Abstract
Background
Excessive sebum production is a factor in acne development. Tazarotene 0.045% lotion has demonstrated reductions in acne lesions and acne-induced sequelae.
Objective
Evaluate efficacy, changes in skin oiliness, and safety with tazarotene 0.045% lotion in participants with moderate-to-severe acne and oily skin.
Methods
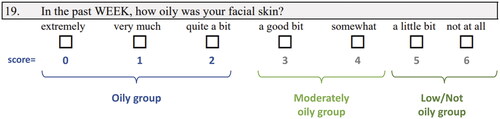
In two phase 3, double-blind, 12-week studies (NCT03168321; NCT03168334), participants aged ≥ 9 years with moderate-to-severe acne were randomized 1:1 to once-daily tazarotene 0.045% lotion or vehicle lotion (N = 1614). This pooled, post hoc analysis included only participants self-categorized with oily skin at baseline on the Acne-Specific Quality of Life questionnaire item 19 (scores: 0 [extremely oily] to 6 [not at all oily]). Inflammatory/noninflammatory lesion counts, treatment success, skin oiliness, treatment-emergent adverse events (TEAEs), and cutaneous safety/tolerability were evaluated.
Results
In all participants with oily skin (n = 793), tazarotene provided greater reductions in inflammatory/noninflammatory lesions (p < 0.001, both) and greater treatment success rates versus vehicle (p < 0.01) at week 12. Over two-thirds of polymeric lotion-treated participants had subjective skin oiliness reductions by week 12, with around a third reporting ‘low/not’ oily skin. Tazarotene TEAE rates were similar to the overall population.
Conclusions
Once-daily treatment with tazarotene 0.045% polymeric emulsion lotion may help improve patient-perceived skin oiliness in those with moderate-to-severe acne.
Keywords:
Introduction
Oily skin is a frequent complaint of dermatology patients with or without acne (Citation1,Citation2). Patients with oily skin may be concerned with excessive brightness/shininess, a ‘greasy’ appearance, and enlarged pores, which can negatively affect quality of life (Citation1–4). Higher rates of facial sebum production may be associated with larger pore sizes (Citation5). Skin oiliness and pore size may differ by race, with some studies demonstrating larger amounts of sebum secretion or larger pore sizes in Black patients (Citation1,Citation6,Citation7) and others finding no difference (Citation8,Citation9). Sebum composition and production can also vary by age and gender, and in patients with or without acne (Citation10–14).
Sebum plays an important role in skin health by protecting against moisture loss, providing antimicrobial activity, and delivering antioxidants to the skin surface (Citation15,Citation16). Excessive sebum production, however, interferes with follicular keratinization in the pilosebaceous unit and leads to pore blockage (Citation15,Citation17). Sebum composition may also be altered by abnormal proliferation of Cutibacterium acnes (C. acnes) which can induce production of proinflammatory mediators in sebocytes and cause inflammation (Citation15,Citation18). Increased sebum production and inflammation, as well as follicular proliferation of C. acnes and abnormal keratinization are all factors implicated in the pathophysiology of acne vulgaris (Citation19,Citation20).
Topical retinoids block several inflammatory pathways and promote normal desquamation (Citation15,Citation21), making them a mainstay of acne treatment (Citation20,Citation21). Additionally, retinoid receptors are expressed in sebocytes, and topical retinoids may inhibit differentiation and lipid synthesis (Citation22). Retinoid use, however, can be limited by cutaneous irritation. Vehicles formulated with emollients/moisturizers may reduce this irritation (Citation21) and naturally oily skin may also provide a protective effect. The topical retinoid tazarotene 0.1% cream has been shown to reduce apparent facial pore size (Citation23), though whether it has a sebum-suppressing effect is unknown (Citation1). A lower-dose 0.045% tazarotene polymeric lotion also demonstrated efficacy in reducing acne lesions, with good tolerability and safety profiles in data from two phase 3 clinical trials of patients with moderate-to-severe acne (Citation24,Citation25), as well as in patients with skin of color (Citation26). Furthermore, the unique formulation of the tazarotene 0.045% polymeric emulsion lotion may minimize irritation by allowing for simultaneous delivery of emollients/humectants and lower drug concentrations of the active ingredient compared with other commercially available formulations (Citation27).
The objective of these pooled post hoc analyses was to evaluate the efficacy, changes in skin oiliness, and safety and tolerability of tazarotene 0.045% lotion in participants with moderate-to-severe acne and self-reported oily skin. Because of possible differences by race in pore size and skin oiliness, data were also analyzed for participants who self-identified as Black.
Methods
Study design and participants
These analyses include data pooled from two previously described identically designed, phase 3, multicenter, double-blind, randomized, vehicle-controlled, parallel-group studies (NCT03168334 and NCT03168321) (Citation24,Citation25). Briefly, eligible patients were aged ≥ 9 years with moderate-to-severe facial acne (a score of 3 or 4 on the Evaluator’s Global Severity Score [EGSS]; ) and inflammatory and noninflammatory lesion counts between 20–50 and 25–100, respectively, with ≤ 2 facial nodules. Participants were equally randomized to tazarotene 0.045% lotion or vehicle lotion applied to the face once daily for 12 weeks.
Table 1. Evaluator’s Global Severity Score.
All studies were approved by relevant independent ethics committees or institutional review boards at each study site and were conducted in accordance with the International Conference on Harmonization, the Declaration of Helsinki, Good Clinical Practice Guidelines, and local regulations. All participants or their legal guardians provided written informed consent; participants under the legal age of consent provided assent.
Study assessments
For each study, efficacy evaluations included investigator-assessed inflammatory lesions, noninflammatory lesions, and treatment success (defined as the proportion of participants achieving ≥2-grade reduction from baseline in EGSS and a score of 0 [clear] or 1 [almost clear]). Self-reported changes in skin oiliness were also assessed using the Acne-Specific Quality of Life (Acne-QoL) questionnaire item 19, which is scored from 0 (extremely oily) to 6 (not at all oily; ) (Citation28). Safety evaluations comprised investigator-assessed cutaneous safety (scaling, erythema, hypopigmentation, and hyperpigmentation) and participant-assessed tolerability (itching, burning, stinging) using a 4-point scale (0 = none, 1 = mild, 2 = moderate, and 3 = severe). Adverse events (AEs) and serious adverse events (SAEs) were also monitored throughout the studies.
Statistical and subgroup analyses
For this post hoc analysis, participants were categorized by self-reported skin oiliness at baseline on the Acne-QoL questionnaire item 19; only participants scoring 0–2 (oily skin; ) at baseline were included. In addition, a subset of participants with oily skin who self-reported race as Black were analyzed separately. The intent-to-treat (ITT) population was defined as all participants who were randomized and received study drug. The safety population included all randomized participants who used study medication or vehicle at least once with a minimum of one post-baseline evaluation.
Least-squares (LS) mean percent changes from baseline in inflammatory and noninflammatory lesion counts by visit, treatment success at week 12, and changes in self-reported skin oiliness from baseline to week 12 were analyzed for all oily skin participants. The subgroup of Black participants with oily skin was also analyzed for changes in skin oiliness and cutaneous safety and tolerability. As significant skewness was found for lesion count changes, a nonparametric method was used to rank transform data prior to performing an analysis of covariance (ANCOVA), with a factor of treatment and the respective baseline lesion count as a covariate. Logistic regressions using Firth’s Penalized Likelihood were performed to analyze treatment success, with a factor of treatment group. Statistical significance was defined as p ≤ 0.05, determined using 2-tailed tests of the null hypothesis. Efficacy values were adjusted for multiple imputations. Missing data for lesion counts and treatment success were estimated using the Markov Chain Monte Carlo method. All statistical analyses were performed in SAS® version 9.3 (SAS Institute, Cary, NC) or later. Dosing compliance (defined as participants missing ≤ 5 consecutive days of dosing and applying 80–120% of expected applications), changes in skin oiliness scores, and assessments of cutaneous safety and tolerability were summarized using descriptive statistics. AEs were recorded and classified using the Medical Dictionary for Regulatory Activities terminology. Imputations were not made for missing safety data.
Results
Participant disposition and demographics
Of 1614 participants in the ITT population of the two pooled phase 3 studies, 1610 had data at baseline and week 12 on Acne-QoL questionnaire item 19. Of these, 736 (45.7%) had oily skin at baseline and were included in these analyses. They had a mean age of 21.6 years, and the majority were female and White (). Approximately, 90% had an EGSS score of 3 (moderate) at baseline. Of 261 participants in the ITT population who identified as Black, 150 (57.4%) had oily skin. They were slightly older than the overall oily skin group (mean age: 25.2 years) and a greater percentage were female (83% vs. 73%). The majority (94.7%) had moderate acne at baseline. Over 90% of all participants with oily skin and Black participants with oily skin were compliant in both treatment groups.
Table 2. Demographic and baseline characteristics of all participants with oily skin (ITT population, pooled).
Efficacy
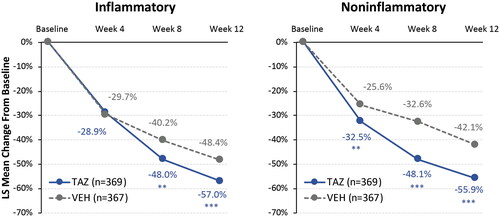
In all participants with oily skin, tazarotene 0.045% lotion provided over 55% reductions in lesion counts from baseline to week 12. These reductions were significantly greater than those observed with vehicle lotion in inflammatory (LS means: −57.0% vs. −48.4%; p < 0.001) and noninflammatory lesion counts (−55.9% vs. −42.1%; p < 0.001; ). Treatment success rates at week 12 were significantly higher for all tazarotene-treated oily skin participants compared with vehicle, with almost a third of tazarotene-treated participants achieving treatment success (29.8% vs. 19.2%; p < 0.01). These acne improvements are similar to those seen in the overall phase 3 pooled population for lesion reductions (tazarotene vs. vehicle; inflammatory: −57.9% vs. −47.8%; noninflammatory: −56.0% vs. −42.0%; p < 0.001, both) and treatment success rates at week 12 (30.4% vs. 17.9%; p < 0.001) (Citation24).
Figure 2. Reductions in acne lesion counts by visit in all participants with oily skin (ITT population, pooled). **p < 0.01; ***p < 0.001 vs. vehicle. Multiple imputation used to impute missing values. Overall phase 3 pooled population at week 12: inflammatory TAZ – 57.9% and VEH – 47.8%; noninflammatory: TAZ – 56.0% and VEH – 42.0%; p < 0.001, both (Citation24). ITT: intent to treat; LS: least squares; TAZ: tazarotene 0.045% lotion; VEH: vehicle lotion.
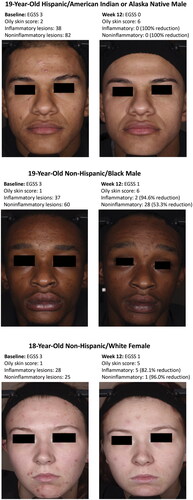
Over 70% of participants in the overall oily skin population reported an improvement in skin oiliness to ‘moderately’ or ‘low/not’ oily with both tazarotene 0.045% lotion and vehicle; a numerically greater percentage of participants reported an improvement to ‘low/not’ oily skin with tazarotene than vehicle (). Compared with the overall oily skin population, a numerically higher percentage of Black participants with oily skin saw improvements in skin oiliness. Over three quarters of tazarotene-treated Black participants reported an improvement in skin oiliness to ‘moderately’ or ‘low/not’ oily skin, compared with about two thirds of vehicle-treated participants (). Images depicting acne improvements with tazarotene 0.045% lotion are shown in .
Figure 3. Improvements in oily skin from baseline to week 12 (ITT population, pooled). Categories based on Acne-Specific Quality of Life questionnaire item 19 scores at baseline: low/not oily (score = 5–6); moderately oily (score = 3–4); oily (score = 0–2; see ). No imputation of missing data. N values indicate participants with ‘oily’ skin at baseline who also had data at week 12. ITT: intent to treat; TAZ: tazarotene 0.045% lotion; VEH: vehicle lotion.
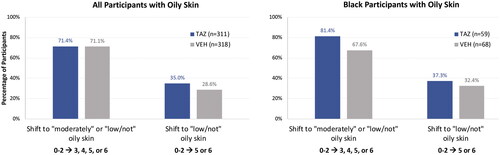
Figure 4. Improvements with tazarotene 0.045% lotion in participants with oily skin. The oily skin score is derived from the Acne-Specific Quality of Life questionnaire item 19: low/not oily (score = 5–6); moderately oily (score = 3–4); oily (score = 0–2; see ). A greater oily skin score indicates reductions in skin oiliness. Individual results may vary. EGSS: Evaluator’s Global Severity Score.
Safety
Treatment-emergent AE (TEAE) rates with tazarotene in all oily-skin participants () were similar to those observed in the overall tazarotene-treated population (n = 779; any TEAE: 27.9% vs. 26.8% overall; treatment-related TEAE: 11.7% vs. 11.3% overall) (Citation25). Most TEAEs were mild in severity and the majority were considered unrelated to treatment. A total of 1.7% of tazarotene-treated participants with oily skin discontinued the study or study drug compared with 2.8% of tazarotene-treated participants overall (Citation25).
Table 3. Treatment-emergent adverse events through week 12 in all participants with oily skin (safety population, pooled).
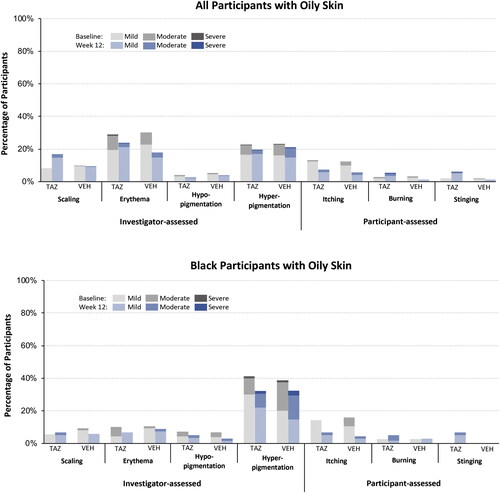
For the cutaneous safety and tolerability assessments, most (≥71%) tazarotene-treated participants with oily skin reported ratings of none (0) at baseline and week 12; of those participants who had any signs/symptoms at baseline or week 12, fewer than 1% reported ratings of severe (3; ). As expected, transient increases in mild-to-moderate itching, burning, stinging, scaling, and erythema were observed at weeks 2–8 (data not shown) and mirrored results observed in the overall population (Citation25). Only erythema and hyperpigmentation were reported in >20% of participants in either treatment group at baseline (28.9% and 22.5%, respectively); by week 12, fewer tazarotene-treated participants reported any erythema (23.7%) or hyperpigmentation (19.5%; ). Similar trends were observed in the tazarotene-treated Black participants with oily skin, though there were greater improvements in hyperpigmentation by week 12 than the overall oily skin population (hyperpigmentation at baseline and week 12: 41.4% and 32.2% Black subgroup vs. 22.6% and 19.6% overall oily skin). Further, Black participants with oily skin had fewer reports of erythema and scaling at baseline or week 12 than the overall population with oily skin ().
Figure 5. Cutaneous safety and tolerability at baseline and week 12 (safety population, pooled). No imputation of missing data. N values: all participants with oily skin baseline: TAZ n = 359, VEH, n = 353; week 12: TAZ n = 312, VEH n = 318; Black participants with oily skin baseline: TAZ n = 70, VEH n = 75; week 12: TAZ n = 59, VEH n = 68. TAZ: tazarotene 0.045% lotion; VEH: vehicle lotion.
Discussion
Excessive sebum production is one of several factors involved in the pathophysiology of acne (Citation19,Citation20). These post hoc analyses of data pooled from two phase 3 trials showed that tazarotene 0.045% polymeric emulsion lotion, applied once daily for 12 weeks, improved acne symptoms and perceived skin oiliness and was well tolerated in participants with oily skin and moderate-to-severe acne, including Black participants.
In the overall oily skin population, tazarotene 0.045% lotion provided over 55% reductions in inflammatory and noninflammatory lesion counts at week 12. Earlier improvements than with vehicle were also demonstrated, with significantly greater reductions from baseline noted at weeks 4 and 8 in noninflammatory and inflammatory lesion counts, respectively. In addition, almost one third of tazarotene-treated participants achieved treatment success at week 12 vs. less than one quarter of those treated with vehicle. These results are similar to those observed in the previously published overall pooled phase 3 studies (Citation24), and demonstrate that tazarotene 0.045% lotion is also efficacious in patients with acne and oily skin.
As facial acne and oily skin can both negatively affect quality of life (Citation3,Citation4,Citation29,Citation30), patients may appreciate an efficacious acne treatment that can also reduce skin oiliness. Some acne treatments, such as topical retinoids (Citation22) or topical clascoterone 1% cream (androgen receptor inhibitor) (Citation31), are postulated to have a sebum-suppressing effect, but clinical data supporting this have not been published. In the tazarotene 0.045% phase 3 studies, almost half (45.7%) of participants with moderate-to-severe acne reported having oily skin at baseline, while only 16.0% reported having low or not oily skin. By week 12, almost three quarters of all participants with oily skin improved to ‘moderately’ or ‘low/not’ oily skin with tazarotene 0.045% lotion or vehicle lotion.
To our knowledge, these are the first published results on the impact of a topical retinoid on oily skin in acne patients. Interpretation of these clinical data is challenging, however, given limitations in study design and analysis. These phase 3 studies were not powered for oily skin analyses, though this limitation exists for all post hoc analyses from clinical trials. Additionally, skin oiliness was measured via participant self-report as an item from the Acne-QoL questionnaire, which is a tool validated to measure overall patient quality of life and not oily skin specifically. Finally, participants may have changed their skin care routine while enrolled in the studies (i.e. cleansed/moisturized more or less often).
Nevertheless, improvements in oiliness with tazarotene lotion observed in these analyses may have been due in part to inhibition of sebocyte differentiation or lipid synthesis (Citation22) and/or more likely the unique, non-greasy excipients/emulsifiers contained in the polymeric emulsion lotion vehicle. This emulsion technology provides fast, uniform, and complete release of the humectants, oil droplets, and other excipients contained in the vehicle onto the skin (Citation27). Indeed, improvements in skin oiliness were similar with both tazarotene and vehicle lotion in the overall oily skin population, with 71% of participants in both treatment groups experiencing improvements. This suggests that the emulsification process, which releases the excipients and mixes with the surface lipids, results in a less oily and greasy feel and appearance of the skin even with the enhanced hydration provided by the added moisturizers. The exact nature, chemistry, and rheology of this process is still under investigation. Reductions in oiliness with tazarotene 0.045% lotion are particularly of interest given that topical retinoid use is associated with irritation and dryness (Citation21).
Sebum production varies greatly between individuals, but factors that may affect skin oiliness include sex, race, and climate (Citation1). Although males typically produce more sebum than females (Citation12,Citation14), it is noteworthy that in the current analyses there were more females in the oily skin group than in the overall population (73% vs. 66%, respectively) (Citation24). It is possible that women find oily skin to be more troublesome than men and were therefore more likely to report greater skin oiliness at baseline. Studies have shown that women with acne tend to report worse quality of life than men with acne (Citation29,Citation30), though it is unclear how much of this is attributable to skin oiliness. A separate post hoc analysis of data pooled from these phase 3 studies of tazarotene 0.045% lotion showed that females had worse acne-related quality of life scores at baseline and greater improvements after 12 weeks of tazarotene treatment than males (Citation30). In the present studies, tazarotene 0.045% lotion improved perceived skin oiliness over 12 weeks; quality of life, however, was not analyzed for this subgroup of participants with oily skin. To our knowledge, there is little published data on the impact of topical retinoids on oily skin and quality of life, and thus, this topic may warrant further research.
In these analyses, tazarotene 0.045% lotion demonstrated good safety and tolerability in participants with oily skin. Rates of TEAEs and treatment-related TEAEs were similar to those observed in the overall tazarotene-treated population (Citation25), and fewer than 2% of tazarotene-treated participants with oily skin discontinued the study or study drug. Over 70% of participants with oily skin treated with tazarotene lotion reported scores of none (0) on all cutaneous safety and tolerability assessments at baseline and week 12, and any increases in severity at weeks 2–8 were transient.
Although acne pathophysiology is believed to be the same across ethnic/racial groups, differences in skin structure and acne-related sequelae such as post-inflammatory hyperpigmentation and scarring may require a more individualized treatment approach, including assessment of racial characteristics and skin color (Citation32). When Black participants with oily skin were analyzed separately in the current analyses, over three-quarters treated with tazarotene reported an improvement in skin oiliness to ‘moderately’ or ‘low/not’ oily skin. While the overall oily skin population showed similar improvements with tazarotene and vehicle, a numerically greater percentage of Black participants experienced improvements with tazarotene vs. vehicle (81.4% vs. 67.6%). In terms of cutaneous safety and tolerability, tazarotene-treated Black participants with oily skin showed similar trends to the overall oily skin population; however, improvements in hyperpigmentation were greater in Black participants by week 12 and there were fewer reports of erythema and scaling at baseline or week 12. These results mirror those from the pooled phase 3 studies which demonstrated that tazarotene 0.045% lotion is efficacious and safe in Black participants with moderate-to-severe acne, with reductions in inflammation-related sequelae such as erythema and hyperpigmentation (Citation26).
Conclusions
In two pooled phase 3 studies, over two-thirds of participants with oily skin treated with tazarotene 0.045% or vehicle polymeric emulsion lotion – including Black participants – had subjective reductions in skin oiliness by week 12, with around a third reporting low or not oily skin. In addition, tazarotene lotion showed efficacy and safety in the treatment of moderate-to-severe acne in participants with oily skin, with outcomes similar to the overall study population. Once-daily treatment with tazarotene 0.045% polymeric emulsion lotion is effective in treating acne and may help to improve patient-perceived skin oiliness in those with moderate-to-severe acne.
Acknowledgments
Medical writing support was provided by Lynn M. Anderson, PhD of Prescott Medical Communications Group (Chicago, IL) with financial support from Ortho Dermatologics.
Disclosure statement
Emil A Tanghetti has served as speaker for Novartis, Ortho Dermatologics, Sun Pharma, Lilly, Galderma, AbbVie, and Dermira; served as a consultant/clinical studies for Hologic, Ortho Dermatologics, and Galderma; and is a stockholder for Accure. Joshua A Zeichner has served as advisor, consultant, or speaker for AbbVie, Allergan, Dermavant, Dermira, EPI Health, Galderma, Incyte, Johnson and Johnson, L'Oreal, Ortho Dermatologics, Pfizer, Procter and Gamble, Regeneron, Sun Pharma, UCB, Unilever, and Vyne. Michael Gold has acted as an investigator, advisor, speaker, and consultant for Ortho Dermatologics. Neil Sadick has served on advisory boards, as a consultant, investigator, speaker, and/or other and has received honoraria and/or grants/research funding from Almirall, Actavis, Allergan, Anacor Pharmaceuticals, Auxilium Pharmaceuticals, Bausch Health, Bayer, Biorasi, BTG, Carma Laboratories, Cassiopea, Celgene Corporation, Cutera, Cynosure, DUSA Pharmaceuticals, Eclipse Medical, Eli Lilly and Company, Endo International, EndyMed Medical, Ferndale Laboratories, Galderma, Gerson Lehrman Group, Hydropeptide, Merz Aesthetics, Neostrata, Novartis, Nutraceutical Wellness, Palomar Medical Technologies, Prescriber’s Choice, Regeneron, Roche Laboratories, Samumed, Solta Medical, Storz Medical AG, Suneva Medical, Vanda Pharmaceuticals, and Venus Concept. Fran E Cook-Bolden has served as consultant, speaker, investigator for Galderma, LEO Pharma, Almirall, Cassiopea, Ortho Dermatologics, Investigators Encore, Foamix, Hovione, Aclaris, and Cutanea. Leon H Kircik has acted as an investigator, advisor, speaker, and consultant for Ortho Dermatologics. Linda Stein Gold has served as investigator/consultant or speaker for Ortho Dermatologics, LEO Pharma, Dermavant, Incyte, Novartis, AbbVie, Pfizer, Sun Pharma, UCB, Arcutis, and Lilly. Jonathan S Weiss is a consultant, speaker, advisor, and/or researcher for AbbVie, Ortho Dermatologics, Janssen Biotech, Dermira, Almirall, Brickell Biotech, DermTech, and Scynexis. Stephen K Tyring has acted as an investigator for Ortho Dermatologics. James Q Del Rosso has served as a consultant, investigator, and/or speaker for Ortho Dermatologics, Abbvie, Amgen, Arcutis, Dermavant, EPI Heath, Galderma, Incyte, LEO Pharma, Lilly, MC2 Therapeutics, Pfizer, Sun Pharma, and UCB. Eric Guenin is an employee of Ortho Dermatologics and may hold stock and/or stock options in its parent company.
Data availability statement
Data are available upon request.
Additional information
Funding
References
- Endly DC, Miller RA. Oily skin: a review of treatment options. J Clin Aesthet Dermatol. 2017;10(8):49–55.
- Maia Campos PMBG, Melo MO, Mercurio DG. Use of advanced imaging techniques for the characterization of oily skin. Front Physiol. 2019;10:254.
- Arbuckle R, Atkinson MJ, Clark M, et al. Patient experiences with oily skin: the qualitative development of content for two new patient reported outcome questionnaires. Health Qual Life Outcomes. 2008;6:80.
- Wu Y, Niu Y, Zhong S, et al. A preliminary investigation of the impact of oily skin on quality of life and concordance of self-perceived skin oiliness and skin surface lipids (sebum). Int J Cosmet Sci. 2013;35(5):442–447.
- Roh M, Han M, Kim D, et al. Sebum output as a factor contributing to the size of facial pores. Br J Dermatol. 2006;155(5):890–894.
- Pappas A, Fantasia J, Chen T. Age and ethnic variations in sebaceous lipids. Dermatoendocrinol. 2013;5(2):319–324.
- Rawlings AV. Ethnic skin types: are there differences in skin structure and function? Int J Cosmet Sci. 2006;28(2):79–93.
- Grimes P, Edison BL, Green BA, et al. Evaluation of inherent differences between African American and White skin surface properties using subjective and objective measures. Cutis. 2004;73(6):392–396.
- Sugiyama-Nakagiri Y, Sugata K, Hachiya A, et al. Ethnic differences in the structural properties of facial skin. J Dermatol Sci. 2009;53(2):135–139.
- Youn SW, Park ES, Lee DH, et al. Does facial sebum excretion really affect the development of acne? Br J Dermatol. 2005;153(5):919–924.
- Pappas A, Johnsen S, Liu JC, et al. Sebum analysis of individuals with and without acne. Dermatoendocrinol. 2009;1(3):157–161.
- Luebberding S, Krueger N, Kerscher M. Skin physiology in men and women: in vivo evaluation of 300 people including TEWL, SC hydration, sebum content and skin surface pH. Int J Cosmet Sci. 2013;35(5):477–483.
- Camera E, Ludovici M, Tortorella S, et al. Use of lipidomics to investigate sebum dysfunction in juvenile acne. J Lipid Res. 2016;57(6):1051–1058.
- Rahrovan S, Fanian F, Mehryan P, et al. Male versus female skin: what dermatologists and cosmeticians should know. Int J Womens Dermatol. 2018;4(3):122–130.
- Makrantonaki E, Ganceviciene R, Zouboulis C. An update on the role of the sebaceous gland in the pathogenesis of acne. Dermatoendocrinol. 2011;3(1):41–49.
- Sakuma TH, Maibach HI. Oily skin: an overview. Skin Pharmacol Physiol. 2012;25(5):227–235.
- Moradi Tuchayi S, Makrantonaki E, Ganceviciene R, et al. Acne vulgaris. Nat Rev Dis Primers. 2015;1:15029.
- Zouboulis CC, Jourdan E, Picardo M. Acne is an inflammatory disease and alterations of sebum composition initiate acne lesions. J Eur Acad Dermatol Venereol. 2014;28(5):527–532.
- Jappe U. Pathological mechanisms of acne with special emphasis on propionibacterium acnes and related therapy. Acta Derm Venereol. 2003;83(4):241–248.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973.e33.
- Leyden J, Stein-Gold L, Weiss J. Why topical retinoids are mainstay of therapy for acne. Dermatol Ther (Heidelb). 2017;7(3):293–304.
- Zouboulis CC, Picardo M, Ju Q, et al. Beyond acne: current aspects of sebaceous gland biology and function. Rev Endocr Metab Disord. 2016;17(3):319–334.
- Kang S, Krueger GG, Tanghetti EA, et al. A multicenter, randomized, double-blind trial of tazarotene 0.1% cream in the treatment of photodamage. J Am Acad Dermatol. 2005;52(2):268–274.
- Tanghetti EA, Werschler WP, Lain E, et al. Novel polymeric lotion formulation of once-daily tazarotene (0.045%) for moderate-to-severe acne: pooled phase 3 analysis. J Drugs Dermatol. 2020;19(3):272–279.
- Tanghetti EA, Werschler WP, Lain T, et al. Tazarotene 0.045% lotion for once-daily treatment of moderate-to-severe acne vulgaris: results from two phase 3 trials. J Drugs Dermatol. 2020;19(1):70–77.
- Bhatia N, Weiss JS, Sadick N, et al. Novel polymeric tazarotene 0.045% lotion for moderate-to-severe acne: pooled phase 3 analysis by race/ethnicity. J Drugs Dermatol. 2020;19(7):727–734.
- Tanghetti EA, Stein Gold L, Del Rosso JQ, et al. Optimized formulation for topical application of a fixed combination halobetasol/tazarotene lotion using polymeric emulsion technology. J Dermatolog Treat. 2021;32(4):391–398.
- Martin AR, Lookingbill DP, Botek A, et al. Health-related quality of life among patients with facial acne – assessment of a new acne-specific questionnaire. Clin Exp Dermatol. 2001;26(5):380–385.
- Skroza N, Tolino E, Mambrin A, et al. Adult acne versus adolescent acne: a retrospective study of 1,167 patients. J Clin Aesthet Dermatol. 2018;11(1):21–25.
- Kircik LH, Lain E, Gold M, et al. Effects of tazarotene 0.045% lotion on quality of life in patients with moderate-to-severe acne. J Drugs Dermatol. 2020;19(11):1086–1092.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156(6):621–630.
- Alexis AF, Woolery-Lloyd H, Williams K, et al. Racial/ethnic variations in acne: implications for treatment and skin care recommendations for acne patients with skin of color. J Drugs Dermatol. 2021;20(7):716–725.