; min: 8.4, max: 253.8; median 15.75) and after (mean ± SD = 44.05 ± 94.25; min: 7.6, max: 379.7; median 13.7). Once again, the findings were not deemed statistically significant (Wilcoxon test, variables: survivin pre, survivin post, p = 0.737) ().
Table 2. Survivin descriptive statistics for basal cell carcinoma (BCC) group, squamous cell carcinoma (SCC) group and both groups combined.
Evaluation of serum CRP levels
Regarding the BCC group, difference between the levels before (mean ± SD = 3.07 ± 2.57; min: 0.3, max: 9.2; median 2.67) and after (mean ± SD = 3.31 ± 4.38; min: 0.23, max: 15.10; median 2.24) the operation did not appear to be statistically significant (split by group, Wilcoxon test, variables: CRP pre, CRP post, p = 0.721).
The same case was observed in the SCC group, as levels of CRP before (mean ± SD = 3.07 ± 3.64; min: 0.24, max: 11.9; median 1.59) and after (mean ± SD = 1.62 ± 1.02; min: 0.15, max: 3.13; median 1.65) intervention were not found to be statistically significant (split by group, Wilcoxon test, variables: CRP pre, CRP post, p = 0.203).
Lastly, the difference between the medians before (mean ± SD = 3.07 ± 3.07; min: 0.24, max: 11.9; median 2.4) and after (mean ± SD= 2.46 ± 3.17; min: 0.15, max: 15.10; median 1.90) surgery was, yet again, not of statistical significance (Wilcoxon Test, Variables: CRP pre, CRP post, p = 0 .313) ().
Table 3. C-reactive protein (CRP) descriptive statistics for basal cell carcinoma (BCC) group, squamous cell carcinoma (SCC) group and both groups combined.
Paired samples T-tests were performed for all three cases above. After logarithmic transformation, the assumption normal distribution was satisfied (p > 0 .05 in both Kolmogorov– Smirnov and Shapiro–Wilk tests) for all cases. The results, again, showed no statistical significance between the means of the CRP before and after operation. More specifically for the BCC group (mean ± SD = 0.061 ± 0.217; df = 9): p = 0.396; for the SCC group (mean ± SD= 0.129 ± 0.606; df = 9): p = 0.515; while for both Groups combined (mean ± SD = 0.096 ± 0.445; df = 19): p = 0.348 ().
Table 4. C-reactive protein (CRP) paired differences for basal cell carcinoma (BCC) group, squamous cell carcinoma (SCC) group and both groups combined.
Association between age and non-melanoma skin cancers
Despite the insignificance of our findings, it is still worth to observe any associations between the variables of age and group () as well as gender and group ().
Table 5. Association between group and age.
Table 6. Association between group and gender.
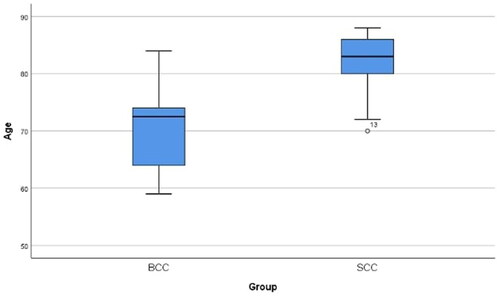
Upon inspection of survivin descriptive statistics for both BCC and SCC groups combined, we notice a high association between age and group: Eta = 0.837, Eta2 = 0.6999 which means that 69.9% of the variation of the dependent variable (group) is explained by the independent variable (age). The difference of the age between the two groups is visible in the Boxplot that follows (). As for the association between Gender and Group: Lambda= 0.100 which is considered weak and p = 0.796 indicates that there is no statistical significance.
Discussion
The aforementioned investigation was a pilot study aiming to estimate the serum levels of survivin and CRP among patients with non-melanoma skin cancers pre and postoperatively.
The study of Foote et al. proved that each year of life was associated with an increased risk of 2% for BCC and 4% for SCC (Citation13). Another epidemiological study of Andrale et al. demonstrated that the average age of BCC and SCC patients was 70.3 and 74.4 years, old respectively (Citation14). Moreover, the international cancer registry in Ireland in 2017 revealed that the median age of diagnosis for BCC was 68 whereas for SCC it was 76 years old (Citation15).
The present findings showing no statistically significant correlation of survivin and CRP levels in peripheral blood, to BCC and/or SCC before and after surgical excision, would suggest that these proteins cannot be used as biomarkers measured in patient’s serum for these skin malignancies.
Although this pilot study encompassed only 20 patients, our statistical analysis supporting that non-melanoma skin cancer generally affect patients older than 50 years old with SCC to occur in even older patients, permits us to assume that our findings were more or less reliable.
To the best of our knowledge, survivin levels measurement in non-melanoma skin cancer patients’ serum have not been previously evaluated. Therefore, it would be quite interesting to our point of view to consider the utility of this protein as a future prognostic biomarker in skin malignancies with the additional evaluation of these biomarkers in biopsy samples from these patients. In addition, more comprehensive large-scale studies would be recommended to determine whether this protein’s serum levels could have any prognostic role for non-melanoma skin cancers.
Disclosure statement
Dr. Tampouratzi reports personal fees from AbbVie, personal fees from LEO Pharma, personal fees from Janssen, personal fees from Sanofi, personal fees from Genesis Pharma, personal fees from UCB, personal fees from Mylan and personal fees from Novartis, outside the submitted work.
Dr. Asonitis reports there are no competing interests to declare.
Dr. Katsantonis reports personal fees from Abbvie, personal fees from UCB, personal fees from LEO Pharma, personal fees from Novartis, outside the submitted work.
Mr. Talaiporou reports there are no competing interests to declare.
Dr. Sfaelos reports there are no competing interests to declare.
Data availability statement
The data that support the findings of this study are available from the corresponding author, ET, upon reasonable request.
References
- Khan Z, Khan AA, Yadav H, et al. Survivin, a molecular target for therapeutic interventions in squamous cell carcinoma. Cell Mol Biol Lett. 2017;22:8.
- Yan W, Wistuba II, Emmert-Buck MR, et al. Squamous cell carcinoma similarities and differences among anatomic sites. Am J Cancer Res. 2011;1:275–300.
- Fahradyan A, Howell AC, Wolfswinkel EM, et al. Updates on the management of non-melanoma skin cancer. Healthcare (Basel). 2017;5(4):82.
- Santarelli A, Mascitti M, Russo LL, et al. Survivin based treatment strategies for squamous cell carcinoma. Int J Mol Sci. 2018;19(4):971.
- Dallaglio K, Marconi A, Pincelli C. Survivin a dual player in healthy and diseased skin. J Invest Dermatol. 2012;132(1):18–27.
- Velculescu VE, Madden SL, Zhang L, et al. Analysis of human transcriptomes. Nat Genet. 1999;23(4):387–388.
- Andersen MH, Svane IM, Becker JC, et al. The universal character of the tumor associated antigen survivin. Clin Cancer Res. 2007;13(20):5991–5994.
- Albers AE, Chen C, Koberle B, et al. Stem cells in squamous head and neck cancer. Crit Rev Oncol Hematol. 2012;81(3):224–240.
- Tan DW, Jensen KB, Trotter MW, et al. Single cell gene expression profiling reveals fuctional heterogeneity of undifferentiated human epidermal cells. Development. 2013;140(7):1433–1444.
- Chiodino C, Cesinaro AM, Ottani D, et al. Communication: expression of the novel inhibitor of apoptosis survivin in normal and neoplastic skin. J Invest Dermatol. 1999;113(3):415–418.
- Bowen AR, Hanks AN, Murphy KJ, et al. Proliferation, apoptosis and survivin expression in keratinocystic neoplasms and hyperplasias. Am J Dermatopathol. 2004;26:177–181.
- Park HR, Min SK, Cho HD, et al. Expression profiles of p63, p53, survivin and hTERT in skin tumors. J Cutan Pathol. 2004;31(8):544–549.
- Foote JA, Harris RB, Giuliano AR, et al. Predictors for cutaneous basal and squamous cell carcinoma among actinically damaged adults. Int J Cancer. 2001;95(1):7–11.
- Andrade P, Brites MM, Vieira R, et al. Epidemiology of BCC and SCC in a department of dermatology- a 5 year review. An Bras Dermatol. 2012;87(2):212–219.
- Cancer trends no. 34 - National Cancer Registry Ireland. July 2017.