Abstract
Purpose: Successful treatment of periungual warts presents a clinical challenge, as many are refractory or represent following conventional treatments. The use of destructive therapies, such as cryotherapy, may even cause permanent nail dystrophy. Materials and methods: Here, we present a series of nine cases in which intralesional cidofovir was used for recalcitrant periungual warts between July 2020 and July 2022 at the University of California, Los Angeles. Results: Following a mean of 2.7 treatments (SD = 0.87), 100% of patients (n = 9) saw improvement in the appearance of their warts, and 77.8% (n = 7) had near to complete resolution. Few self-resolving local reactions occurred, including pain, edema, erosion, blister formation, and discoloration at the proximal nail fold. All reactions resolved within weeks of treatment and required no additional treatment. Conclusions: Intralesional cidofovir treatment of recalcitrant periungual warts is well tolerated and provides unmatched results. Given the risks of traditional therapies to the nail, intralesional cidofovir should be considered as a first-line therapy for periungual warts. Randomized clinical trials are necessary, in the future, to adequately understand the effectiveness of intralesional cidofovir.
Introduction
Periungual warts present around the nailbed as discrete, pinhead-sized translucent lesions that grow to pea-sized, rough lesions that may become inflamed or fissured. Notably, these may be refractory or reoccur following treatment. Traditional destructive therapies, such as cryotherapy, may cause permanent nail dystrophy. Successful treatment of periungual warts presents a therapeutic challenge (Citation1).
Cidofovir is an antiviral therapy approved originally for cytomegalovirus retinitis [Citation1]. More recently, effectiveness in treating refractory cutaneous warts with intralesional cidofovir (ILC) has been reported in case reports, a retrospective review, and a retrospective study with a comparison to sodium tetradecyl sulfate [Citation2–5]. The drug is a nucleotide analog that incorporates viral DNA and stimulates apoptotic cell death [Citation1]. Cidofovir should be avoided in patients with severe sulfonamide or probenecid hypersensitivity [Citation2].
Methods
Here, we present a series of cases in which ILC was used for recalcitrant periungual warts at the University of California Los Angeles between July 2020 and July 2022. Cidofovir (75 mg/mL) was diluted with 1% lidocaine with 1:100,000 epinephrine to create a 25 mg/mL solution. The solution was injected directly into the body of the wart, no deeper than the superficial dermis, with 0.1 mL serial punctures. In each treatment session, 0.2–0.4 mL were needed. Patients were seen back no sooner than 4 weeks for reinjection if necessary. Improvement in wart appearance was patient-reported. A resolution was determined by the clinician’s exam. Patient characteristics and treatment outcomes are summarized (). This study was approved by the institutional review board at the University of California Los Angeles.
Table 1. Characteristics and treatment outcomes for patients with recalcitrant periungual warts treated with intralesional cidofovir.
Results
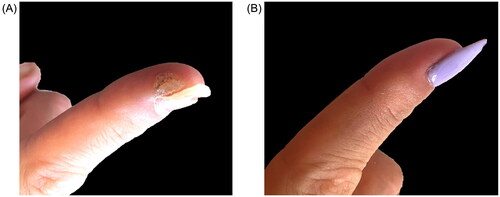
Of the patients treated (n = 9), 88.9% had previous cryotherapy treatments and 100% tried three or more different wart treatments prior to ILC. After a mean of 2.7 treatments (SD = 0.87), 100% of warts improved and 77.8% were nearly or completely resolved. Three patients saw 95% improvement after a single treatment. A representative treatment response is shown (). Reported local reactions were pain (n = 2), edema (n = 2), erosion (n = 1), blister formation at the injection site (n = 1), and discoloration of the proximal nail fold (n = 1). All local reactions were self-limited and required no additional treatment.
Discussion
To date, successful ILC treatment for cutaneous warts of primarily of palmoplantar locations has been reported in patients across two case reports and two retrospective reviews [Citation2–5]. Here, we report the first case series focused on ILC’s efficacy and tolerability for the treatment of periungual warts. Effective concentrations reported in the existing literature for all cutaneous warts range from 7.5 to 25 mg/mL, with most reporting use of 15 mg/mL [Citation2–5]. Comparing our findings with those of others, patients require an average of two to four injections for significant improvement or resolution. Other possible side effects may include a burning sensation, or pruritus, however, all are self-limiting [Citation2,Citation5]. The tolerance of ILC is excellent and the results are unmatched for recalcitrant warts.
In this series of cases, ILC was successful in resolving most recalcitrant periungual warts after one to four treatment sessions. Limitations of this case series include a small number of patients and follow-up restricted to 6 months for most cases with complete resolution. Patients with partial resolution continued treatment beyond the study window. Even considering these limitations, our results suggest that ILC can be successful in patients with periungual warts that have proven recalcitrant to standard therapy. ILC should not be used in patients with severe sulfonamide or probenecid hypersensitivity [Citation2]. No systemic side effects were observed in this case series nor reported in the literature for intralesional administration. Application site reactions can occur and should be explained to patients prior to use. Given the risks to the nail of traditional therapies, we submit that ILC should be considered as a first-line therapy for periungual warts. To adequately understand the effectiveness of ILC, randomized clinical trials are necessary.
Ethical approval
The present work was approved by the Institutional Review Board at UCLA, IRB #22-001583.
Patient consent
Consent for the publication of the patient photographs was obtained at the time of article submission. A copy of the submission was made available for the patient.
Disclosure statement
No potential conflict of interest was reported by the author(s).. No data from this manuscript has been published elsewhere.
Data availability statement
The data that support the findings of this study are available from the corresponding author, JD, upon reasonable request.
Additional information
Funding
References
- Coremans G, Snoeck R. Cidofovir: clinical experience and future perspectives on an acyclic nucleoside phosphonate analog of cytosine in the treatment of refractory and premalignant HPV-associated anal lesions. Expert Opin Pharmacother. 2009;10(8):1343–1352.
- Anshelevich EE, Barbieri JS, Kovarik CL. Intralesional cidofovir for treatment of recalcitrant warts in both immunocompetent and immunocompromised patients: a retrospective analysis of 58 patients. J Am Acad Dermatol. 2021;84(1):206–207.
- Oh BH. Cidofovir intralesional injection for recalcitrant common warts: a comparison with sodium tetradecyl sulfate intralesional injection. Ann Dermatol. 2020;32(4):273–279.
- Blouin MM, Cloutier R, Noël R. Intralesional cidofovir in the treatment of cutaneous warts in a renal transplant patient. J Cutan Med Surg. 2012;16(6):462–464.
- Broganelli P, Chiaretta A, Fragnelli B, et al. Intralesional cidofovir for the treatment of multiple and recalcitrant cutaneous viral warts. Dermatol Ther. 2012;25(5):468–471.