Abstract
Background
Intralesional corticosteroid administration (ICA) is a first-line therapy in keloid treatment. However, its clinical results are still highly variable and often suboptimal. Treatment results may strongly be influenced by various ways of ICA.
Objective
To explore the prevailing practice of ICA in keloid treatment among dermatologists and plastic surgeons in the Netherlands.
Methods
The survey was constructed based on a scoping review on ICA in keloid treatment. Members of the Dutch Society for Plastic surgery and the Dutch Society for Dermatology and Venereology were asked to participate.
Results
One hundred and thirty-six responses were obtained. One hundred and thirty (95.6%) participants used triamcinolone acetonide. The majority (54.7%) did not use local anesthesia for pain reduction. Reported corticosteroid dosing that one would inject in one specific keloid differed by a factor of 40. Treatment intervals varied from 1 week to more than 8 weeks. The keloid center was most often injected (46.9%), followed by subepidermal (18.0%).
Conclusions
A wide variety in ICA for keloids is noted among dermatologists and plastic surgeons, even in a limited geographic region and when evidence points toward an optimal way of treatment. Future studies and better implementation of existing evidence may reduce variation in ICA and optimize its treatment results.
Introduction
Keloids are fibroproliferative disorders resulting from chronic inflammation in the dermis and often cause pain, pruritus, a tight sensation, cosmetic concerns, and occasionally movement restriction (Citation1,Citation2). Keloids are associated with an impacted quality of life (Citation3,Citation4). Intralesional corticosteroid administration (ICA) is a first-line therapy in the current practice of keloid treatment. However, clinical results of this treatment are still highly variable and often suboptimal (Citation5,Citation6). Treatment results may strongly be influenced by clinicians’ preferences, such as the type, volume, and concentration of the corticosteroid; the number of treatment sessions; the treatment interval; and the syringe and needle size. Moreover, the manual injection technique is highly operator-dependent. A recent scoping review revealed incomplete reporting and substantial heterogeneity on ICA in keloids among randomized controlled trials (RCTs) (Yin et al. Amsterdam UMC, 2022, unpublished observation). We hypothesized that considerable variance exists in the current clinical practice as well. The aim of this survey study was to explore the prevailing practice of ICA in keloid treatment among dermatologists and plastic surgeons in the Netherlands.
Methods
We conducted a cross-sectional survey study among dermatologists and plastic surgeons in the Netherlands. The study followed the Consensus-Based Checklist for Reporting of Survey Studies (CROSS-checklist, Supplementary material 1).
Survey design
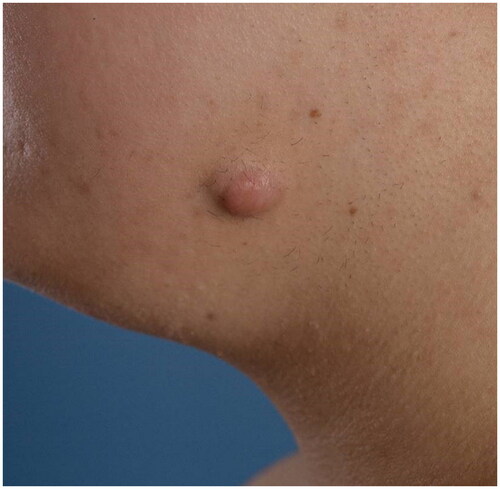
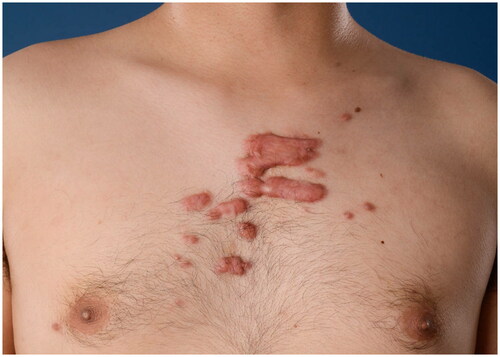
The survey questions and answers were constructed based on a scoping review on ICA in keloid treatment and other relevant studies found by an extensive search in PubMed, Ovid MEDLINE, Ovid EMBASE, and CENTRAL (Yin et al. Amsterdam UMC, 2022, unpublished observation). The survey consisted of four multiple-choice demographic questions and 11 treatment questions. Demographic questions elaborated on the medical specialty of training, years of experience as dermatologist or plastic surgeon, practice setting, and number of keloid treatments per month. Treatment questions focused on (1) drugs and dosing, including the type, volume, and concentration of the corticosteroid; local anesthesia; the number of treatment sessions; and the treatment interval, (2) equipment, including the syringe and needle size, and (3) the manual injection technique, including level of injection, speed of infiltration, and endpoint of infiltration. Two clinical cases were used for determining the estimated number of treatment sessions and corticosteroid dosing, as calculated by the volume multiplied by the concentration. The first case is a patient with a small keloid of 15 × 10 mm on the left mandibula (). The second case is a patient with multiple keloids on the chest (). The option ‘Other’ with a write-in box was provided as answer option where appropriate. The survey was concluded by a free-response item. All questions concerned intralesional corticosteroid needle injection. Jet injectors were outside the scope of this paper. All questions were critically revised by three plastic surgeons (PZ, FN, and OL) and one dermatologist (AW) experienced in keloid treatment. After that, a pilot group of one experienced dermatologist, two plastic surgeons, and two residents individually evaluated question clarity and identified potential (technical) problems individually. The physicians of this pilot group were not particularly specialized in keloid treatment and were representative of the study population. The complete questionnaire is provided in Supplementary material 2. The questions were compiled into an online survey using a secured online survey software platform (Alchemer, Louisville, CO).
Data collection methods
Permission was obtained from the Dutch Society for Dermatology and Venereology (NVDV) and the Dutch Society for Plastic surgery (NVPC) to survey its members. An online link to the survey was sent by the societies to all of their members by e-mail. One reminder was sent to the members of the NVPC. No reminder could be sent to the members of the NVDV, as a result of internal regulations. Data were collected between 28 March 2022 and 16 May 2022. All participants voluntarily consented to participating in the survey study. Data collection took place anonymously. The IP address and geographical location were registered for each response. Nevertheless, multiple participations by one person cannot be ruled out.
Data analysis
Only responses of dermatologists, plastic surgeons, and residents dermatology and plastic surgery were included. Responses of incomplete questionnaires were also included. Open-ended answers obtained from the write-in boxes were categorized into original responses when appropriate. Data analysis was performed using SPSS (IBM SPSS Statistics 26, Armonk, NY). Frequency distributions were created for all response variables. Bivariate cross-tabulations were used to compare treatment variables with demographic variables. Comparison between academic and nonacademic specialists, plastic surgeons and dermatologists, and between specialists treating more and fewer than 10 keloids per month was done using the Pearson chi-squared test. The relationship between the number of treatment sessions and corticosteroid dosing was investigated using the Spearman rank correlation coefficient. A p-value less than .05 is considered statistically significant.
Results
Three hundred and seventy-four plastic surgeons, 600 dermatologists, and 232 residents were asked to participate. A total of 136 responses were obtained. Eight respondents (5.8%) did not complete the questionnaire. The response rate was calculated to be 12% among both dermatologists and plastic surgeons. Of all respondents, 73 (53.7%) were dermatologists, 46 (33.8%) were plastic surgeons, and 17 (12.5%) were dermatology or plastic surgery residents (). Forty-five (33.1%) participants practiced in an academic setting, either part-time or full-time. Most participants treated less than three keloids per month (60.2%), followed by 3–10 keloids per month (34.4%). Only seven (5.5%) participants treated more than 10 keloids per month (). When the option ‘Other’ was chosen, a specification was required. These open-ended answers are presented in Supplementary material 3.
Table 1. Demographic variables.
Drugs and dosing
One hundred and thirty (95.6%) participants used triamcinolone acetonide (TAC) (Kenacort, Kenalog) most frequently as corticosteroid. For 70 (54.7%) participants, local anesthesia was not part of the ICA treatment regimen. Forty-four (34.4%) participants mixed the local anesthetic with the corticosteroid, three participants injected the local anesthetic before ICA, and three participants used anesthetic cream before ICA ().
Table 2. Type of corticosteroid and local anesthetics, dosing, treatment sessions, treatment interval.
Two clinical cases were used for determining the estimated number of treatment sessions and corticosteroid dosing. The latter was calculated by multiplying the volume with the concentration that participants would use per case. For a small keloid of 15 × 10 mm on the mandibula (), 0.5 mL was the used median volume (IQR 0.7) and 10 mg/mL was the most frequently (35.9%) noted concentration. The effective dose varied from 1 mg to 40 mg. Considering treatment sessions, 105 (82.0%) participants would apply 1–3 sessions for the small keloid (). For the multiple keloids on the chest (), 3 mL was the used median volume (IQR 2.3) and 40 mg/mL was the most frequently (50%) noted concentration. The effective dose varied from 10 mg to 400 mg. Considering treatment sessions, 70 participants (54.7%) would use 4–6 sessions for the multiple keloids, 38 (29.7%) participants would apply more than six sessions, and 10 (7.8%) participants would apply less than four sessions. Eight participants would not treat the multiple keloids with intralesional corticosteroids.
The treatment interval chosen most frequently was six weeks (48.4%), followed by 4 weeks (23.4%) and 8 weeks (18.0%) ().
Equipment
Sixty-eight (53.1%) participants always used the same syringe for corticosteroid injection in keloids, of which the 1 mL (41.2%) and 2 mL (35.3%) syringes were used most often (range: 0.45–5 mL). Concerning needle sizes, 54 (42.2%) participants used the 25-gauge (orange, 0.50 mm) needle, followed by the 30-gauge (yellow, 0.30 mm) needle (13.3%) and the 27-gauge (medium grey, 0.40 mm) needle (9.4%). A minority had no preference for a specific syringe size (10.9%) or needle size (5.5%). A substantial proportion indicated that the type of keloid determines the choice of syringe size (34.4%) and needle size (12.5%) ().
Table 3. Syringe size, needle size, and manual injection technique.
Manual injection techniques
Sixty (46.9%) participants mentioned injecting into the center of the keloid, followed by subepidermal (18.0%) and a combination of the center and subepidermal (12.5%) (). Twenty-one (16.4%) participants injected either below the center of the keloid or below the center combined with another level of the keloid. Four participants mentioned not to consider the injection level during ICA. During infiltration, the speed and drug distribution were considered by 26 (20.3%) and 77 (60.2%) participants as important factors. Observation of blanching was chosen as the endpoint of infiltration by 104 (81.3%) respondents ().
Subanalyses
No statistical differences were found for treatment variables between medical specialists treating more and fewer than 10 keloids per month, nor between academic and nonacademic specialists. When comparing plastic surgeons and dermatologists, treatment differences were only found for the treatment interval (p=.01). Compared to dermatologists, plastic surgeons more often opted for treatment intervals longer than 4 weeks (82.2% versus 68.2%, p=.025), longer than 5 weeks (82.2% versus 65.2%, p=.013), and longer than 6 weeks (37.8% versus 15.2%, p=.004). The number of treatment sessions was not related to corticosteroid dosing for the small keloid (Spearman’s rho = 0.03, p=.80), nor for the multiple keloids (Spearman’s rho= −0.14, p=.16).
Discussion
This survey study explored the prevailing practice of ICA in keloid treatment among dermatologists and plastic surgeons in the Netherlands. We noticed a wide variety in the current clinical practice of this first-line therapy among the respondents.
Available intralesional corticosteroids include TAC, betamethasone, dexamethasone, and (methyl)prednisolone (Citation7). In line with the literature (Yin et al. Amsterdam UMC, 2022, unpublished observation), TAC is also the most frequently used corticosteroid among respondents. This may be the result of practical reasons, such as drug availability and familiarity with the drug. Additionally, TAC may be regarded as the preferred intralesional corticosteroid in keloid treatment, based on the evidently larger available (clinical) literature compared to other available intralesional corticosteroids and its favorable pharmacokinetic properties (Citation7–10). When comparing different corticosteroids, it has been reported that TAC has the property to remain in a suspension in the tissue for a long time and has a longer duration of action than betamethasone or prednisolone (Citation7–10), and thus TAC may be a more effective treatment of the keloid, which is a chronic disorder. On the other hand, more local adverse events including hypopigmentation, atrophy, and telangiectasia occur following TAC injection, compared to the betamethasone-acetate-phosphate suspension (Citation10).
ICA can be painful, which affects therapy adherence (Citation11). Reducing pain during ICA should be aimed. Most of the respondents do not use local anesthesia. One-third of the respondents mentioned the use of local anesthesia, predominantly mixing the local anesthetic with the corticosteroid. However, based on an RCT comparing topical 2.5% lidocaine and 2.5% prilocaine (EMLA) cream, a 1:1 mixture of 1% lidocaine and TAC, and placebo, diluting the intralesional corticosteroid with a local anesthetic is not recommended for pain reduction (Citation12). According to this RCT, neither of the local anesthetics alleviated pain during injection. However, EMLA cream did alleviate needle puncture pain, whereas the lidocaine mixture did not. Local infiltration anesthesia prior to ICA was not evaluated in this study, nor in other clinical studies in English literature. Even though RCTs mentioned skin cooling to be effective for pain reduction (Citation13,Citation14), none of the respondents reported skin cooling as part of their treatment regimen. The speed of injection was only chosen by 20.3% of the respondents as an important factor for injection. However, more care for slow drug infiltration may be needed, as the speed of injection has shown to be more essential in the cause of pain than the properties of the substance being injected (Citation15).
Respondents were asked to estimate the volume and concentration of the corticosteroid and the number of treatment sessions they would apply for two types of keloids. Notably, the effective dose, as calculated by volume multiplied by concentration varied by a factor of 40 for both keloids. Similarly, this large variability in corticosteroid dosing was also found in the literature, where dosing per area varied by a factor of 20 in RCTs (Yin et al. Amsterdam UMC, 2022, unpublished observation). For both keloids presented in the survey, the chosen number of treatment sessions also varied largely between the respondents and did not correspond to corticosteroid dosing. The effective dose per area and the number of treatment sessions are essential factors that define treatment effect. Although different keloids may react differently to corticosteroid treatment, the highly variable clinical results of ICA could at least partly be explained by the large differences in dosing regimens.
Six weeks was the most frequently opted treatment interval, followed by four weeks and eight weeks. The optimal treatment interval was studied by Aluko-Olokun et al. in 16 keloids by weekly volume measurements after TAC injection (Citation16). Based on this study, the most optimal treatment interval should be 4 weeks or shorter, as the volume reduction was found to be most profound within the first two weeks after a single TAC injection. In addition, they observed that the volume reduction between 3 and 4 weeks was no longer significant, and a reversal in trend was seen between 4 and 5 weeks, which even accelerated between 5 and 6 weeks (p≤.05) (Citation16). Moreover, itching often recurs within four weeks of TAC injection, indicating a potential benefit of a shorter interval. In RCTs, four weeks was the most frequently used treatment interval, followed by three weeks and two weeks (Yin et al. Amsterdam UMC, 2022, unpublished observation). Differently from these data, the treatment interval in the clinical practice generally appears to be longer, especially among plastic surgeons, which may be the result of crowded outpatient services.
In contrast to RCTs, which scarcely mention syringe and needle sizes (Yin et al. Amsterdam UMC, 2022, unpublished observation), the majority of respondents did mention having preferences for specific syringe and needle sizes. One-third (34.4%) and 12.5% of the participants opted that the choice of syringe and needle size should depend on the type of keloid. Notably, syringe and needle diameter are closely correlated to corticosteroid dosing. The treating physician should realize that larger syringes generate lower injection pressure, and their use may result in inadequate drug delivery. On the other hand, smaller syringes generate higher injection pressure, which increases the risk of drug delivery into the surrounding healthy tissue. However, smaller syringes may generally be indicated, as most keloids, especially naïve ones, are very firm and the operators’ thumb force may not be sufficient to generate the necessary pressure for adequate drug infiltration (Citation17). Considering needle size, injecting with thinner needles may be less painful. However, thinner needles tend to occlude easier. A 30-gauge needle has an inner diameter of 0.16 mm. It has been reported that in TAC 40 mg/mL and betamethasone phosphate/betamethasone acetate 6 mg/mL, particles sizes of >50 µm (0.05 mm) account for 4–12% and 1–3% of the total distribution, respectively (Citation18,Citation19). The larger the number and size of particles, the larger the chance of needle occlusion, especially when the orifice is smaller.
Currently, there is no evidence from clinical studies indicating the optimal level of injection. Whereas some preclinical studies suggest that ICA should take place in the superficial dermis and in the margin of the keloids (Citation20,Citation21), the Japan Scar Workshop consensus described that the injection target should be the deepest part and/or the periphery of the lesion, where the inflammation is particularly pronounced (Citation22). It mentioned that the solid central fibrotic mass of the lesion should not be injected, because the drug will not infiltrate the tissue adequately and the rising pressure in the dense tissue may cause pain (Citation23). In contrary to this consensus, 60 (46.9%) participants of our survey mentioned injecting into the center of the keloid. Only 16.4% of the participants inject below the center of the keloid. It should be noted that deep injection may increase the risk of subcutaneous atrophy. Important factors in preventing subcutaneous atrophy chosen by >50% of respondents are the level of injection, dose adjustment, and number of treatment sessions.
There are some limitations to this study. First of all, all responses are only from the Netherlands, thus data are limited to one geographic region. Additionally, the estimated response rate of our study is 12%, which is to be expected for surveys based on voluntary participation and comparable to the response rate of an earlier internet-based survey on the evaluation, prevention, and treatment of (hypertrophic) scars and keloids (Citation24). Nevertheless, the risk for selection bias leads to limited generalizability of the results. However, to our knowledge, this is the first survey study in the English literature to assess the clinical practice of all aspects of ICA in keloid treatment and we managed to acquire a reasonable sample size of physicians that shared their treatment regimen.
To summarize, a wide variety exists in the current clinical practice of ICA in keloid treatment among dermatologists and plastic surgeons in the Netherlands, even when evidence points toward an optimal way of treatment. To optimize the therapeutic effect of this first-line treatment, we should focus on the ways intralesional corticosteroids are administered. Future (experimental) studies should further prove the relevance and effect of various ways of ICA. Additionally, better implementation of existing evidence may also reduce variation in ICA and optimize its treatment results.
Supplemental Material
Download Zip (407.2 KB)Disclosure statement
All authors declared to have no disclosures.
References
- Ogawa R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int J Mol Sci. 2017;18(3):606.
- Furtado F, Hochman B, Ferrara SF, et al. What factors affect the quality of life of patients with keloids? Rev Assoc Med Bras (1992). 2009;55(6):700–704.
- Bijlard E, Kouwenberg CA, Timman R, et al. Burden of keloid disease: a cross-sectional health-related quality of life assessment. Acta Derm Venereol. 2017;97(2):225–229.
- Kassi K, Kouame K, Kouassi A, et al. Quality of life in Black African patients with keloid scars. Dermatol Reports. 2020;12(2):8312.
- Wang CJ, Ko JY, Chou WY, et al. Extracorporeal shockwave therapy for treatment of keloid scars. Wound Repair Regen. 2018;26(1):69–76.
- Kaushal V, Kumar S, Brar BK, et al. Comparative evaluation of therapeutic efficacy and safety of intralesional triamcinolone acetonide injection vs intralesional radiofrequency with intralesional triamcinolone acetonide in treatment of keloids. Dermatol Ther. 2020;33(6):e13919.
- Callen JP. Intralesional corticosteroids. J Am Acad Dermatol. 1981;4(2):149–151.
- Syed F, Bayat A. Superior effect of combination vs. monotherapy in keloid disease: a comparative in vitro analysis of glucocorticoids. Wound Repair Regen. 2012;20(2):A41.
- Delaney TJ, Rowlingson JC, Carron H, et al. Epidural steroid effects on nerves and meninges. Anesth Analg. 1980;59(8):610–614.
- Jarratt MT, Spark RF, Arndt KA. The effects of intradermal steroids on the pituitary–adrenal axis and the skin. J Invest Dermatol. 1974;62(4):463–466.
- Muneuchi G, Suzuki S, Onodera M, et al. Long-term outcome of intralesional injection of triamcinolone acetonide for the treatment of keloid scars in Asian patients. Scand J Plast Reconstr Surg Hand Surg. 2006;40(2):111–116.
- Usanakornkul A, Burusapat C. A topical anesthetic and lidocaine mixture for pain relief during keloid treatment: a double-blind, randomized controlled trial. Dermatol Surg. 2017;43(1):66–73.
- Jongkajornpong N, Wattanawong K. The efficacy of skin cooling for pain relief during intralesional steroid injection for keloid treatment: a randomized cross-over study. J Med Assoc Thailand. 2021;104(11):1752–1757.
- Wang X, Wu X, Liu K, et al. Topical cryoanesthesia for the relief of pain caused by steroid injections used to treat hypertrophic scars and keloids. Medicine. 2017;96(43):e8353.
- Ono N. Pain-free intralesional injection of triamcinolone for the treatment of keloid. Scand J Plast Reconstr Surg Hand Surg. 1999;33(1):89–91.
- Aluko-Olokun B, Olaitan AA, Ladeinde AL, et al. Determination of the optimal frequency of injection of triamcinolone: monitoring change in volume of keloid lesions following injection of 40 mg of triamcinolone. Eur J Plast Surg. 2016;39(2):119–124.
- Vo A, Doumit M, Rockwell G. The biomechanics and optimization of the needle-syringe system for injecting triamcinolone acetonide into keloids. J Med Eng. 2016;2016:5162394.
- Benzon HT, Chew TL, McCarthy RJ, et al. Comparison of the particle sizes of different steroids and the effect of dilution: a review of the relative neurotoxicities of the steroids. Anesthesiology. 2007;106(2):331–338.
- Tiso RL, Cutler T, Catania JA, et al. Adverse central nervous system sequelae after selective transforaminal block: the role of corticosteroids. Spine J. 2004;4(4):468–474.
- Jiao H, Zhang T, Fan J, et al. The superficial dermis may initiate keloid formation: histological analysis of the keloid dermis at different depths. Front Physiol. 2017;8:885.
- Syed F, Ahmadi E, Iqbal SA, et al. Fibroblasts from the growing margin of keloid scars produce higher levels of collagen I and III compared with intralesional and extralesional sites: clinical implications for lesional site-directed therapy. Br J Dermatol. 2011;164(1):83–96.
- Ogawa R, Akita S, Akaishi S, et al. Diagnosis and treatment of keloids and hypertrophic scars—Japan Scar Workshop Consensus Document 2018. Burns Trauma. 2019;7:39.
- Ogawa R, Tosa M, Dohi T, et al. Keloids and hypertrophic scars can be cured completely. Wound Repair Regen. 2019;27(5):A2.
- Lumenta DB, Siepmann E, Kamolz LP. Internet-based survey on current practice for evaluation, prevention, and treatment of scars, hypertrophic scars, and keloids. Wound Repair Regen. 2014;22(4):483–491.