Dear Editor,
Atopic dermatitis (AD) is a chronic inflammatory skin condition that reduces quality of life (Citation1). Treatment options range from older drugs such as cyclosporine to monoclonal antibodies (mAb) and small molecules (Citation2). The mAb dupilumab was effective and safe in AD registration studies (Citation3). Tralokinumab was also effective and safe for AD with a lower rate of conjunctivitis than seen with dupilumab (Citation4). The small molecule upadacitinib is even more effective than dupilumab (Citation5), but has more side effects (including acne and CK and lipid increases) and recommended clinical and laboratory monitoring (Citation5).
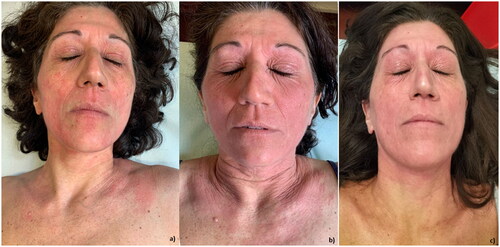
AD can be resistant to multiple treatments. We report a 52-year-old female patient followed at the University Dermatology Clinic of Turin. The patient presented with AD from birth, with concomitant conjunctivitis and allergic rhinitis, and—having failed treatment with topical calcineurin inhibitors and steroids, systemic steroids, and cyclosporine in January 2019—started dupilumab. At 4 months, EASI had decreased from 30 to 3, but face and neck involvement, high impairment of quality of life (QoL), and pruritus persisted (Dermatology Life Quality Index [DLQI] from 14 to 13 and Number Rating Scale of Pruritus [NRSp] from 5 to 8). During subsequent visits, facial involvement persisted, pruritus progressive worsened, and numerous conjunctivitis episodes occurred. In April 2021, given the high disease burden (EASI17, DLQI 13 NRSp 6) and side effects, we opted to switch to upadacitinib 30 mg daily. At two months, EASI was 2, DLQI and NRSp were 1 and 0, respectively, but facial involvement persisted. At 4 months into upadacitinib treatment, pruritus and QoL worsened (NRSp 5 and DLQI 7); at subsequent follow-up visits AD worsened, too. During treatment with upadacitinib, there were numerous episodes of arthralgias and cutaneous infectious events, including a right upper lip abscess, right shoulder cystic lesion, and numerous episodes of chalazion that required repeated antibiotic therapy and reduction of upadactinib dosage to 15 mg per daydie. In March 2022 in consideration of adverse events and worsening AD (EASI 7, NRSp 10, DLQI22), after ruling out lymphomatous pathology by biopsy and histological examination, upadacitinib was discontinued. In May 2022 in consideration of EASI 24, tralokinumab was started according to the approved dosage (600 mg at baseline and 300 mg every 2 weeks thereafter). At the September 2022 follow-up visit, an initial skin response with persistent facial involvement (EASI 3) was observed while maintaining an unchanged impact on PROs (DLQI 24 and NRSp 10) (). The patient is currently continuing therapy with tralokinumab.
Figure 1. (a) The patient at the end of dupilumab therapy: extensive erythematous involvement of the face and neck. (b) The patient at the end of upadacitinib therapy: erythema and lichenification of the face, neck, and neckline. (c) First follow-up after initiation of tralokinumab: initial reduction in the extent of erythematous lesions and reduction in lichenification at the face.
New treatments for AD have revolutionized the lives of patients with AD; however, some individuals may be resistant to multiple treatments (Citation6). Our patient had AD from birth with associated atopic comorbidities (Citation7); we speculate that this particular endotype is predisposing to greater resistance to treatment, but the data in the literature for the moment do not support this suggestion. Cutaneous lymphoma can mimic AD but was excluded by biopsy in our patient; poor treatment adherence is another potential cause of apparently ‘resistant’ AD. Multi-treatment resistant atopic patients may not be uncommon in the future; more data are needed to identify factors predictive or characteristic of treatment resistance.
Informed consent
The patients in this manuscript have given written informed consent to publication of their case details.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Mastorino L, Cantafio Duò VL, Vecco C, et al. Impact of comorbidities in the response of atopic patients treated with dupilumab: a real-life study up to 36 weeks. Acad Dermatol Venereol. 2022;36(12):e1021–e1023.
- Drucker AM, Morra DE, Prieto-Merino D, et al. Systemic immunomodulatory treatments for atopic dermatitis: update of a living systematic review and network meta-analysis. JAMA Dermatol. 2022;158(5):523–532.
- Miniotti M, Lazzarin G, Ortoncelli M, et al. Impact on health-related quality of life and symptoms of anxiety and depression after 32 weeks of Dupilumab treatment for moderate-to-severe atopic dermatitis. Dermatol Ther. 2022;35(5):e15407.
- Wollenberg A, Blauvelt A, Guttman-Yassky E, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184(3):437–449.
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157(9):1047–1055.
- Mastorino L, Roccuzzo G, Dapavo P, et al. Patients with psoriasis resistant to multiple biological therapies: characteristics and definition of a difficult-to-treat population. Br J Dermatol. 2022;187(2):263–265.
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143(1):1–11.

