Abstract
Background
Dose reduction of biologics for psoriasis is applied in daily practice, although guidelines are lacking. Striving for clear criteria is important, as it leads to a consistent application of dose reduction.
Objective
To achieve consensus on criteria for biologic dose reduction in psoriasis patients with stable and low disease activity.
Methods
An online Delphi procedure (eDelphi) was conducted. Dutch dermatologists were invited to participate in a maximum of 3 voting rounds. Proposed statements were selected based on literature review and included criteria for the application of dose reduction and dosing schedules. Biologic dose reduction was defined as ‘application of injection interval prolongation’. Proposed statements were rated using a 9-point Likert scale; consensus was reached when ≥70% of all voters rated ‘agree’ (7–9) and <15% rated ‘disagree’ (1–3).
Results
A total of 27 dermatologists participated and reached a consensus on 15 recommendations over 2 voting rounds. Agreed statements included criteria for dose reduction eligibility, criteria for dose reduction (dis)continuation, and dosing schedules for adalimumab, etanercept, and ustekinumab. Based on the eDelphi outcomes, an algorithm fit for implementation in current practice was developed.
Conclusions
Recommendations of this national consensus process can guide clinicians, and consequently their patients, toward consistent application of biologic dose reduction.
Introduction
Biologics are effective but expensive drugs for patients with moderate-to-severe psoriasis [Citation1]. As psoriasis is a chronic disease with a large impact on a patient’s quality of life, lifelong treatment is mostly needed for long-term disease control. Treatment with a fixed dose may however not be necessary for patients with good treatment responses, as some patients may be overtreated [Citation2]. Dose reduction (DR) of biologics for psoriasis patients with low disease activity is a possible solution for more efficient use. Overtreatment might be prevented and healthcare costs can be reduced when striving for the lowest effective dose [Citation3,Citation4]. Guidance is however needed, as DR could theoretically lead to loss of disease control.
Dose reduction by injection interval prolongation of adalimumab, etanercept, and ustekinumab is possible in patients with low disease activity without losing disease control, but success rates differ based on success definition, DR strategy, and study design [Citation3,Citation5–7]. In a previously conducted randomized trial, no differences in persistent disease flares were observed between patients on DR vs. patients on the standard maintenance dose of adalimumab, etanercept, or ustekinumab [Citation8]. Most studies described a minimal treatment duration and/or stable low disease activity of 6–12 months prior to DR, and the biologic dose was mostly reduced gradually in fixed steps leading to 67% and 50% of the original dose. Regaining adequate treatment responses after the resumption of the standard dose in case of relapse due to DR was described in several studies [Citation7,Citation9–11]. For the relatively newer biologics (e.g., IL-17 and IL-23 inhibitors), data are sparse [Citation12–15].
At present, DR is performed in daily practice but clear criteria have not been elaborated in clinical guidelines [Citation6,Citation7,Citation16–18]. A recently performed national survey among 114 dermatologists in the Netherlands showed that biologic DR was already practiced by the majority of respondents, most frequently for the biologics adalimumab, ustekinumab, and etanercept [Citation19]. There was a variation in the used criteria for starting and stopping DR. In total, 78% of all respondents felt the necessity of a guideline on biologic DR, with scientific evidence and practical advice. Internationally, a survey among 53 dermatologists revealed that 66% performed DR, mainly for the biologics adalimumab, etanercept, ustekinumab, and secukinumab [Citation20]. Again, the criteria for the application of DR differed between respondents. Among the barriers to the application of DR was the lack of guidelines or scientific evidence.
For further uptake of biologic DR into clinical practice, it is important to strive for clear criteria that guide healthcare professionals, and consequently their patients, towardsafe application of DR. As criteria for applying DR and the actual manner of reducing dosages varied between studies and among dermatologists, agreement upon criteria for practicing DR should be achieved among involved healthcare professionals, supported by the existing evidence. Therefore, the aim of the current study was to achieve consensus among Dutch dermatologists on criteria for biologic DR in psoriasis patients and propose an algorithm fit for implementation in current practice.
Materials and methods
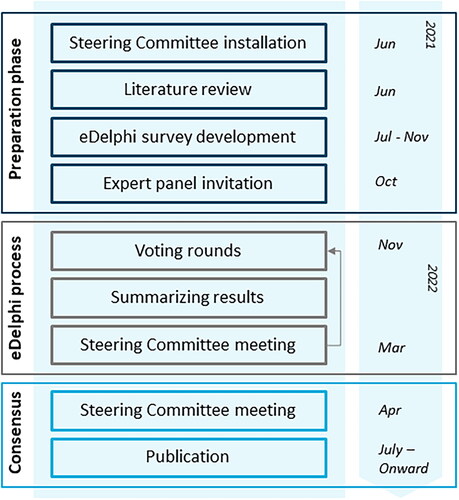
An online Delphi procedure (further referred to as ‘eDelphi’) was conducted. The Delphi approach comprises sequential questionnaires answered by experts. Gradually, consensus evolves as the range of answers decreases and the group converges toward a consensus opinion over the course of several rounds [Citation21,Citation22]. See for a graphical overview of the study design.
This study was conducted according to the ICH GCP guidelines, and the principles of the Declaration of Helsinki. Data collection was performed in accordance with the Dutch Act on Implementation of General Data Protection. The need for ethical approval was waived by the Medical Ethical Committee Arnhem-Nijmegen (2021-12627). Consent to participate was assumed through self-registration and round completion. Consent to be acknowledged in this publication was specifically sought. Reporting followed the Standards for Quality Improvement Reporting Excellence (SQUIRE) 2.0 guidelines [Citation23].
Preparation phase and eDelphi survey development
A Steering Committee (SC) (L.S., E.B., A.E., S.M., S.W., J.R., E.J.) was installed in order to provide guidance and feedback on the eDelphi process. Members were experts with a background in psoriasis-related research and/or clinical practice leadership and representatives of the Dutch Association for Dermatology and Venereology. The SC advised on the development and refinement of the eDelphi statements, determined consensus criteria, and deliberated on proposals for revision of statements. Dermatologist members of the SC (E.B., S.M., E.J.) were allowed to vote during the eDelphi rounds. Patient representatives (I.E., A.P.) were delegated by the Dutch National Psoriasis Patient Association and were asked to review the consensus outcomes in order to incorporate the patient perspective.
A literature review was conducted in order to identify possible relevant outcomes for the application of DR in daily practice. We used the results of a previously conducted scoping review on DR of biologics in adult patients with psoriasis [Citation5]. The search strategy of this review was updated until June 2021. Selection criteria forinclusion of studies and format of data extraction complied with the used methods from the scoping review [Citation5]. After obtaining results from the literature review, the eDelphi statements were drafted. Additionally, results from a previously conducted survey among Dutch dermatologists regarding used criteria for the application of DR in clinical practice were incorporated in the eDelphi statements [Citation19]. Dose reduction was defined as ‘the application of injection interval prolongation’ and was aimed at psoriasis patients with stable and low disease activity. Dose reduction by means of decreasing the absolute dosage in milligrams was excluded due to limited available evidence on this strategy [Citation5]. Within the eDelphi statements, criteria for starting DR, DR (dis)continuation, and DR schedules per biologic were proposed. Here, it was aimed to define criteria for selecting patients with stable low disease activity. Moreover, criteria for guidance of patients on DR were proposed in order to allow timely action to prevent loss of disease control. Based on previous literature and current practice, included disease activity measures used for DR eligibility criteria were the Psoriasis Area and Severity Index (PASI), Physicians Global Assessment (PGA), and impact on the patient’s quality of life measured with Dermatology Life Quality Index (DLQI). Proposed thresholds for these outcome measures were based on prevailing national treatment targets [Citation17], and on a previously conducted multicentre, randomized controlled trial in the Netherlands, as this was the only available randomized controlled trial designed for DR evaluation [Citation11]. Upper limits of proposed thresholds included PASI 5, PGA 0-2, and DLQI 5, and were chosen in order to provide some room for the application of DR in daily practice, as the accepted or reachable level of disease activity might differ between patients. Note that the proposed thresholds were not treatment targets in this context, but were defined as critical thresholds to advise DR discontinuation. Proposed dosing intervals were also based on the previous trial [Citation11]. When developing the statements, it was aimed to only include the biologics adalimumab, etanercept, and ustekinumab, as for these biologics most evidence regarding DR was available [Citation5,Citation11]. Infliximab was excluded as DR might lead to an increased risk of infusion reactions [Citation24]. Statements were written in Dutch.
In order to optimize response rates and practicability, it was aimed to include a maximum number of 20–25 statements. Each statement should be rated on a 9-point Likert scale, with 1–3 labeled ‘not important/disagree’, 4–6 labeled ‘important but not critical/neither agree nor disagree’ and 7–9 labeled ‘critical/agree’. With each statement, a blank text box for comments was provided and participants were asked to suggest additional items. The questionnaire was accompanied by background information on the Delphi process and on how candidate items have been selected. Consensus on any statement required ≥70% of all voters to rate the outcome with a score of 7–9 (agree) and <15% to rate the outcome with a score of 1–3 (disagree) [Citation25–27].
Participants and recruitment
Dermatologists experienced in treating psoriasis patients with biologics were recruited through the Dutch Association for Dermatology and Venereology by an invitation e-mail. Within the invitation e-mail, it was noted that experience with biologic treatment was mandatory in order to participate. Respondents to the invitation were invited for the first eDelphi round. The sample size was not pre-defined.
eDelphi process
The questionnaire was distributed by the Dutch Association for Dermatology and Venereology using a password-protected web-based survey system: Survio (www.survio.com). The survey was pilot tested before going live.
A maximum of 3 rounds of online Delphi voting were planned. The total number of rounds depended on whether consensus was achieved or not. In case no consensus was reached after 3 rounds, a consensus meeting would be held with all participants in order to resolve the remaining disagreements. Participants were asked to rate each item within the questionnaire. All questions were mandatory to answer. To reduce the risk of attrition bias, the importance of completing all eDelphi rounds was highlighted to all participants at each round. Participants were asked to complete each round within 4 weeks. Reminder e-mails were sent weekly to increase the response rate. Round 1 included participant characteristics (age, gender, position, type of practice/hospital, years of experience with treatment of psoriasis patients with biologics).
After each voting round, answers were analyzed in order to determine whether consensus on statements was reached based on the pre-stated consensus criteria. Results were discussed with the SC. It was decided which ‘non-consensus’ statements and/or participants’ other suggestions should continue to the next round and when necessary, statements were re-defined by the SC based on participants’ comments. Consensus could not be overturned by the SC.
In each subsequent round, participants were asked to re-score non-consensus items. Feedback and results of the previous round were provided. Participants who did not participate in, or did not complete the previous round were not invited to the subsequent round. The total number of participants who completed the survey was recorded as the number of participants for each round. After the eDelphi exercise, the results were shared with all participants.
Analysis
Collected data was pseudonymized by the use of unique numerical identifiers. Results were password protected. Data were imported into Microsoft Excel for analysis. Descriptive analysis was used to summarize responses and determine if consensus thresholds were reached. Statements were translated into English for the final report.
Results
In October 2021, 850 dermatologists were invited to participate in the eDelphi process. A total number of 44 dermatologists experienced with biologic treatment for psoriasis registered for participation, of which 27 eventually participated in the first eDelphi round. In total, 2 voting rounds took place. In round 2, all 27 dermatologists of round 1 participated. The demographics of participants are presented in . Years of experience in prescribing biologics exceeded the number of years of working experience as a dermatologist, as respondents could have had experience with prescribing biologics during their residency.
Table 1. Demographic characteristics of participants in the eDelphi survey.
In round 1, conducted from 22 November 2021 until 14 January 2022, 15 statements were presented to the participants. See Appendix S1 for the survey. In total, three reminders were sent. After round 1, agreement was achieved on 10 items regarding DR eligibility, (dis)continuation, and dosing schedules for adalimumab and etanercept. See for the results.
Table 2. Results of the eDelphi consensus: criteria for biologic dose reduction in patients with psoriasis.
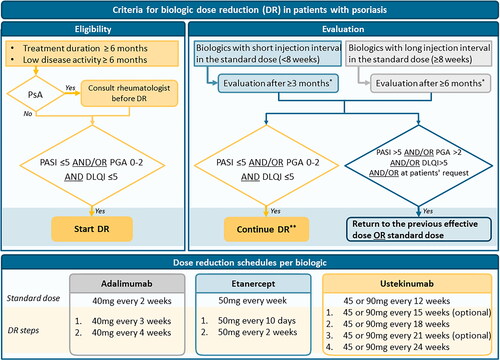
Within the first round, agreed criteria for DR eligibility and (dis)continuation included the following thresholds for disease activity measures: PASI ≤5, PGA 0–2, and DLQI ≤5. Some participants commented however to prefer using lower PASI scores (≤2–3) and/or PGA scores (0–1). It was agreed that outpatient visits should not be performed more frequently when applying DR, although participants commented that patients should be instructed to contact the clinic in case of disease flares. The proposed dosing schedules of two steps leading to 67% and 50% of the standard dose were agreed upon for adalimumab and etanercept. For ustekinumab, agreement was not achieved in the first round and participants suggested the use of intermediate DR steps. In addition, the option of reducing the dose of ustekinumab from 90 to 45 mg every 12 weeks was suggested. As the consensus was aimed at DR by means of interval prolongation, this option was not included.
After round 1, five non-consensus items were revised and proceeded to round 2. See Table S1 for an overview of revised statements including the rationale. Revisions were made to the criteria on the minimal duration of low disease activity before starting DR, and minimal treatment duration before consideration of further DR. Moreover, DR steps for ustekinumab were adjusted: intermediate DR steps were added to the proposed schedule, resulting in four subsequent DR steps for this biologic. Based on participants’ comments, the statement which indicated cautiousness for consideration of DR of the newer biologics was rephrased in a way that DR of the newer biologics could be considered in individual patients.
Round 2 was conducted from 7 March 2022 until 31 March 2022. Two reminders were sent. See Appendix S2 for the survey. Of the 5 presented revised statements, agreement was achieved on all items. All final results are presented in . No new statements were proposed by participants. An algorithm based on the agreed criteria is presented in .
Figure 2. Algorithm for biologic dose reduction (DR) in patients with psoriasis based on the consensus. DR: dose reduction; PASI: Psoriasis Area and Severity Index; PGA: Physician Global Assessment; PsA: psoriatic arthritis; DLQI: Dermatology Life Quality Index. *DR can be discontinued at any time point in case of increased psoriasis or at the patient’s request. **Continue DR: next step DR or continue lowered dose. For dosing schedules see the lowest part of the algorithm.
Discussion
By using an eDelphi consensus process involving Dutch dermatologists, consensus was reached within 2 eDelphi rounds on all 15 statements regarding criteria for the application of biologic DR by means of interval prolongation in patients with psoriasis. In addition, a clear algorithm was developed, ready for use in clinical practice.
Agreed criteria for eligibility and (dis)continuation of biologic DR included thresholds of outcome measures (PASI, PGA, DLQI). By using these criteria, timely action can prevent disease flares in case of loss of treatment response. Thresholds were based on a literature review and on prevailing national treatment targets [Citation17]. The PGA was added to the PASI in order to provide a more practical tool for clinicians in daily practice, as PASI measurements can be time-consuming. Of note, thresholds were defined with the aim to select patients with low disease activity and should not be considered treatment goals or targets. It can be debated if the proposed thresholds are low enough, as in the field of psoriasis more stringent targets were described in recent years [Citation28,Citation29]. Some respondents indicated that they preferred to use lower thresholds, for example, PASI 2 or 3 instead of 5. From our experience, however, the acceptable level of disease activity can be different for each individual patient [Citation8]. In addition, the thresholds are upper limits and hence, the option to start DR with lower scores as well is provided. Moreover, combining PASI with DLQI scores will probably lead to patients with lower PASI scores initiating DR, as can be seen from the included patients in the CONDOR trial (median PASI at starting DR 1.8) [Citation11]. It should also be emphasized that the aim of the used thresholds is to select patients with stable and low disease activity and that DR should only be initiated within a shared-decision making approach.
Within the eDelphi process, DR schedules for the biologics adalimumab, etanercept, and ustekinumab were proposed, as most evidence regarding the DR of these biologics was available [Citation5,Citation11]. Of note, very long-term data regarding the DR of these biologics still needs to follow and more insight into predictors for successful DR is warranted [Citation5]. The newer biologics (i.e., IL-17 and IL-23 inhibitors) were excluded due to limited scientific evidence on DR effects. To our best knowledge, few studies regarding the DR of the IL-17 inhibitors and IL-23 inhibitors were available [Citation12,Citation14,Citation15,Citation30,Citation31]. First, a statement was included describing cautiousness for DR of the newer biologics, but participants commented that DR for the newer biologics was already applied in daily practice. In the second round, the statement was revised in order to provide more room for application of DR for the newer biologics. However, the statement still indicated some caution because more evidence needs to follow, as the risk remains that future evidence could provide disappointing results. In case more evidence appears, it should be decided whether current criteria and dosing regimens can also be applied to the newer agents.
For ustekinumab, it was suggested to reduce the dose from 90 mg every 12 weeks to 45 mg every 12 weeks in case of adequate response. It was decided not to include a statement regarding this option, as there is limited literature available on this option, and the consensus was aimed at DR by means of interval prolongation. Moreover, this intervention would lead to a reduction of 50% of the original dose, which is a relatively large step. Future studies could consider exploring further options for DR including reducing dosages in milligrams per injection, and DR guided by drug levels based on evidence from therapeutic drug monitoring studies [Citation32].
To our best knowledge, this is the first consensus on criteria for biologic DR in psoriasis. Another strength is that by design, the risk of bias was minimized as our anonymous eDelphi approach avoided possible dominance by any of the participants. Furthermore, implementation of the final results will be promoted due to collaboration with the Dutch Association for Dermatology and Venereology. The main limitation is the low number of respondents. Demographic characteristics showed however those participants represent both general and academic practice and were experienced in the treatment of psoriasis patients with biologics, as the median reported time of experience with prescription of biologics was 11 years. As results comply with the results of a previous survey among Dutch and international dermatologists [Citation19,Citation20], we believe that the agreed criteria can be incorporated into clinical practice. As stated above, another limitation is that no statements regarding DR of the newer biologics (e.g., IL-17 and IL-23 inhibitors) were formulated due to the scarcity of scientific evidence on DR of these agents. However, one statement on how to handle DR in these drugs was added. Furthermore, exclusion of patients in the voting process can be considered a limitation, although patients’ representatives reviewed the consensus outcomes. Further elaboration on the patient perspective regarding biologic DR is however important for further implementation of DR strategies. This is an important issue for future work.
Another important next step could be to reach international consensus and enhance uptake of the proposed criteria for biologic DR in guidelines. Dermatologists worldwide indicated the lack of guidelines on biologic DR as main barrier to the application of DR [Citation20]. However, ideal criteria might differ between countries due to differences in commonly used disease activity measures and differences in cultural and healthcare organizational aspects. Future research could therefore aim at defining international consensus on criteria for biologic DR, for which the current consensus might form a basis.
In conclusion, recommendations resulting from this national consensus process can guide clinicians, and consequently their patients, toward consistent application of biologic DR in daily clinical practice. Further implementation and uptake of biologic DR in (inter)national clinical guidelines is important for future perspective.
Supplemental Material
Download PDF (245.3 KB)Acknowledgments
The authors acknowledge the key role played by the Dutch Association for Dermatology and Venereology in the organization of the eDelphi survey and would like to thank the Dutch National Psoriasis Patient Association for their contributions. The authors would also like to thank all participating dermatologists who took part in the eDelphi rounds, among them: M. B. A. van Doorn, P. I. Spuls, M. P. M. Andriessen, D. Vellinga, M. de Groot, S. R. P. Dodemont, A. L. Nguyen, E. A. Dowlatshahi, R. R. Keijsers, D. N. H. Enomoto, L. A. A. Gerbens, S. van de Scheur, M. A. de Rie, P. P. M. van Lumig, J. H. J. Hendricksen-Roelofzen, E. T. Hamers. All those named have given their consent to be acknowledged.
Disclosure statement
L. S. van der Schoot carries out clinical trials for Janssen and Novartis and received speaking fees from Janssen and Eli Lilly. All funding is not personal but goes to the independent Research Fund of the Department of Dermatology of the Radboud University Medical Center Nijmegen, The Netherlands. E. M. Baerveldt received speaking fees from Abbvie, Janssen, and Novartis and attended advisory boards from Janssen. Fees were paid directly to the institution. M. M. B. Seyger received grants from/was involved in clinical trials from Abbvie, Amgen, Celgene, Eli Lilly, Janssen, Leo Pharma, and Pfizer. She served as a consultant for Abbvie, Eli Lilly, Janssen, Leo Pharma, Novartis, Pfizer, and UCB. Fees were paid directly to the institution. S. L. Wanders received speaking fees from Abbvie. Fees were paid directly to the institution. J. M. P. A. van den Reek carries out clinical trials for AbbVie, Celgene, and Janssen; has received speaking fees/attended advisory boards from AbbVie, BMS, Almirall and Janssen and has received reimbursement for attending a symposium from Celgene and AbbVie. All funding is not personal but goes to the independent research fund of the Department of Dermatology of the Radboud University Medical Center Nijmegen, the Netherlands. E. M.G. J. de Jong has received research grants for the independent research fund of the Department of Dermatology of the Radboud University Medical Center Nijmegen, the Netherlands from AbbVie, Pfizer, Novartis, Janssen Pharmaceuticals and Leo Pharma, and has acted as consultant and/or paid speaker for and/or participated in research sponsored by companies that manufacture drugs used for the treatment of psoriasis including AbbVie, Janssen Pharmaceuticals, Novartis, Lily, Celgene, Leo Pharma, UCB and Almirall. All funding is not personal but goes to the independent research fund of the Department of Dermatology of the Radboud University Medical Center Nijmegen, the Netherlands. The other authors declare no conflict of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol. 2020;156(3):258–269.
- Menting SP, Coussens E, Pouw MF, et al. Developing a therapeutic range of adalimumab serum concentrations in management of psoriasis: a step toward personalized treatment. JAMA Dermatol. 2015;151(6):616–622.
- Di Altobrando A, Magnano M, Offidani A, et al. Deferred time of delivery of biologic therapies in patients with stabilized psoriasis leads to a ‘perceived satisfaction’: a multicentric study. J Dermatolog Treat. 2020;29:1–5.
- Atalay S, Reek J, Otero M, et al. Health economic consequences of a tightly controlled dose reduction strategy for adalimumab, etanercept and ustekinumab compared with standard psoriasis care: a cost-utility analysis of the CONDOR study. Acta Derm Venereol. 2020;100(19):adv00340.
- Michielsens CAJ, van Muijen ME, Verhoef LM, et al. Dose tapering of biologics in patients with psoriasis: a scoping review. Drugs. 2021;81(3):349–366.
- Llamas-Velasco M, Daudén E. Reduced doses of biological therapies in psoriasis may increase efficiency without decreasing drug survival. Dermatol Ther. 2020;33(6):e14134.
- Sanz-Gil R, Pellicer A, Montesinos MC, et al. Improved effectiveness from individualized dosing of self-administered biologics for the treatment of moderate-to-severe psoriasis: a 5-year retrospective chart review from a Spanish university hospital. J Dermatolog Treat. 2020;31(4):370–377.
- Atalay S, van der Schoot LS, Vandermaesen L, et al. Evaluation of a one-step dose reduction strategy for adalimumab, etanercept and ustekinumab in patients with psoriasis in daily practice. Acta Derm Venereol. 2021;101(5):adv00463.
- Hansel K, Bianchi L, Lanza F, et al. Adalimumab dose tapering in psoriasis: predictive factors for maintenance of complete clearance. Acta Derm Venereol. 2017;97(3):346–350.
- Piaserico S, Gisondi P, De Simone C, et al. Down-titration of adalimumab and etanercept in psoriatic patients: a multicentre observational study. Acta Derm Venereol. 2016;96(2):251–252.
- Atalay S, van den Reek J, den Broeder AA, et al. Comparison of tightly controlled dose reduction of biologics with usual care for patients with psoriasis: a randomized clinical trial. JAMA Dermatol. 2020;156(4):393.
- Reich K, Puig L, Szepietowski JC, et al. Secukinumab dosing optimization in patients with moderate to severe plaque psoriasis: results from the randomised, open-label OPTIMISE study. Br J Dermatol. 2020;182(2):304–315.
- Papp KA, Gordon KB, Langley RG, et al. Impact of previous biologic use on the efficacy and safety of brodalumab and ustekinumab in patients with moderate-to-severe plaque psoriasis: integrated analysis of the randomized controlled trials AMAGINE-2 and AMAGINE-3. Br J Dermatol. 2018;179(2):320–328.
- Ye LR, Yan BX, Chen XY, et al. Extended dosing intervals of ixekizumab for psoriasis: a single-center, uncontrolled, prospective study. J Am Acad Dermatol. 2022;86(6):1348–1350.
- Gisondi P, Maurelli M, Bellinato F, et al. Is risankizumab as needed administration a good option for patients with plaque psoriasis? J Euro Acad Dermatol Venereol. 2022;36(9):e713–e715.
- Hamadah IR, Al Raddadi AA, Bahamdan KA, et al. Saudi practical guidelines on biologic treatment of psoriasis. J Dermatolog Treat. 2015;26(3):223–229.
- Nast A, Smith C, Spuls PI, et al. EuroGuiDerm guideline on the systemic treatment of psoriasis vulgaris - part 1: treatment and monitoring recommendations. J Eur Acad Dermatol Venereol. 2020;34(11):2461–2498.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072.
- van Muijen ME, van der Schoot LS, Bovenschen HJ, et al. Dosisvermindering van biologics voor psoriasis. Nederlands Tijdschrift Voor Dermatologie en Venereologie. 2021;31(1):22–26.
- van Muijen ME, van der Schoot LS, van den Reek J, et al. Attitudes and behaviour regarding dose reduction of biologics for psoriasis: a survey among dermatologists worldwide. Arch Dermatol Res. 2022;314(7):687–695.
- Sinha IP, Smyth RL, Williamson PR. Using the Delphi technique to determine which outcomes to measure in clinical trials: recommendations for the future based on a systematic review of existing studies. PLoS Med. 2011;8(1):e1000393.
- Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67(4):401–409.
- Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (standards for QUality improvement reporting excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986–992.
- Reich K, Wozel G, Zheng H, et al. Efficacy and safety of infliximab as continuous or intermittent therapy in patients with moderate-to-severe plaque psoriasis: results of a randomized, long-term extension trial (RESTORE2). Br J Dermatol. 2013;168(6):1325–1334.
- Gerbens LA, Boyce AE, Wall D, et al. TREatment of ATopic eczema (TREAT) Registry Taskforce: protocol for an international Delphi exercise to identify a core set of domains and domain items for national atopic eczema registries. Trials. 2017;18(1):87.
- De Bruin-Weller M, Biedermann T, Bissonnette R, et al. Treat-to-target in atopic dermatitis: an international consensus on a set of core decision points for systemic therapies. Acta Derm Venereol. 2021;101(2):adv00402.
- Thorlacius L, Ingram JR, Villumsen B, et al. A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol. 2018;179(3):642–650.
- Mahil SK, Wilson N, Dand N, et al. Psoriasis treat to target: defining outcomes in psoriasis using data from a real-world, population-based cohort study (the British Association of Dermatologists Biologics and Immunomodulators Register, BADBIR). Br J Dermatol. 2020;182(5):1158–1166.
- Armstrong AW, Siegel MP, Bagel J, et al. From the medical board of the national psoriasis foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76(2):290–298.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–1328.
- Schwensen JF, Clemmensen A, Sand C, et al. Effectiveness and safety of secukinumab in 69 patients with moderate to severe plaque psoriasis: a retrospective multicenter study. Dermatol Ther. 2017;30(6):e12550.
- Liau MM, Oon HH. Therapeutic drug monitoring of biologics in psoriasis. Biologics. 2019;13:127–132.