Abstract
Background
The risk of SARS-CoV-2 infection does not appear to be increased for psoriasis patients using biologics compared to those on other treatments, but evidence is still limited.
Objectives
(1) to estimate the prevalence of SARS-CoV-2 infection in patients with psoriasis, (2) to compare SARS-CoV-2 infection rates for different psoriasis treatments groups (biologic vs. systemic conventional vs. topical therapy) corrected for confounders and (3) to describe patients with severe COVID-19 for all treatment groups.
Methods
In this cross-sectional cohort study all patients received a questionnaire to gather data on psoriasis treatment, SARS-CoV-2 infections and related risk factors. Simultaneously, they underwent a blood test to screen for antibodies to SARS-CoV-2 N-antigen. Prevalence of SARS-CoV-2 infections was calculated and logistic regression and Cox proportional-hazards models were performed to determine the association between treatment group and SARS-CoV-2 infection risk, corrected for confounders. Patients with severe COVID-19 disease were described and the mortality rate per treatment group was calculated for the target population.
Results
Patients were included between April 12 2021 and October 31 2021. Of 551 patients, 59 (10.7% (CI95% 8.3–13.6)) had experienced a SARS-CoV-2 infection, based on questionnaire data combined with serological data. In our study cohort, corrected for confounders, biologic or non-biologic systemic therapy users did not appear to have increased SARS-CoV-2 infection risk compared to patients using other treatment. Only 4 hospitalizations (0.7% (CI95% 0.2–1.0) were reported in our study population and no ICU admissions were reported. The rough mortality rate in the target cohort was 0.32% (CI95% 0.13–0.66) in all treatment groups.
Conclusions
Corrected for risk-mitigating behavior and vaccination status, a higher SARS-CoV-2 incidence for biologics or non-biologics systemics compared to other treatments could not be proven. Severe cases were infrequent in all treatment groups. This finding further strengthens treatment recommendations that systemic therapies for patients with psoriasis do not require preventive cessation for reduction of SARS-CoV-2 infection risk.
Introduction
Psoriasis is a chronic inflammatory skin disease that affects over 60 million people worldwide with an overall prevalence range between 0.5 and 1.5% depending on geographical differences (Citation1). For moderate to severe psoriasis, treatment with conventional systemic therapies (e.g. methotrexate, ciclosporin-A and fumaric acid esters) and biologics (TNF-alpha-, IL-12/23-, IL17- and recently IL23-inhibitors) are standard of care. Hypothetically, the immunosuppressive effects of these systemic therapies could lead to an increase in symptomatic SARS-CoV-2 infection-rate and COVID-19 severity. Moreover, the heavier burden of cardiovascular comorbidities and obesity in patients with psoriasis may worsen COVID-19 outcomes compared to the general population. To date, multiple studies described SARS-CoV-2 incidence in psoriasis patients, especially of patients treated with biologics. Surprisingly, the hospitalization rate in these studies was low (Citation2–8). Current data suggests that patients with psoriasis have similar SARS-CoV-2 infection rates and outcomes compared to the general population (Citation9). Biologics do not appear to be related to an increased risk for (severe) COVID-19, and TNF-inhibitors even seem to be related to a lower risk for adverse COVID-19 outcomes compared to no treatment or conventional systemic drugs for psoriasis and other immune-mediated diseases (Citation10). However, patients with biologics have reported stricter risk-mitigating behavior than the general population, which might lead to an underestimation of the aforementioned risks (Citation11,Citation12). In addition, published studies on this topic generally lack confirmation by serological tests, hence did not include asymptomatic and mild infections.
The National Psoriasis Foundation COVID-19 task force, amongst others, does not advise psoriasis patients to stop their biologic or conventional systemic treatment because of the COVID-19 pandemic (Citation9). However, it has been shown that patients discontinue and/or modify their therapies autonomously due to various reasons (Citation13). With increasing evidence, the recommendations may be strengthened, especially without the possible distorting effects of shielding behavior and vaccination effects.
The aim of this study was to (1) estimate the prevalence of SARS-CoV-2 infection in a real-world population of patients with psoriasis based on a combination of questionnaires (PCR confirmed infections) and serological data, (2) determine the association between psoriasis treatments groups (biological therapies (BT) vs. non-biologic systemic therapies (ST) vs. topical therapy (TT)) and SARS-CoV-2 infection rates, corrected for confounders including risk-mitigating behavior and vaccination and (3) to describe patients with severe COVID-19 disease.
Patients and methods
The PsoCovid study is a multi-center cross-sectional cohort study of psoriasis patients who received psoriasis treatment between March 2020-October 2021 in 3 Dutch hospitals (Bravis hospital, Radboud University Medical Center and Rijnstate hospital). The study was approved by the Ethics Committee (CMO Arnhem-Nijmegen) and conducted in accordance with the declaration of Helsinki. After written informed consent, each patient received a questionnaire and blood was drawn once. Included were adults ≥18 years that received BT, ST or TT between March 2020 until October 2021. Patients could be included irrespective of their vaccination history, because the vaccine-elicited antibodies that are measured are not directed against the SARS-CoV-2 N-antigen. Exclusion criteria were immunosuppressive or immunomodulatory treatment for indications other than psoriasis, or immunocompromised due to another reason than psoriasis treatment (e.g. receiving chemotherapy).
Patients who reported having received a biologic for >3 consecutive months were assigned to the BT group, and patients who were treated with non-biologic systemic therapies for >3 consecutive months were assigned to the ST group. In case biologics and conventional systemic drugs were used concurrently ánd both for >3 months, patients were assigned to the BT group. Patients treated with topical therapy were assigned to the TT group. For the patients with a SARS-CoV-2 infection with a known date, concordance was calculated between treatment assignment according to the above mentioned rules and treatment at moment of infection.
Variables collected in the questionnaire were: age, sex, weight, body mass index (BMI), intoxications, relevant comorbidities (psoriatic arthritis, severe cardiologic, pulmonary, renal, liver or hematological disease, immune deficiency disorder, auto-immune disease, HIV/AIDS, recent malignancy, diabetes mellitus, organ transplantation), and co-medication. Specifically for psoriasis, disease duration, psoriasis treatment as of March 2020 (start COVID-19 pandemic) until study measurement date, start and stop dates per treatment and reasons to discontinue psoriasis treatment were collected. With respect to COVID-19, the following variables were collected: prior test-confirmed SARS-CoV-2 infection (date, symptoms, severity, treatment, hospitalization), suspicion for COVID-19 (untested), risk factors including occupational risk, home setting, risk-mitigating behavior during pandemic, vaccination status, date of first vaccination, number of vaccinations, type of vaccination.
Serological testing
Serum samples were first screened with the highly sensitive Wantai SARS-CoV-2 total antibody ELISA (Beijing Wantai Biological Pharmacy Enterprise Co., Beijing China) to determine if antibodies against SARS-CoV-2 were present. Positive samples were tested with Luminex xMAP SARS-CoV-2 Multi-Antigen Antibody assay combined IgG anti-N, anti-S and anti-RBD test (Luminex Corporation, Den Bosch, the Netherlands). If the SARS-CoV-2 IgG anti-N signal was higher than the threshold determined by the manufacturer (700 AU/ml), the patient was classified as having a past SARS-CoV-2 infection.
Study size
An a priori power calculation was made to test for a difference between SARS-CoV-2 infection rate among treatment groups. With an estimated prevalence of 13% in ST, 10% in BT and 7% in TT users; a power of 80% and α = 0.05, the estimated sample size was determined to be 1500.
Statistical methods
Analyses were performed in SPSS version 25.0 (IBM, Armonk, NY, USA). A p-value < .05 was considered significant. Baseline patient and treatment characteristics for the total study group, and split per treatment group were displayed using descriptive statistics (N (%), mean ± standard deviation (SD) in case of continuous parametric data, or median (interquartile range (IQR)) in case of non-parametric data). Number of missings per variable are described in table legends.
Logistic regression analysis was performed to assess and compare the risk for SARS-CoV-2 infection (dependent variable) for each treatment group (ST/BT/TT). The dependent outcome was a composite end-point for SARS-CoV-2 infection: a reported positive PCR test and/or serological test was considered as ‘positive’ for SARS-CoV-2 infection. In case both outcomes were negative, patients were classified as having had no past SARS-CoV-2 infection. If serological data was not available, only the questionnaire result (PCR yes/no) was taken into account. The regression analyses described below were performed with the composite endpoint, but also sensitivity analyses were performed with only considering PCR-confirmed SARS-CoV-2 infections as cases.
Univariate logistic regression analysis was performed to test if solitary variables other than treatment class, were associated with the dependent variable (SARS-CoV-2 infection). In a multivariable logistic regression model, variables associated with a p-value ≤ .2 with the dependent outcome ánd those variables considered clinically relevant were added to the model to check if this changed the association between treatment class and SARS-CoV-2 infection. In the models, the independent variable ‘COVID-19 vaccination’ was expressed as the proportion of time with at least one vaccine dose (starting 14 days after first vaccination), to assign weight to the period that patients were protected for (severe) infections.
Cox proportional-hazards models were built for sensitivity analyses, to also take into account the time until SARS-CoV-2 infection. For the patients with only a serologic-confirmed infection (without a known date), the median duration of occurrence of the PCR-confirmed infections was used. Again, analyses were repeated, corrected for the potential confounders identified in the primary (logistic regression) analysis. Additional sensitivity analyses were performed to correct for vaccination: Cox regression with ‘time until first vaccination’ incorporated as time varying confounder (time between start pandemic and moment of first vaccination plus 14 days), and Cox regression stratified for unvaccinated and vaccinated time.
To address survivorship bias, all deceased patients between start pandemic until study closure (October 31 2021) from the identified target population with SARS-Cov-2 infection as cause of death were systematically identified from the electronic patient records. The mortality rate in the total target population was calculated to provide a rough estimation of the overall COVID-19 mortality risk, as well as for all treatment groups.
Results
Study patient and disease characteristics
Patients were included between April 12 2021 and October 31 2021. We identified 3113 patients with psoriasis from the electronic patients records and registries of the three participating medical centers of which 2177 were found eligible, and 588 patients (27% response rate) completed the questionnaire. Thirty-seven patients were excluded based on their questionnaire answers (e.g. reported to use other immunosuppressive drugs), leading to 551 patients with questionnaire data () of which 481 also handed in a blood-sample. The a-priori calculated sample size was not reached despite repeated invitations (email/phone).
Figure 1. Inclusion flowchart. BT: biologic therapies; ST: systemic therapies; TT: topical therapies.
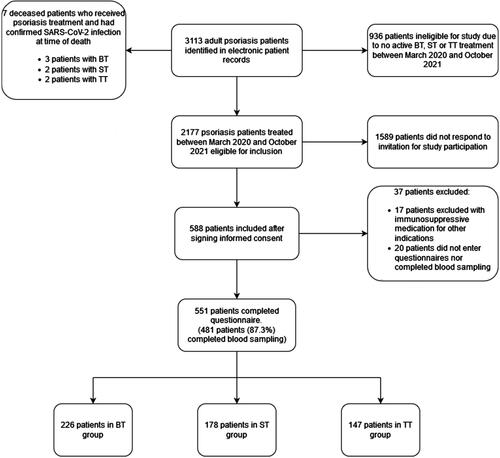
presents the patient characteristics of the 551 included patients. The BT group consisted of 226 patients (41.2%, 95% confidence interval (CI) 36.9–45.1) of whom 27 patients (11.9%, 95%CI 8.0–16.9) also used methotrexate. The ST group consisted of 178 patients (32.3%, 95%CI 28.4–36.4) and the TT group of 147 patients (26.6%, 95%CI 23.0–30.6). presents questionnaire data on vaccination status and past SARS-CoV-2 infection in our overall study population. ‘Having at least one vaccination’ was reported by 425 patients (77.1%, 95%CI 73.4–80.6) at the moment of inclusion. Fifty-two (9.4%, CI95% 7.1–12.2) patients reported prior SARS-CoV-2 infection as confirmed by a PCR-test. and 10.7% (95%CI 8.3–13.6) based on the composite endpoint (PCR and serum test, ). Four of the included patients (0.8%, CI95% 0.2–19.9) reported hospitalization due to COVID-19, but none to an intensive care unit. Detailed patient characteristics of admitted patients are presented in .
Table 1. Patient and treatment characteristics of patients included in PsoCovid (n = 551).
Table 2. Information on vaccination and infection with SARS-CoV-2 of patients included in PsoCovid – results from questionnaires (n = 551).
Table 3. Interpretation blood test (n = 551 with questionnaire completed; n = 482 with serum antibody test and questionnaire completed) per treatment group.
SARS-CoV-2 infection period prevalence compared for treatments groups
Per treatment group, 15 (6.6% CI95% 3.8–10.7) of the BT, 17 (9.6% CI95% 5.7–14.9) of the ST and 20 (13.6% CI95% 8.5–20.2) of the TT users reported a positive PCR test. There was 100% concordance for treatment at the moment of infection with the treatment group (BT/ST/TT) these patients were assigned to. As for the composite endpoint, 19 (8.4% CI95% 5.1–12.8) in the BT group, 19 (10.7% CI95% 6.5–16.1) in the ST group, and 21 (14.2% CI95% 9.1–21.0) in the TT group had experienced a past SARS-CoV-2 infection. Stricter risk-mitigating behavior than indicated by national guidelines (e.g. complete quarantine or shielding) was reported by 32.1% (CI95% 26.0–38.7) BT, 28.1% (21.6–35.3) ST, and 29.2% (21.9–37.3) TT users, which was not significantly different between groups (Chi-squared test, p = 0.651) ().
Table 4. SARS-CoV-2 infections and behavior split per treatment group – results of questionnaires and serum antibody tests.
Forty-four patients who reported a PCR confirmed SARS-CoV-2 infection also had blood drawn for the study. Notably, 20/44 (45.4% CI95% 30.4–61.2) did not have measurable IgG anti-N in blood. This indicates that these patients had either not developed antibodies to SARS-CoV-2 (i.e. mild infection or false positive PCR) or these antibodies had decreased to undetectable levels. The period between infection and serological test was significantly longer for patients with prior positive PCR test and negative serology (222 ± 62 days, ≈7 months), compared to patients with prior positive PCR test and positive serology (160 ± 86 days; ≈5 months, p = 0.020). Age, sex, weight, BMI and treatment class were not different between these groups (data available upon request). The Pearson correlation coefficient for days between infection and serological test (anti-N level) was low (R = 0.429 (p = .004).
SARS-CoV-2 infection risk compared for different psoriasis treatments groups (BT vs. ST vs. topical therapy (TT))
presents the results of the logistic regression model to determine the risk difference for SARS-CoV-2 infection (composite endpoint PCR and antibodies in blood). The odds ratio (OR) for BT compared to ST and TT was 0.382 (CI95% 0.182–0.801, p = 0.011 (corrected for vaccination, risk mitigating behavior, sex, age, and weight)), which may indicate a lower risk for SARS-CoV-2 infection for this group compared to other treatments. ST vs. TT/BT showed no lower or increased risk (0.502 CI95% 0.236–1. 065, p = .073). When only TT was used as reference, ORs for BT vs TT and ST vs. TT were similar (see bottom line ).
Table 5. Logistic regression analysis: factors associated with SARS-CoV-2 infection (composite endpoint PCR and serum antibody confirmed cases).
A sensitivity analysis of this model, with only PCR-confirmed infections as cases, showed similar risk estimates (Table s3). Also, sensitivity analyses were performed to assess the effect of treatment on SARS-CoV-2 infection risk with Cox proportional-hazards models in which ‘time until first vaccination’ was handled as a time varying confounder (Table s4a). For the multivariable model, corrected for time until vaccination, risk mitigating behavior, sex, age, and weight, Hazard ratios (HRs) for BT vs. other treatments and ST vs. other treatments remained <1, albeit nonsignificant. Similar results were found for the Cox-regression analysis with only PCR-confirmed infections as cases (Table s4b). Stratification for unvaccinated and vaccinated time showed that 55 out of 59 Sars-Cov-2 infections (events) took place within the ‘unvaccinated’ observation time. The unvaccinated time summed up to 635 observation years and vaccinated time was limited to 58 observation years. Therefore, only the analysis for unvaccinated time was presented, showing similar results with regards to effect of treatment on SARS-CoV-2 infection risk (Table s5).
Severe COVID-19 illness compared between treatment groups
As only four cases (4/551, 511, 0.73%, CI95% 0.20–1.90) of severe COVID-19 were reported (n = 1 BT, n = 1 ST, and n = 2 TT), no regression model was built to analyze the association with treatment class. Patients are described in Table s2. From the total target cohort that was eligible for study participation (n = 2177), seven deceased patients (mortality rate 0.32% (CI95% 0.13–0.66) were identified having died due to SARS-CoV-2 infection and were on psoriasis treatment in the months before the passing (Table s1). Three patients (0.37%, CI95% 0.08–1.1) originated from the total invited BT group (n = 801), 2 (0.27%, CI95% 0.03–0.99) from the ST group (n = 731), and 2 (0.31%, CI95% 0.04–1.1) from the TT group (n = 645).
Discussion
In this cross-sectional cohort study on the prevalence and severity of SARS-CoV-2 infections in patients with psoriasis on biologics (BT), nonbiologic systemic treatments (ST), or topical therapies (TT), 59 patients (10.7% (CI95% 8.3–13.6) experienced a SARS-CoV-2 infection since the start of the pandemic-October, 31 2021, based on the composite endpoint of reported infections (test/PCR confirmed) and serological data. Only 4 hospitalizations (0.7%) in these 551 psoriasis patients were reported and no intensive-care unit admissions. Stricter risk-mitigating behavior was comparable in all treatment groups. No statistically significant risk estimates for SARS-CoV-2 infection comparing BT/ST to other treatments were found, except for one analysis in which the OR for BT compared to ST and TT was <1 (0.382 (CI95% 0.182–0.801, p = .011).
The low rate of hospitalizations is in line with other studies that conclude a low rate of severe outcomes of COVID-19 disease in patients with psoriasis, regardless of treatment modality (Citation3,Citation5,Citation6,Citation14,Citation15). Patients that deceased after SARS-CoV-2 infection could not be included in the present study. We identified the number of deceased patients due to COVID-19 and calculated proportions based on the total target population. The mortality rate (0.32% total group; 0.37% BT, 0.27% ST and 0.31% TT), was slightly higher than reported in a large study from France, but comparability is hindered by the fact that our study had a substantially longer observation period, included out-of-hospital deaths as well as in-hospital deaths, and also that our smaller sample sizes decreased precision of our estimate. In the study from France, systemic treatments for psoriasis were not associated with increased risk of in-hospital mortality (Citation15).
The finding that biologics use was associated with lower SARS-CoV-2 infection risk is described in various studies in psoriasis and other immune-mediated inflammatory diseases (Citation8,Citation10,Citation12,Citation16), but this association could be confounded by stricter risk-mitigating behavior in patients on biologics during the pandemic (Citation11). We performed analyses with correction for stricter risk-mitigating behavior and vaccination, and also found that neither BT nor ST showed increased risk of SARS-CoV-2 infection compared to psoriasis patients on other treatment. However, some caution is needed due to the risk for sampling bias due to the lower response rate. It has been hypothesized that disease severity of SARS-CoV-2 infections is related to cytokine storm associated with these infections and that various biologicals may have a protective and/or therapeutic effect (Citation17,Citation18). Several cytokines were found to play a role in this cytokine storm including IL-6, IL-10, IL12 and TNF-α, of which some are targeted by biologics for psoriasis (Citation19,Citation20).
One-time serological testing of anti-N-SARS-CoV-2 antibodies was done in addition to questionnaire-reported infections with the aim to decrease the risk of underreporting of cases and detect asymptomatic infections (in case seroconversion was achieved).
A recent systematic review and meta-analysis has shown that 40.5% of the confirmed SARS-CoV-2 infected population is asymptomatic (Citation21).
In 1.3% of patients, SARS-CoV-2 infection was only detected by serology but not noticed by the patient. Surprisingly, in our study, 20 of 44 patients who reported a positive PCR-test, had a negative antibody test (N-SARS-CoV-2 < 700 AE/ml). This phenomenon occurred in all three treatment groups (n = 5 (2.2%) in BT, n = 9 (5.1%) in ST and n = 6 (4.4%) in TT), limiting its impact on the comparison of COVID-19 risk among treatment groups.
In our patients with a previous positive PCR test but a negative serological result, the reported infection was on average 7 months ago compared to 5 months in the group without seroconversion. Treatment group, age, sex and BMI did not differ between patients with a negative vs. positive serological test. In this study, seroconversion to negative results after infection occurred more than in a recent study on healthy unvaccinated volunteers in which 95% of cases with prior PCR-confirmed infection still showed anti-N antibodies after a median of 9 months after infection (Citation22). However, other longitudinal studies have shown that SARS-CoV-2-antibody levels decrease over time after initial infection; most notably in mild cases (Citation23,Citation24). In another study, asymptomatic patients were even shown to develop significantly less antibody responses compared to mild cases (Citation25). This could explain our findings that a substantial part of patients in our cohort had a negative serological test, despite reporting a previous positive PCR-test. Therefore, underreporting of cases is still possible in our study and should be considered a limitation.
Another limitation of this study was that power was not met despite repeated study invitation, which could lead to sampling bias. As nonresponders were not investigated, we do not know if they have different characteristics that could potentially influence our results. Though, general patient characteristics match with what would be expected from a general psoriasis cohort with regards to age, sex, and weight/BMI distribution. Correction for vaccination status is challenging in COVID-19 studies, because patients received vaccines at different time points and with different working mechanisms. Therefore, we performed multiple sensitivity analyses which all showed similar results.
In summary, in this cohort of 551 patients with psoriasis, 59 (10.7% (CI95% 8.3–13.6) had experienced a SARS-CoV-2 infection since the start of the pandemic, of which four patients were hospitalized. Corrected for risk-mitigating behavior and vaccination status, a higher SARS-CoV-2 incidence for biologics or non-biologics systemics compared to other treatments could not be proven. The rough mortality rate was 0.32% (CI95% 0.13–0.66) in all treatment groups of the total target cohort. Our results do not contradict current recommendations to continue biologics and systemic antipsoriatic drugs during the COVID-19 pandemic.
| Abbreviation | ||
| Anti-N | = | antibodies to SARS-CoV-2 nucleocapsid protein |
| Anti-S | = | antibodies to SARS-CoV-2 spike protein |
| Anti-RBD | = | antibodies to SARS-CoV-2 receptor binding domain of spike protein |
| AU/ml | = | arbitrary units per milliliter |
| BT | = | biological immunosuppressive therapies |
| CI | = | confidence interval |
| ELISA | = | enzyme-linked immunosorbent assay |
| HRs | = | hazard rates |
| IQR | = | interquartile range |
| N | = | nucleocapsid protein |
| OR | = | odds ratio |
| PCR | = | polymerase chain reaction |
| SD | = | standard deviation |
| ST | = | conventional systemic immunosuppressive therapies |
| TT | = | topical therapy |
Supplemental Material
Download PDF (222.5 KB)Acknowledgements
The authors thank the participants, investigators, and study staff who made this study possible. We are very grateful for the statistical input of Hans MM Groenewoud and Scott Maurits of the Radboud University.
Disclosure statement
fK.V. Kwee has received a speaking fee from Eli Lilly and has carried out clinical trials for AbbVie, Novartis, Leo Pharma, Eli Lilly and Cellgene. All funding is not personal, but goes to the independent research fund of the department of dermatology of Bravis Hospital, Bergen op Zoom, the Netherlands
J.L. Murk has received speaker fees for educational events organized by Biogen and acted as consultant for Pfizer and Johnson & Johnson.
Q. Yin has carried out clinical trials for AbbVie, Novartis, Leo Pharma and Eli Lilly. All funding is not personal, but goes to the independent research fund of the department of dermatology of Bravis hospital, Bergen op Zoom, the Netherlands
E.M.G.J. de Jong has received research grants for the independent research fund of the department of dermatology of the Radboud university medical center Nijmegen, the Netherlands from AbbVie, BMS, Janssen Pharmaceutica, Leo Pharma, Novartis, and UCB for research on psoriasis and has acted as consultant and/or paid speaker for and/or participated in research sponsored by companies that manufacture drugs used for the treatment of psoriasis or eczema including AbbVie, Amgen, Almirall, Celgene, Galapagos, Janssen Pharmaceutica, Lilly, Novartis, Leo Pharma, Sanofi and UCB. All funding is not personal but goes to the independent research fund of the department of dermatology of Radboud University medical center Nijmegen, the Netherlands.
J M.P.A. van den Reek carried out clinical trials for AbbVie, Celgene and Janssen and has received speaking fees/attended advisory boards from AbbVie, Janssen, BMS, Almirall, LEO Pharma, Novartis, UCB and Eli Lilly and reimbursement for attending a symposium from Janssen, Pfizer, Celgene and AbbVie. All funding is not personal but goes to the independent research fund of the department of dermatology of Radboudumc Nijmegen, the Netherlands.
M Tjioe has carried out clinical trials for Abbvie, Novartis, Eli Lilly, Leo Pharma, Cellgene. All trial funding is not personal but goes to the independent research fund of the department of dermatology of Bravis Hospital Bergen op Zoom, the Netherlands.
He has received speaking fees/attended advisory boards from Novartis, UCB and Pfizer and reimbursement for attending a symposium from UCB.
The other authors declare no conflicts of interests.
IRB approval status: Reviewed and approved by CMO Regio Arnhem-Nijmegen NL76575.091.21
Additional information
Funding
References
- Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397(10281):1301–1315.
- Damiani G, Pacifico A, Bragazzi NL, et al. Biologics increase the risk of SARS‐CoV‐2 infection and hospitalization, but not ICU admission and death: real‐life data from a large cohort during red‐zone declaration. Dermatol Ther. 2020;33(5):2–7.
- Gisondi P, Facheris P, Dapavo P, et al. The impact of the COVID‐19 pandemic on patients with chronic plaque psoriasis being treated with biological therapy: the Northern Italy experience. Br J Dermatol. 2020;183(2):373–374.
- Queiro Silva R, Armesto S, González Vela C, et al. COVID-19 patients with psoriasis and psoriatic arthritis on biologic immunosuppressant therapy vs apremilast in North Spain. Dermatol Ther. 2020;33(6):e13961.
- Yiu ZZN, Harding-Oredugba G, Griffiths CEM, et al. Risk of COVID-19 infection in adult patients with atopic eczema and psoriasis: a single-Centre cross-sectional study. Br J Dermatol. 2021;185(2):441–443.
- Talamonti M, Galluzzo M, Chiricozzi A, PSO-BIO-COVID study group, et al. Characteristic of chronic plaque psoriasis patients treated with biologics in Italy during the COVID-19 pandemic: risk analysis from the PSO-BIO-COVID observational study. Expert Opin Biol Ther. 2021;21(2):271–277.
- Baniandrés-Rodríguez O, Vilar-Alejo J, Rivera R, BIOBADADERM Study Group, et al. Incidence of severe COVID-19 outcomes in psoriatic patients treated with systemic therapies during the pandemic: a biobadaderm cohort analysis. J Am Acad Dermatol. 2021;84(2):513–517.
- Fagni F, Simon D, Tascilar K, et al. COVID-19 and immune-mediated inflammatory diseases: effect of disease and treatment on COVID-19 outcomes and vaccine responses. Lancet Rheumatol. 2021;3(October):724–736.
- Gelfand JM, Armstrong AW, Bell S, et al. National psoriasis foundation COVID-19 task force guidance for management of psoriatic disease during the pandemic: version 2—advances in psoriatic disease management, COVID-19 vaccines, and COVID-19 treatments. J Am Acad Dermatol. 2021;84(5):1254–1268.
- Izadi Z, Brenner EJ, Mahil SK, Psoriasis Patient Registry for Outcomes, Therapy and Epidemiology of COVID-19 Infection (PsoProtect); the Secure Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD); and the COVID-19 Global Rheumatology Allianc, et al. Association between tumor necrosis factor inhibitors and the risk of hospitalization or death among patients with Immune-Mediated inflammatory disease and COVID-19. JAMA Netw Open. 2021;4(10):e2129639–17.
- Mahil SK, Yates M, Langan SM, PsoProtect, CORE-UK study groups, et al. Risk-mitigating behaviours in people with inflammatory skin and joint disease during the COVID-19 pandemic differ by treatment type: a cross-sectional patient survey. *Br J Dermatol. 2021;185(1):80–90.
- Mahil SK, Dand N, Mason KJ, PsoProtect study group, et al. Factors associated with adverse COVID-19 outcomes in patients with psoriasis—insights from a global registry–based study. J Allergy Clin Immunol. 2021;147(1):60–71. Jan
- Bragazzi NL, Riccò M, Pacifico A, et al. COVID-19 knowledge prevents biologics discontinuation: data from an italian multicenter survey during RED-ZONE declaration. Dermatol Ther. 2020;33(4):e13508.
- Brazzelli V, Isoletta E, Barak O, et al. Does therapy with biological drugs influence COVID-19 infection? Observational monocentric prevalence study on the clinical and epidemiological data of psoriatic patients treated with biological drugs or with topical drugs alone. Dermatol Ther. 2020;33(6):1–5.
- Penso L, Dray-Spira R, Weill A, et al. Psoriasis-related treatment exposure and hospitalization or in-hospital mortality due to COVID-19 during the first and second wave of the pandemic: cohort study of 1 326 312 patients in France*. Br J Dermatol. 2022;186(1):59–68.
- Jones ME, Kohn AH, Pourali SP, et al. The use of biologics During the COVID-19 pandemic. Dermatol Clin. 2021;39(4):545–553. Oct
- Sun X, Wang T, Cai D, et al. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020;53(January):38–42.
- Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8(6):e46–7–e47.
- Kim JS, Lee JY, Yang JW, et al. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021;11(1):316–329.
- Luo XH, Zhu Y, Mao J, et al. T cell immunobiology and cytokine storm of COVID-19. Scand J Immunol. 2021;93(3):0–2.
- Ma Q, Liu J, Liu Q, et al. Global percentage of asymptomatic SARS-CoV-2 infections among the tested population and individuals with confirmed COVID-19 diagnosis: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(12):e2137257–18.
- Alejo JL, Mitchell J, Chang A, et al. Prevalence and durability of SARS-CoV-2 antibodies among unvaccinated US adults by history of COVID-19. JAMA - J Am Med Assoc. 2022;327(11):1083–1085.
- Muecksch F, Wise H, Batchelor B, et al. Longitudinal serological analysis and neutralizing antibody levels in coronavirus disease 2019 convalescent patients. J Infect Dis. 2021;223(3):389–398.
- Van Elslande J, Oyaert M, Ailliet S, et al. Longitudinal follow-up of IgG anti-nucleocapsid antibodies in SARS-CoV-2 infected patients up to eight months after infection. J Clin Virol. 2021;136:104763–104765.
- Shirin T, Bhuiyan TR, Charles RC, et al. Antibody responses after COVID-19 infection in patients who are mildly symptomatic or asymptomatic in Bangladesh. Int J Infect Dis. 2020;101:220–225.

