?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Cell-based therapies are popular in the field of reconstructive surgery. The stromal vascular fraction (SVF), comprised of mature adipocytes or blood, reportedly has a regenerative effect; however the mechanism remains unclear. This study aimed to prove the viability and effectiveness of using SVF in scar treatment.
Methods
This prospective double-blind study involved 20 patients who visited an outpatient clinic for 2 years, from July 2016 to July 2018, and underwent scar revision for traumatic or surgical scars. After scar revision surgery performed by a single surgeon, patient scars were divided into experimental and control sides. The subcutaneous layer of the experimental and control sides were injected with 0.1 mL/cm of SVF and normal saline, respectively. Each side was evaluated using the Patient and Observer Scar Assessment Scale (POSAS) before and six months after the surgery.
Results
Of the 20 patients who underwent scar revision surgery and SVF treatment, 4 dropped out for personal reasons. In 11 of 12 POSAS items, the experimental side showed significant improvements compared to the control side.
Conclusions
Although more research is needed, autologous SVF is a valuable source of regenerative medicine that can be swiftly and inexpensively prepared from human fat tissue.
Introduction
A scar is an abnormal remodeling of a wound that can be caused by trauma, burns, or surgery (Citation1,Citation2). Due to their cosmetic appearance, scars impair patients’ self-esteem and may cause functional discomfort, such as pain, itchiness, and a restriction of joint movement due to contracture, thereby reducing normal social function (Citation2). Therefore, various medical treatments have been used to prevent and treat scars (Citation3). Approximately 12 million lacerations are sutured every year in the United States alone, with 250 million surgical incisions made worldwide, and more than 20 billion USDs are spent on scar management every year (Citation4). In addition, 170,000 scar revisions have been performed per year in the United States alone (Citation5), and several treatments, such as topical and intralesional therapies, have been also used in parallel, but the effects are limited (Citation3).
Since autologous fat grafting was first introduced, it has been widely used to cover volume defects in both cosmetic and reconstruction areas (Citation6) and has further been applied to skin rejuvenation (Citation7) and hand surgery (Citation8). Autologous fat grafts have been useful for scar treatment (Citation9–11), but few studies have been conducted on scars, such as acne scars (Citation12), and these studies have not been able to elucidate the mechanism (Citation13).
The stromal vascular fraction (SVF) is a substance made up of mature adipocytes or blood that has been removed from the patient’s fat tissue, and it has been reported to have a regenerative effect. However, the evidence is limited and controversial. Therefore, this study aimed to demonstrate the effect of administering the SVF to scars during scar revision surgery and prove its viability in scar treatment.
Methods
This study was approved by the institutional review board of Chungnam National University Hospital (Chungnam National University Hospital No. 2016-08-024). Patients provided written informed consent for the publication and the use of the images. This prospective double-blind study included 20 patients with scars due to trauma or surgery from among the patients who visited the outpatient clinic for over 6 months between July 2016 and June 2018. The scar revision surgery was performed by a single surgeon. Patients’ scars were divided in half along their total length after the surgery. Half the scars were in the experimental treatment group, and 0.1 mL/cm of SVF extracted with Smart-X® (DongKoo Bio & Pharma Co. Ltd., Seoul, Korea) was injected into the subcutaneous layer of the scar; the other half of the scars were in the control group, into which 0.1 mL/cm of saline was injected.
Random allocation
After dividing the scar in half along the total length, the part furthest from the trunk was called the distal part, and the part nearest the trunk was called the proximal part. One of the co-researchers used dice to randomly determine which of these would be assigned to the experimental group (if the dice roll was odd, the proximal part was the experimental group, and if the dice roll was even, the distal part was the experimental group), and they were recorded and managed. The assignee participated only in the randomization and was excluded from other study processes; the assignment process was not shared with the other researchers and patients. For double-blindness, the operator and patients did not know which side of the scars belonged to which treatment (SVF or saline). This was recorded and analyzed by a co-researcher, excluding the assignee, and the patients and operators took pictures in a standardized environment before surgery, immediately after surgery, and 6 months after surgery.
Patient selection criteria
The selection criteria were as follows: age between 19 and 65 years, scars due to trauma or surgery, follow-up during the test period, and a total scar length of more than 2 cm. All patients had scars for more than a year. The exclusion criteria were as follows: patients who refused to or did not fill out the consent form, those with scars from malignant tumor removal surgery, pregnant and lactating women, those with psychiatric problems, those with keloid disease or collagen and elastic fiber diseases, and if the clinical trial director judged that it was difficult to conduct the clinical trial. The purpose and contents of the clinical trial were explained to patients before the trial, and they voluntarily signed the written consent and the human material research consent forms.
Surgical procedure and SVF preparation
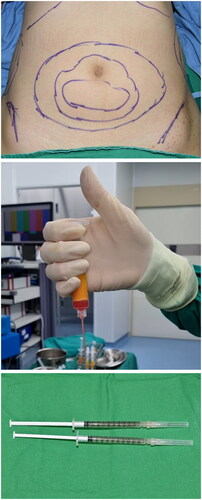
All surgeries were performed under local anesthesia and sedation. Autologous fat was harvested from the patient’s abdomen and flank (). The tumescent solution was prepared by mixing 1 mL epinephrine (1 mg/mL), 20 mL lidocaine 2% (20 mg/mL), and 2 mL hyaluronidase (1,500 IU/mL) in 1 L Hartmann’s solution. Subsequently, a stab incision was made in the patient’s navel with a number 11 blade, and the prepared tumescent solution was widely infiltrated in both the abdomen and flank. After waiting for 20 min, fat was harvested from the abdomen and flank using a blunt cannula and centrifuged at 1,000 RPM (110 G) for 3 min to obtain pure fat, from which the serum and oil were removed (). After mixing a collagenase I solution with the fat at a 1:1 ratio, the mixture was stirred in an incubator at 37 °C (Vision Scientific Co, Ltd., Daejeon, Korea) for 30 min. Then, the enzyme-treated fat was mixed with the Smart-X® Kit (DongKoo Bio & Pharma. Co. Ltd., Seoul, Korea) and centrifuged for 3 min at 3,000 RPM (1,006 × G) to obtain the SVF cell layer at the bottom. After centrifugation, the upper stopper of the component separator was removed, and a syringe was connected to move the supernatant and fat layer to the upper syringe, retaining the SVF cell layer at the bottom. Then, using a separate syringe, the clean washing solution (normal saline) was moved to the component separator and centrifuged again at 3,000 RPM (1,006 G) for 3 min; this was repeated three times. Then, a small amount of supernatant fluid and SVF were extracted by connecting a syringe (in this case, the SVF was suspended in the supernatant fluid) and passed through a filter to obtain the SVF. The average number of SVF cells isolated was approximately ().
Figure 1. (A) Preoperative design for tumescent injection and fat harvesting. (B) The serum is removed from the centrifuged fat, (C) creating the desired quantity (approximately 2 cc) of the stromal vascular fraction.
After administering local anesthesia, excision of the scar, elevation of the flaps on both sides without tension, layer-by-layer suturing, and the scar revision were performed.
Injection of SVF (experimental treatment group) and normal saline (control group)
After the scar revision was completed, 0.1 mL of SVF per 1 cm of the total scar length was injected into the subcutaneous layer in the experimental group, and 0.1 mL of normal saline per 1 cm was injected in the control group in the same manner.
Clinical evaluation: patient and observer scar assessment scale
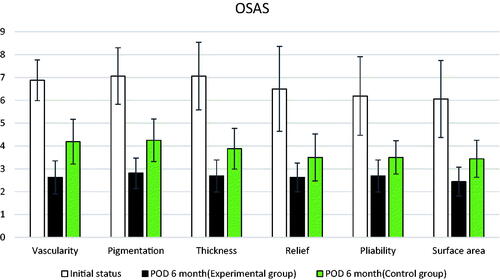
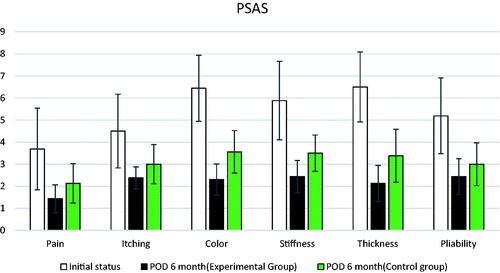
Both the patients and observers used the Patient and Observer Scar Assessment Scale (POSAS) to evaluate and record scars before and 6 months after the surgery in both groups. The POSAS (Citation14) is widely used worldwide as a valid tool for assessing scars by both observers and patients (Citation15). The POSAS consists of two scales: observer and patient ().
Table 1. The Patient and Observer Scar Assessment Scale (POSAS).
Side effects
During the follow-up period of up to 6 months after the surgery, we evaluated the presence of pain, bleeding, infection, fat necrosis, skin necrosis, systemic allergic or anaphylactic reaction, fever, headache, muscle pain, and fatigue.
Statistical analysis
Using SPSS Statistics version 26 (IBM Corp., Armonk, NY, USA), the POSAS scores of the control and experimental treatment groups were compared for each item using an independent t-test at 6 months after the surgery. Statistical significance was set at p < .05.
Results
Of the 20 patients who underwent scar revision and SVF surgery, 16 were followed up with after 6 months; four patients dropped out for personal reasons. The baseline demographics and characteristics of the 16 patients are shown in .
Table 2. Patient demographics.
The scars in both groups showed improvement compared to before surgery. The scars in the experimental treatment group improved significantly compared to those in the control group in 11 of the 12 total items (six in the observer scar assessment scale, six in the patient scar assessment scale) in the POSAS scale following evaluation before and 6 months after surgery (p <.05; ). On the observer scar assessment scale, the experimental treatment group showed a statistically significant improvement compared to the control group in all items (vascularity, pigmentation, thickness, relief, liability, and surface area). Except for the pliability item on the patient scar assessment scale, the scar was significantly better in all other items (pain, itching, color, stiffness, and thickness). The results are shown in and with a bar plot and error bars. The overall score was significantly improved in the experimental group compared to in the control group in both the patient and observer scales. There was also a visible difference in the appearance of scars between the experimental treatment and control sides ( and ). Except for two patients who complained of mild pain two days after the surgery, no patient experienced side effects, such as bleeding, infection, fat necrosis, skin necrosis, systemic allergic or anaphylactic reaction, fever, headache, muscle pain, or fatigue during the six-month follow-up period.
Figure 4. (A) A scar that occurred after trauma to the leg 6 years prior. (B) Immediate postoperative image. The yellow section is the control side and the red section is the experimental treatment side. (C) Six months postoperatively. The scar appears less visible on the experimental treatment side than on the control side.
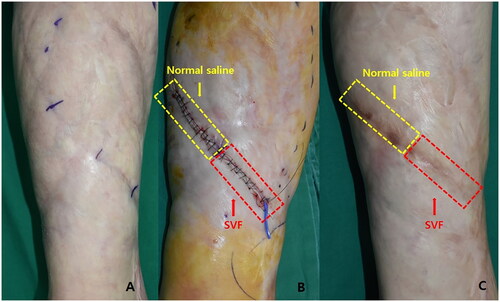
Figure 5. (A) Hypertrophic scar after Cesarean surgery 2 years prior. (B) Immediate postoperative image. The yellow section is the control side and the red section is the experimental treatment side. (C) Six months postoperatively. The scar appears less visible on the experimental treatment side than on the control side.
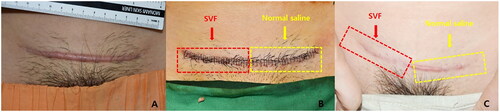
Table 3. Results of the POSAS scale comparing pre-and 6-months post-surgery.
Discussion
In this study, the 6-month follow-up following scar revision surgery revealed better results after treatment with SVF than those in the control group.
Scar formation is an inevitable consequence of wound healing in which the normal skin is replaced by fibrous tissue. The esthetic appearance of a scar is the most important criterion for determining surgical outcomes. Both surgical and nonsurgical techniques, used either alone or in combination, can be used for scar revision. Although many techniques have been described to minimize scarring, none can completely eliminate scars (Citation16).
Currently, cell-based therapies are popular in the field of reconstructive surgery. The SVF is a component of adipose tissue that contains a range of cells, including stem cells, which can differentiate into many types of cells and tissues (Citation17). Lipoaspirate products are easily accessible to surgeons via liposuction, and adipose-derived stem cells (ADSCs) and the SVF are gaining traction in reconstructive surgery and esthetics. The SVF has an advantage over ADSCs in clinical applications because it does not require a culture process and thus does not require United States Food and Drug Administration approval, making it readily available. As a result, expanding the clinical indications for the SVF would benefit a large number of patients (Citation18).
Research is being conducted to determine the mechanistic process functioning at the cellular level. Epidermal growth factor (EGF) is one of the most important growth factors in skin wound healing and epithelialization. EGF induces keratinocytes and fibroblasts to migrate, grow, and accelerate wound healing. In addition, wound healing genes, including vascular endothelial growth factor-A, EGF, fibroblast growth factor-2, and connective tissue growth factor precursor, are upregulated in the SVF compared with in adipose-derived mesenchymal stem cells. With these factors, SVF implantation drastically accelerates wound closure and increases cellularization and re-epithelialization (Citation19).
Clinically, the SVF has been investigated as a possible therapeutic option for many medical disorders, including scar revision and wound healing (Citation12,Citation13,Citation20–25). Although several studies have demonstrated no significant effect (Citation26), some recent results have indicated that it is beneficial for acne scars (Citation12,Citation22), acute cutaneous wounds (Citation23), skin rejuvenation (Citation7), temple augmentation (Citation27), cicatricial ectropion (Citation28), and burns (Citation25).
Surowiecka reported that the use of medical devices like radiofrequency and laser in conjunction with autologous-derived injectables, like SVF and platelet-rich plasma, in scar therapy is safe and can facilitate both cosmetic and functional outcomes (Citation29). Vinci et al. performed fat grafting on scars and analyzed the results using the POSAS; quality improvements were observed in all treated scars, both cosmetically and functionally, and pain relief and increased scar elasticity were clinically assessable in all cases (Citation9). van Dongen et al. showed that, except for the degree of pigmentation, both the fat grafting and nano fat-enriched fat grafting groups had equivalent Visual Analog Scale and Vancouver Scar Scale sub-scores. In the group that received fat grafts supplemented with nano fat, the pigmentation level decreased (Citation24). These results are consistent with this study’s 6-month findings. This shows that the favorable effects on early wound healing are not caused by intact adipocytes, but rather by adipocyte-derived chemicals.
Using these qualities at various stages of wound healing, Lee et al. established the therapeutic efficacy of SVF in the surgical management of constricted and depressed scars and explained their findings (Citation30). Although no significant changes were observed in the vascularity, all patients in the test group demonstrated improvements in several scar assessment scales, particularly in terms of the height and pliability.
Similar to the above studies, autologous SVF transplantation was performed during scar revision surgery in this study, and the results were evaluated using the POSAS. The POSAS items included the vascularity, pigmentation, thickness, reliability, pliability, and surface area, and significant differences between the experimental treatment and control groups were confirmed for all these items. In addition, significant results were obtained that proved the effectiveness of the SVF for all items, except the pliability, among pain, itching, color, stiffness, thickness, and pliability corresponding to the POSAS.
In addition, the SVF accelerates early wound healing in the first six months (Citation24), suggesting that the SVF might be a valid option for treating acute cutaneous wounds. The current guidelines for treating acute wounds are based on limiting bacterial growth, lowering edema, and maintaining hydration. Occlusive and semi-occlusive dressings, procedural interventions including surgical debridement and primary closure, and topical therapy, such as antiseptics and antibacterial medications, are examples of typical procedures. The SVF can be included in advances in tissue regeneration, including engineered skin substitutes (Citation23).
In this study, we observed the clinical effects of the SVF on the scar quality. Our study’s strength was that the design allowed for the comparison of the wounds as a paired sample, and all patients underwent the procedure by a single surgeon. Therefore, we suggest that intralesional SVF injections can improve the scar quality.
However, this study had some limitations. First, a relatively small number of participants were included in the study. Second, the location, etiology, and onset of scars were not considered. The effects of the SVF based on the location, etiology, and timing of the scar development has yet to be determined. Finally, the follow-up period was short. More research is needed to evaluate if the outcomes of long-term follow-ups are comparable to those of this short-term follow-up study.
Although there is controversy regarding the effect of the SVF, this process has factors that help wound healing increase; therefore, if further research is conducted, the SVF may develop into a viable option to aid in scar revision.
Conclusions
Autologous SVF is a valuable source of regenerative medicine that can be swiftly and inexpensively prepared from human fat tissue. Moreover, it demonstrates good short-term clinical outcomes without serious side effects. However, the absence of a long-term follow-up and the small sample size are limitations of this study, indicating that more research with a large sample size and long-term follow up of clinical outcomes is needed. While the SVF has shown promise in scar remodeling, additional research is needed to completely understand its usefulness and safety. It is also crucial to note that the findings of various studies may not apply to all patients, and the efficacy of the SVF may vary depending on the specific characteristics of the scar or wound as well as the individual patient.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research, supporting data is not available.
Additional information
Funding
References
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453(7193):314–321.
- Niessen FB, Spauwen PH, Schalkwijk J, et al. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg. 1999;104(5):1435–1458.
- Khansa I, Harrison B, Janis JE. Evidence-based scar management: how to improve results with technique and technology. Plast Reconstr Surg. 2016;138(3 Suppl):165s–178s.
- Block L, Gosain A, King TW. Emerging Therapies for scar prevention. Adv Wound Care. 2015;4(10):607–614.
- Lim AF, Weintraub J, Kaplan EN, et al. The embrace device significantly decreases scarring following scar revision surgery in a randomized controlled trial. Plast Reconstr Surg. 2014;133(2):398–405.
- Coleman SR. Structural fat grafting: more than a permanent filler. Plast Reconstr Surg. 2006;118(3 Suppl):108s–120s.
- Pattayadeekul T, Pawcsuntorn T, Nararatwanchai T. The efficacy and safety of autologous stromal vascular fraction transplantation for infraorbital skin rejuvenation: a clinical prospective study. J Cosmet Dermatol. 2022;21(1):220–226.
- Nseir I, Delaunay F, Latrobe C, et al. Use of adipose tissue and stromal vascular fraction in hand surgery. Orthop Traumatol Surg Res. 2017;103(6):927–932.
- Klinger M, Caviggioli F, Klinger FM, et al. Autologous fat graft in scar treatment. J Craniofac Surg. 2013;24(5):1610–1615.
- Fredman R, Katz AJ, Hultman CS. Fat grafting for burn, traumatic, and surgical scars. Clin Plast Surg. 2017;44(4):781–791.
- Condé-Green A, Marano AA, Lee ES, et al. Fat grafting and adipose-derived regenerative cells in burn wound healing and scarring: a systematic review of the literature. Plast Reconstr Surg. 2016;137(1):302–312.
- Nilforoushzadeh MA, Heidari-Kharaji M, Alavi S, et al. Transplantation of autologous fat, stromal vascular fraction (SVF) cell, and platelet-rich plasma (PRP) for cell therapy of atrophic acne scars: clinical evaluation and biometric assessment. J Cosmet Dermatol. 2022;21(5):2089–2098.
- Stachura A, Paskal W, Pawlik W, et al. The use of Adipose-Derived Stem Cells (ADSCs) and Stromal Vascular Fraction (SVF) in skin scar treatment-A systematic review of clinical studies. J Clin Med. 2021;10(16):3637.
- Draaijers LJ, Tempelman FR, Botman YA, et al. The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg. 2004;113(7):1960–1965.
- van de Kar AL, Corion LUM, Smeulders MJC, et al. Reliable and feasible evaluation of linear scars by the patient and observer scar assessment scale. Plast Reconstr Surg. 2005;116(2):514–522.
- Garg S, Dahiya N, Gupta S. Surgical scar revision: an overview. J Cutan Aesthet Surg. 2014;7(1):3–13.
- Liu W, Shi K, Zhu X, et al. Adipose tissue-derived stem cells in plastic and reconstructive surgery: a bibliometric study. Aesthetic Plast Surg. 2021;45(2):679–689.
- Ataman MG, Uysal CA, Ertas NM, et al. The effect of adipose stromal vascular fraction on transverse rectus abdominis musculocutaneous flap: an experimental study. J Plast Surg Hand Surg. 2016;50(5):272–280.
- Chae DS, Han S, Son M, et al. Stromal vascular fraction shows robust wound healing through high chemotactic and epithelialization property. Cytotherapy. 2017;19(4):543–554.
- Jeon HJ, Choi DH, Lee JH, et al. A prospective study of the efficacy of cell-assisted lipotransfer with stromal vascular fraction to correct contour deformities of the autologous reconstructed breast. Aesthetic Plast Surg. 2021;45(3):853–863.
- Laloze J, Fievet L, Desmouliere A. Adipose-derived mesenchymal stromal cells in regenerative medicine: state of play, current clinical trials, and future prospects. Adv Wound Care. 2021;10(1):24–48.
- Behrangi E, Moradi S, Ghassemi M, et al. The investigation of the efficacy and safety of stromal vascular fraction in the treatment of nanofat-treated acne scar: a randomized blinded controlled clinical trial. Stem Cell Res Ther. 2022;13(1):298.
- Lee MH, Kang BY, Wong CC, et al. A systematic review of autologous adipose-derived stromal vascular fraction (SVF) for the treatment of acute cutaneous wounds. Arch Dermatol Res. 2022;314(5):417–425.
- van Dongen JA, van Boxtel J, Uguten M, et al. Tissue stromal vascular fraction improves early scar healing: a prospective randomized multicenter clinical trial. Aesthet Surg J. 2022;42(7):NP477–NP488.
- Yastı AÇ, Akgün AE, Akın M. Use of stromal vascular fraction stem cell therapy for functional and cosmetic outcomes in a young female patient with deep dermal flame burns on the face. Burns Open. 2022;6(3):116–119.
- Koo HT, Jin US. The effect of stromal vascular fraction on scar formation of transverse rectus abdominis muscle flap donor sites: a pilot study. J Wound Manage Res. 2022;18(1):1–10.
- Roshdy OH, Abdallah WI, Farid CI, et al. Stromal vascular fraction improves the durability of autologous fat temple augmentation-A split-face randomized study using ultrasound biomicroscopy. J Plast Reconstr Aesthet Surg. 2022;75(6):1870–1877.
- Tanwar V, Pushker N, Agrawal S, et al. Autologous fat grafting for the correction of cicatricial ectropion. J Plast Reconstr Aesthet Surg. 2022;75(12):4496–4512.
- Surowiecka A. Combined therapies in scar treatment-The role of autologous derived agents in scar remodeling: a series of cases. Dermatol Ther. 2022;35(11):e15877.
- Lee JW, Park SH, Lee SJ, et al. Clinical impact of highly condensed stromal vascular fraction injection in surgical management of depressed and contracted scars. Aesthetic Plast Surg. 2018;42(6):1689–1698.