Dear Editor,
Silicone is a hydrophobic polymeric substance that has been widely used for cosmetic purposes over the last decades. Nowadays, its possible complications make its use questionable. Among them, granulomatous reactions are infrequent and usually present as late complications. These represent a major therapeutic challenge (Citation1,Citation2).
A 63-year-old woman, with no relevant personal history, presented to our outpatient clinic with a one-week facial angioedema and subcutaneous erythematous nodules located on the forehead and cheeks (). Lesions were indurated and painful, suggesting inflammation of the subcutaneous cellular tissue. Ultrasound examination of the skin revealed thickened subcutaneous cell tissue, with hyperechogenic appearance, lacking an individualizable nodular morphology.
Figure 1. (A) Initial lesions the patient presented. Facial angioedema and subcutaneous erythematous nodules located on the forehead and cheeks. (B) Histological examination. Lobar panniculitis with focally granulomatous inflammation containing coalescing ‘holes’ of nonpolarizable clear material suggestive of silicone (hematoxylin–eosin, original magnification ×200). (C) Complete clearance of skin lesions after 1 months under treatment with adalimumab. No lesions have been evidenced in the follow up for the last 26 months.
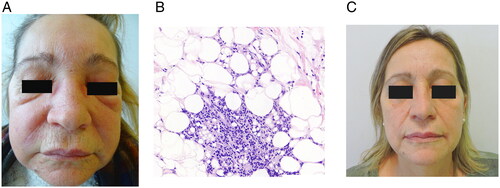
Histopathological examination of incisional biopsies from the cheeks revealed septal and lobar panniculitis with focally granulomatous inflammation and multiple variable-sized coalescing ‘holes’ containing nonpolarizable clear material suggestive of silicone (). On re-questioning the patient, she admitted having undergone a lip filler treatment 20 years ago. Facial magnetic resonance imaging evidenced multiple silicone microspheres migrated from their injection site. Subsequent surgical removal of the latter was not possible.
Initial treatment with oral prednisone at a dosage of 15 mg/day and allopurinol at 200 mg/day was started. No improvement was observed after 4 months of treatment. Intralesional triamcinolone acetonide (40 mg/ml), loratadine 10 mg/day, and hydroxychloroquine (200 mg/12 h) were added to the treatment for the next 4 months without any therapeutic response. Subsequently, allopurinol was suspended, dapsone 100 mg/day was tapered for 3 months, lacking any clinical response. Thereafter, previous therapies were suspended and treatment with adalimumab 40 mg every 2 weeks after an 80 mg loading dose was established, noticing the disappearance of the lesions after one month (). To date, the patient remains asymptomatic, without recurrence of swelling 26 months after the onset of the treatment.
Discussion
Since silicone is a permanent filler, inflammatory reactions can occur even several years after its injection (Citation2). Clinical and pathologic features of silicone granulomatous reactions (SG) are variable (Citation3). Delayed reactions usually present as subcutaneous nodules, recurrent cellulitis or solid edema (Citation3). A diffuse granulomatous reaction with variable-shaped vacuoles is typically observed. Additionally, fungal, bacterial, or mycobacterial infection should be ruled out when SG is suspected (Citation4).
Delayed granulomatous reactions to non-biodegradable fillers are often refractory to treatment. Systemic and local corticosteroids, intralesional 5-fluoruracil, allopurinol, isotretinoin, methotrexate, topical imiquimod or tacrolimus, tetracyclines, and CO2 laser have been described successful in scarce cases. Surgical removal is often problematic taking into account the migratory nature of liquid silicone (Citation3).
Pathogenesis of SG formation remains unclear. Immunologic cross-reaction with T-cell and macrophage activation with release of cytokines, such as interferon gamma and tumor necrosis factor alpha (TNF-α) have been postulated as the most likely pathway. The latter could be triggered by a granulomatous response to secondary-site events like infections or trauma (Citation5,Citation6).
Adalimumab is an IgG1 monoclonal antibody whose origin is completely human, which binds to both soluble and transmembrane TNF-α. It has demonstrated efficacy and safety in the treatment of other granulomatous skin diseases (Citation7). Nevertheless, the role of anti-TNF drugs in the treatment of SG remains barely understood. Regarding the literature, etanercept has been shown to be effective in two cases of factitial panniculitis (Citation8).
Herein, we report a case of granulomatous foreign body reaction to cosmetic filler treated with adalimumab with complete clinical response maintained over time, fact, to our knowledge, previously unreported in the literature. Both the initial rapid response and the favorable and maintained outcome were analogous to those of the best therapeutic options reported. The present response suggests the need for further research of the role of adalimumab and TNF inhibitors in the management of this entity. Consequently, anti-TNF therapy could be an interesting option for its management.
Author contributions
Contributed significantly to this publication: Francisco Javier de la Torre Gomar, Javier Gimeno Castillo, Pau Rosés Gibert, Erika Iglesias Martínez, and Maria Isabel Martínez González.
Consent form
The authors confirm written informed consent from the patients for the use of image and publication of their case details has been given, regarding their privacy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings are available from the corresponding author, JGC, upon reasonable request.
Additional information
Funding
References
- Kim H, Cho SH, Lee JD, et al. Delayed onset filler complication: two case reports and literature review. Dermatol Ther. 2017;30(5):e12513.
- Pérez-Ruiz C, Barabash-Neila R, Zulueta-Dorado T, et al. Adverse granulomatous reaction to silicone filler treated with methotrexate. Dermatol Surg. 2019;45(3):489–492.
- Broly M, Marie J, Picard C, et al. Management of granulomatous foreign body reaction to fillers with methotrexate. J Eur Acad Dermatol Venereol. 2020;34(4):817–820.
- Emer J, Roberts D, Levy L, et al. Indurated plaques and nodules on the buttocks of a young healthy female. J Clin Aesthet Dermatol. 2013;6(3):46–49.
- Lee SK, Kim SM, Cho SH, et al. Adverse reactions to injectable soft tissue fillers: memorable cases and their clinico-pathological overview. J Cosmet Laser Ther. 2015;17(2):102–108.
- Alijotas-Reig J, Fernández-Figueras MT, Puig L. Inflammatory, immune-mediated adverse reactions related to soft tissue dermal fillers. Semin Arthritis Rheum. 2013;43(2):241–258.
- Hong JJ, Hadeler EK, Mosca ML, et al. Off-label uses of TNF-a inhibitors and IL-12/23 inhibitors in dermatology. Dermatol Online J. 2022;27(11).
- Pasternack FR, Fox LP, Engler DE. Silicone granulomas treated with etanercept. Arch Dermatol. 2005;141:3.

