Abstract
Background
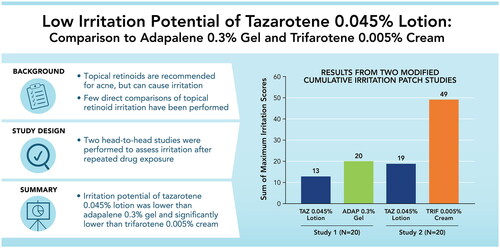
Irritation with topical retinoids presents a significant impediment to acne treatment adherence. Two studies assessed the irritation potential of tazarotene 0.045% lotion versus adapalene 0.3% gel and trifarotene 0.005% cream.
Methods
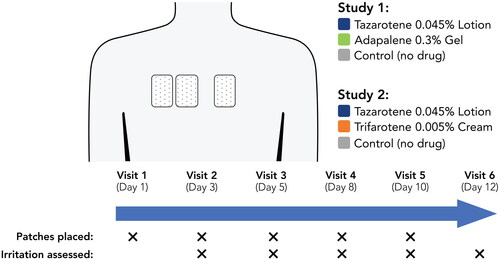
In two double-blind, 12-day modified cumulative irritation patch studies, healthy adults (N = 20 each) had two active patches, containing 0.1 cc of tazarotene 0.045% lotion and either adapalene 0.3% gel (Study 1) or trifarotene 0.005% cream (Study 2), and one control patch (no product) placed on their upper back. Skin irritation was assessed and patches were replaced every 2–3 days.
Results
In Study 1, tazarotene 0.045% lotion and adapalene 0.3% gel were both mildly irritating, though irritation was lower overall with tazarotene 0.045% lotion. In Study 2, significantly greater irritation was observed with trifarotene 0.005% cream than tazarotene 0.045% lotion, beginning two days after the first patch application and at each subsequent visit. In sub-analyses of data from both studies, irritation among participants with acne was similar to the overall study populations.
Conclusions
In two head-to-head studies comparing the irritation potential of third- and fourth-generation retinoids, tazarotene 0.045% lotion was significantly less irritating than trifarotene 0.005% cream and numerically less irritating than adapalene 0.3% gel.
Keywords:
Introduction
Topical retinoids have been used in the treatment of acne for over 50 years and are recommended as first-line acne therapies (Citation1,Citation2). Although efficacious (Citation3), the clinical effectiveness of topical retinoids can be limited by tolerability concerns (Citation2,Citation4). In fact, the very mechanisms by which retinoids address the pathophysiology of acne may contribute to cutaneous irritation (Citation5), potentially leading to perceptions that the tradeoff for greater efficacy is poorer tolerability (Citation6,Citation7).
Tolerability of topical retinoids is impacted by a number of factors, including the retinoid itself, the concentration used, and the vehicle used for its delivery (Citation8), as well as skin hydration and moisturization (Citation5). Third- and fourth-generation topical retinoid formulations use lower drug concentrations, enhanced vehicles, and/or novel retinoids to be clinically efficacious while providing a more patient-friendly tolerability profile (Citation2). For example, adapalene was specifically designed to have a particle size of 3–10 microns to provide preferential delivery into the pilosebaceous unit and is considered one of the best-tolerated topical retinoids (Citation9,Citation10); prescription-strength adapalene 0.3% gel is approved for the treatment of acne in patients aged 12 years and older (Citation11). Tazarotene 0.045% lotion, approved for the treatment of acne in patients 9 years of age and older (Citation12), uses a proprietary polymeric emulsion vehicle to uniformly deliver hydrating excipients onto the skin along with a lower dose of tazarotene than other commercially available 0.1% tazarotene formulations (Citation13). Trifarotene is a first-in-class fourth-generation retinoid, developed to have greater specificity for the gamma subtype of retinoic acid receptors than its predecessors (Citation14). Trifarotene 0.005% cream, indicated for the treatment of facial and truncal acne in patients 9 years of age and older, is the most-recently approved single-agent topical retinoid for acne (Citation2,Citation14–16).
Thorough cross-study comparisons of adverse events associated with topical retinoids have been made (Citation2,Citation3,Citation7), though discerning their relative tolerability from these studies is made difficult by differences in study populations and designs along with differences in definitions and assessments used. Furthermore, there are few studies that have directly compared cutaneous irritation of topical retinoid formulations in a head-to-head fashion (Citation8,Citation17), and fewer still comparing newer retinoid formulations. Two modified cumulative irritation patch test (CIPT) studies, in which each participant served as their own control, directly compared the irritation potential of tazarotene 0.045% lotion versus adapalene 0.3% gel or trifarotene 0.005% cream (Citation15).
Methods
Two identical 12-day modified CIPT studies enrolled adults (≥18 years) with Fitzpatrick skin types I–II and normal upper back skin. At the first study visit, three 2” x 3” Telfa patches covered with Scanpor tape were applied to participants’ upper back (infrascapular region) in a randomized, double-blind fashion. Two of the patches were loaded with active study drugs distributed evenly over the Telfa patch; one control patch contained no study product (). The location of the patches was randomized for each participant. In Study 1, active patches were loaded with 0.1 cc of adapalene 0.3% gel (Differin®; Galderma) or tazarotene 0.045% lotion (Arazlo®; Ortho Dermatologics). In Study 2, active patches were loaded with 0.1 cc of trifarotene 0.005% cream (Aklief®; Galderma) or tazarotene 0.045% lotion. Patches were applied a total of five times using the modification to the traditional daily patch replacement, as first described by Berger and Bowman (Citation18). Study patches were replaced three times weekly to minimize tape irritation. Participants were instructed to keep the patches dry throughout the studies.
At each patch removal (study visits 2–6), irritation at application sites was assessed using scales that have been validated in previous cumulative irritation patch tests for topical fomulations (Citation18,Citation19). Dermal Effects were assessed using an 8-point scale and Other Effects were assessed using a 7-point scale; higher scores for either assessment were indicative of greater irritation (). Patches were not replaced after assessments were made on the final study visit (Visit 6). Scores at each study visit were averaged and analyzed using a Wilcoxon signed-rank test; group differences were considered statistically significant at a p-value of ≤0.05. To compare overall irritation, the maximum irritation score at any visit (Dermal or Other Effects) for each drug was tabulated by individual and summed across participants. Additional sub-analyses were performed for participants with acne. Adverse events were monitored throughout the studies.
Table 1. Assessments of Irritation.
These studies were approved by the Allendale Institutional Review Board (Old Lyme, CT, USA) and were conducted in accordance with principles of Good Clinical Practice and the Declaration of Helsinki. All participants provided written informed consent.
Results
In the first study of adapalene 0.3% gel versus tazarotene 0.045% lotion, all 20 enrolled adults completed the study. They ranged in age from 22–69 years (mean: 50.1 years); all were White, and 19/20 (95%) were female.
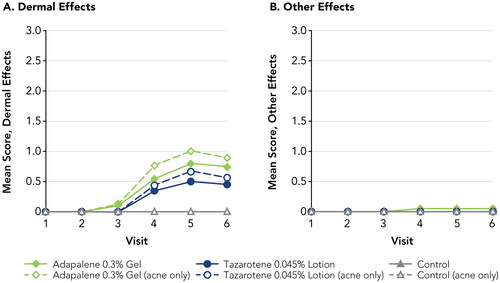
Both tazarotene 0.045% lotion and adapalene 0.3% gel were assessed as minimally irritating, with Dermal Effects mean scores <1 and negligible Other Effects (; Supplemental Table 1). Tazarotene 0.045% lotion was less irritating than adapalene 0.3% gel overall (highest Dermal Effects mean scores: 0.5 and 0.8, respectively), though differences between the drugs were not statistically significant at any assessment. No Dermal Effects or Other Effects were observed at the control patch site at any study visit. No adverse events were reported during the study.
Figure 2. Irritation Potential of Adapalene 0.3% Gel Versus Tazarotene 0.045% Lotion (Study 1). Means, standard deviations, and p-values at all study visits are presented in Supplemental Table 1. Patches were applied at visits 1–5 after any skin assessments were made. Visits 1–6 correspond to study days 1, 3, 5, 8, 10, and 12, respectively. Filled symbols represent the overall study population (N = 20); open symbols represent participants with acne (n = 9). There were no statistically significant differences between adapalene 0.3% gel and tazarotene 0.045% lotion at any study visit. For adapalene 0.3% gel, Dermal Effect scores were significantly greater vs control at visits 4–6 for both the overall and acne-only study populations. For tazarotene 0.045% lotion, Dermal Effects scores were significantly greater vs control at visits 4–6 for the overall study population, and at visit 5 for the acne-only population.
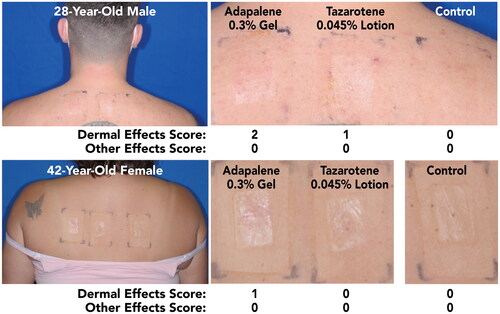
Out of the overall study population, nine participants had acne (22–47 years; mean 37.8 years; 88.9% female). Dermal Effects and Other Effects were similar in this subgroup to the overall study population. Although Dermal Effects scores for both drugs were slightly higher amongst participants with acne, both drugs were still regarded as only mildly irritating, with highest mean scores of 0.67 for tazarotene 0.045% lotion and 1.0 for adapalene 0.3% cream. Images of representative patch application sites at the final assessment in participants with acne are shown in .
Figure 3. Participant Photographs at Final Assessment in Participants With Acne (Study 1; Visit 6; Day 12). Individual results may vary.
In the second study of trifarotene 0.005% cream versus tazarotene 0.045% lotion, 20 adults (22–74 years; mean 51.4 years) were enrolled and completed the study; 18/20 (90%) were White, 2/20 (10%) were Black, and 18/20 (90%) were female.
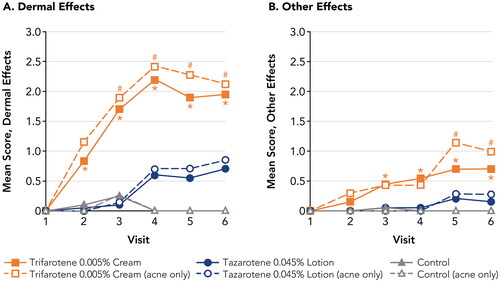
As in Study 1, tazarotene 0.045% lotion was assessed as minimally irritating, with Dermal Effects mean scores <1 and Other Effects mean scores <0.3 at all study visits. In contrast, trifarotene 0.005% cream was assessed as significantly more irritating than tazarotene 0.045% lotion (; Supplemental Table 2). Dermal Effects and Other Effects mean scores were significantly greater with trifarotene 0.005% cream versus tazarotene 0.045% lotion beginning at Visits 2 and 3, respectively, and continuing through the end of the study (p < 0.05, all). The highest irritation scores with trifarotene 0.005% cream were over 3-fold greater than with tazarotene 0.045% lotion (Dermal Effects: 2.20 versus 0.70; Other Effects: 0.70 versus 0.20, respectively). Dermal Effects mean scores were significantly greater for both drugs than at the control patch, beginning at Visit 2 for trifarotene 0.005% cream and Visit 4 for tazarotene 0.045% lotion (2 and 8 days after initial patch placement, respectively) and continuing through the remainder of the study (p < 0.05, all). Other Effects with tazarotene 0.045% lotion were not significantly different from the control patch. No adverse events were reported during the study.
Figure 4. Irritation Potential of Trifarotene 0.005% Cream Versus Tazarotene 0.045% Lotion (Study 2). *p < 0.05, trifarotene 0.005% cream vs tazarotene 0.045% lotion, overall study population (N = 20; filled symbols). #p < 0.05, trifarotene 0.005% cream vs tazarotene 0.045% lotion, acne-only population (n = 7; open symbols). Means, standard deviations, and p-values at all study visits are presented in Supplemental Table 2. Patches were applied at visits 1–5 after any skin assessments were made. Visits 1–6 correspond to study days 1, 3, 5, 8, 10, and 12, respectively. For trifarotene 0.005% cream, Dermal Effects scores were significantly greater vs control at visits 2–6 for the overall study population and at visits 3–6 for the acne-only population; Other Effects scores were significantly greater vs control at visits 3–6 for the overall study population and at visits 5–6 for the acne-only population. For tazarotene 0.045% lotion, Dermal Effects scores were significantly greater vs control at visits 4–6 for both the overall and acne-only study populations; Other Effects scores were not significantly different from control at any study visit.
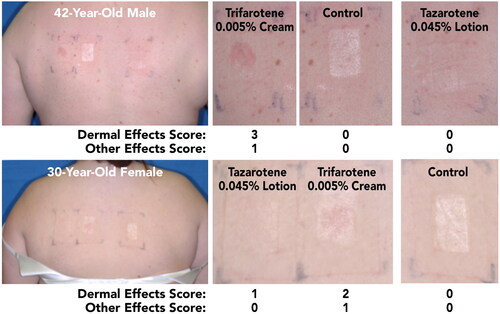
Of the overall study population, 7 participants had acne (22–44 years; mean 36.4 years; 85.7% female; 85.7% White, 14.3% Black). As in Study 1, Dermal Effects and Other Effects were similar in this subgroup to the overall study population, though slightly higher overall. Among these participants, highest Dermal Effects mean scores for tazarotene 0.045% lotion and trifarotene 0.005% cream were 0.86 and 2.43, respectively; mean scores were significantly greater with trifarotene 0.005% cream versus tazarotene 0.045% lotion beginning at Visit 3 and continuing through the remaining study visits (p < 0.05, all). Other Effects mean scores were significantly greater with trifarotene 0.005% cream than with tazarotene 0.045% lotion at the final two assessments (p < 0.05, all; highest mean scores: 1.14, and 0.29, respectively). Images of representative patch application sites at the final assessment in participants with acne are shown in .
Figure 5. Participant Photographs at Final Assessment in Participants With Acne (Study 2; Visit 6; Day 12). Individual results may vary.
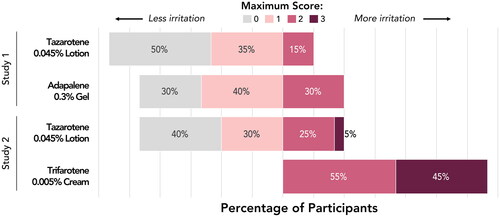
Overall, tazarotene 0.045% lotion was associated with the lowest maximum irritation scores (Dermal or Other Effects; ). Across the two studies, 40–50% of participants experienced no irritation at the tazarotene 0.045% lotion patch site, and an additional 30–35% of participants experienced a maximum irritation score of 1. Adapalene 0.3% gel was only slightly more irritating, with 30% of participants in Study 1 experiencing no irritation and 40% with a maximum score of 1 at the patch site. Trifarotene 0.005% cream was the most irritating formulation. All participants in Study 2 experienced irritation with trifarotene 0.005% cream and all maximum irritation scores were 2 or 3. Summed maximum irritation scores were ∼1.5 times greater with adapalene 0.3% gel than with tazarotene 0.045% lotion (Study 1; 20 versus 13, respectively) and ˜2.6 times greater with trifarotene 0.005% cream than with tazarotene 0.045% lotion (Study 2; 49 versus 19, respectively).
Discussion
Tolerability of topical acne therapy is a key driver of patient acceptance and adherence to treatment, which can impact product efficacy (Citation4,Citation20,Citation21). However, understanding the relative tolerability of topical retinoids is made difficult by a scarcity of head-to-head comparisons (Citation22). This applies not only to the fourth-generation retinoid trifarotene—which has less data on irritation relative to adapalene lotion or gel and tazarotene gel, cream, or foam (Citation2,Citation3)—but also to newer formulations of established retinoids, such as tazarotene 0.045% lotion. As such, two within-subject studies were conducted to evaluate the irritation potential of tazarotene 0.045% lotion versus adapalene 0.3% gel (Study 1) and trifarotene 0.005% cream (Study 2). In these randomized, 12-day modified CIPT studies, tazarotene 0.045% lotion was found to be numerically less irritating than adapalene 0.3% gel and significantly less irritating than trifarotene 0.005% cream.
The strength of these studies is that they allowed for direct, head-to-head comparisons of irritation with tazarotene 0.045% lotion versus adapalene 0.3% gel or trifarotene 0.005% cream within the same patients. The lower overall degree of irritation with tazarotene 0.045% lotion relative to adapalene 0.3% gel in the first study is notable, as adapalene in varying formulations is generally perceived to be among the best-tolerated topical retinoids (Citation3,Citation6,Citation7,Citation10). In the second study, maximal mean Dermal Effects scores with trifarotene 0.005% cream were over 3 times greater than with tazarotene 0.045% lotion. In addition, while half of the participants had no evidence of irritation at the tazarotene 0.045% lotion patch site, all participants had irritation at the trifarotene 0.005% cream patch site, with maximum Dermal Effects or Other Effects scores ranging from 2 to 3. Overall, despite the small number of participants enrolled (N = 20), mean irritation scores with tazarotene 0.045% lotion were found to be significantly lower than with trifarotene 0.005% cream, further underscoring the strength of the head-to-head study design and highlighting the robust differences in irritation potential between these two retinoid formulations. Similar patterns of results in both studies were observed among participants with acne, indicating the lower irritation potential with tazarotene 0.045% lotion.
The methodology used in these studies is standardized and has been widely adopted for the evaluation of irritation for a variety of topical and transdermal applications, including topical retinoids (Citation23–25). Nonetheless, the generalizability of these findings may be limited in that continuous exposure to topical treatments, applied to the back under occlusion, does not reflect the once-daily application indicated for their use in the treatment of acne (Citation11,Citation12,Citation16). While exposure over 12 days is also not commensurate with the duration of topical acne treatment, which can take several months (Citation26), irritation associated with topical retinoids typically peaks within the first few weeks of treatment (Citation5), consistent with the time frame for measuring irritation in these studies. Moreover, significantly greater irritation with trifarotene 0.005% cream versus tazarotene 0.045% lotion was seen as early as two days after initial patch application. Thus, lower rates of irritation with tazarotene 0.045% lotion in these studies were observed during the period in which tolerability of acne treatment may be of greatest concern.
Enrollment in these studies was limited to adults with Fitzpatrick skin types I–II, thus limiting the ability to extrapolate these findings to other demographic groups, including patients with more pigmented skin. However, this restriction was deemed necessary for these cumulative irritation studies, as irritation with acne treatments in people with skin of color carries a higher risk of post-inflammatory hyperpigmentation (PIH), and worsening PIH can lead to hypertrophic and keloidal scarring (Citation27). Though patients with more pigmented skin were not included in this study, the importance of minimizing treatment-related irritation in these patients has been highlighted in a recent consensus publication, which emphasized the importance of considering tolerability of active ingredients and vehicle when choosing acne treatments for patients with skin of color (Citation27). An additional demographic consideration is that participants in these studies were generally older (mean age of ∼ 50 years) than the stereotypical acne patient; because skin changes with age, these results may not reflect irritation that may be experienced by younger patients. It should be noted, however, that the study populations were selected for their amenability to patch testing (eg, Fitzpatrick skin type) rather than for acne treatment. Regardless, irritation assessments for each drug were similar for participants with and without acne. Moreover, although incidence of acne may be highest in people under 18 years of age, the majority of acne patients are adults (Citation28) and acne prevalence among adults has been increasing (Citation29). This is particularly true among females (Citation30), for whom irritation associated with topical treatment may be especially concerning (Citation31).
These studies were designed to demonstrate the irritation potential of topical retinoids under exaggerated conditions. In clinical practice, irritation can be reduced by minimizing exposure (eg, applying the least amount of medication to cover the affected area, or washing off the medication after a short period) and/or subsequent application of moisturizers. Nonetheless, topical retinoids that are inherently less irritating may promote effective treatment by reducing the need for additional procedures that may negatively impact treatment adherence and/or efficacy.
In the head-to-head comparisons reported here, tazarotene 0.045% lotion was numerically less irritating than adapalene 0.3% gel, one of the best-tolerated topical retinoids for acne, and significantly less irritating than trifarotene 0.005% cream. Findings were similar for the overall study populations and in subpopulations of participants with acne. The low irritation potential of tazarotene 0.045% lotion may encourage patient acceptance and adherence to treatment and, as a result, greater likelihood of acne improvement.
Supplemental Material
Download PDF (77.5 KB)Acknowledgements
Medical writing support was provided by Kevin Corcoran, PhD and Jacqueline Benjamin, PhD of Prescott Medical Communications Group with financial support from Ortho Dermatologics.
Disclosure statement
Zoe Draelos received funding from Ortho Dermatologics to conduct the studies presented here.
Data availability statement
Data are available upon request.
References
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973.e33.
- Baldwin H, Webster G, Stein Gold L, et al. 50 Years of topical retinoids for acne: evolution of treatment. Am J Clin Dermatol. 2021;22(3):315–327.
- Kolli SS, Pecone D, Pona A, et al. Topical retinoids in acne vulgaris: a systematic review. Am J Clin Dermatol. 2019;20(3):345–365.
- Sevimli Dikicier B. Topical treatment of acne vulgaris: efficiency, side effects, and adherence rate. J Int Med Res. 2019;47(7):2987–2992.
- Thiboutot DD, Rosso JQ. Acne vulgaris and the epidermal barrier. J Clin Aesthet Dermatol. 2013;6(2):18–24.
- Tolaymat L, Dearborn H, Zito PM. Adapalene. Treasure Island (FL). StatPearls Publishing; 2022.
- Culp L, Moradi Tuchayi S, Alinia H, et al. Tolerability of topical retinoids: are there clinically meaningful differences among topical retinoids? J Cutan Med Surg. 2015;19(6):530–538.
- Leyden J, Grove G, Zerweck C. Facial tolerability of topical retinoid therapy. J Drugs Dermatol. 2004;3(6):641–651.
- Shroot B. Pharmacodynamics and pharmacokinetics of topical adapalene. J Am Acad Dermatol. 1998;39(2 Pt 3):S17–S24.
- Rigopoulos D, Ioannides D, Kalogeromitros D, et al. Comparison of topical retinoids in the treatment of acne. Clin Dermatol. 2004;22(5):408–411.
- Differin® (adapalene) gel 0.3%. US prescribing information. Fort Worth, TX: Galderma; 2012.
- Arazlo® (tazarotene) lotion 0.045%. US prescribing information. Bridgewater, NJ: Ortho Dermatologics; 2021.
- Tanghetti EA, Stein Gold L, Rosso, et al. Optimized formulation for topical application of a fixed combination halobetasol/tazarotene lotion using polymeric emulsion technology. J Dermatolog Treat. 2021;32(4):391–398.
- Cosio T, Di Prete M, Gaziano R, et al. Trifarotene: a current review and perspectives in dermatology. Biomedicines. 2021;9(3):237.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80(6):1691–1699.
- Aklief® (trifarotene) 0.005% cream. US prescribing information. Fort Worth, TX: Galderma; 2019.
- Draelos ZD, Tanghetti EA, Guenin E. Vehicle formulation impacts tolerability and patient preference: comparison of tretinoin branded lotion and generic cream. J Drugs Dermatol. 2022;21(8):875–880.
- Berger RS, Bowman JP. A reappraisal of the 21-day cumulative irritation test in man. J Toxicol: Cutan Ocular Toxicol. 1982;1(2):109–115.
- Kircik LH, Green L, Guenin E, et al. Dermal sensitization, safety, tolerability, and patient preference of tazarotene 0.045% lotion from five clinical trials. J Dermatolog Treat. 2022;33(4):2241–2249.
- Eicher L, Knop M, Aszodi N, et al. A systematic review of factors influencing treatment adherence in chronic inflammatory skin disease - strategies for optimizing treatment outcome. J Eur Acad Dermatol Venereol. 2019;33(12):2253–2263.
- Hoffman LK, Bhatia N, Zeichner J, et al. Topical vehicle formulations in the treatment of acne. J Drugs Dermatol. 2018;17(6):s6–s10.
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild-to-moderate acne vulgaris: systematic review and network meta-analysis. Br J Dermatol. 2021;185(3):512–525.
- Suzuki K, Castelli M, Komaroff M, et al. Pharmacokinetic profile of the asenapine transdermal system (HP-3070). J Clin Psychopharmacol. 2021;41(3):286–294.
- Galvin SA, Gilbert R, Baker M, et al. Comparative tolerance of adapalene 0.1% gel and six different tretinoin formulations. Br J Dermatol. 1998;139 Suppl 52:34–40.
- Brand B, Gilbert R, Baker MD, et al. Cumulative irritancy comparison of adapalene gel 0.1% versus other retinoid products when applied in combination with topical antimicrobial agents. J Am Acad Dermatol. 2003;49(3 Suppl):S227–S32.
- Eichenfield DZ, Sprague J, Eichenfield LF. Management of acne vulgaris: a review. JAMA. 2021;326(20):2055–2067.
- Alexis AF, Woolery-Lloyd H, Williams K, et al. Racial/ethnic variations in acne: implications for treatment and skin care recommendations for acne patients with skin of color. J Drugs Dermatol. 2021;20(7):716–725.
- Yentzer BA, Hick J, Reese EL, et al. Acne vulgaris in the United States: a descriptive epidemiology. Cutis. 2010;86(2):94–99.
- Skroza N, Tolino E, Mambrin A, et al. Adult acne versus adolescent acne: a retrospective study of 1,167 patients. J Clin Aesthet Dermatol. 2018;11(1):21–25.
- Dreno B, Thiboutot D, Layton AM, et al. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29(6):1096–1106.
- Zeichner JA, Baldwin HE, Cook-Bolden FE, et al. Emerging issues in adult female acne. J Clin Aesthet Dermatol. 2017;10(1):37–46.