Dear Editor,
The use of intravenous ertapenem is included in the HS ALLIANCE working group review as a treatment option in selected severe patients, but its use is at the discretion of the treating physician (evidence level 4, grade of recommendation C) (Citation1). It is essential to determine which severely affected hidradenitis supurativa (HS) patients would benefit, when would be the ideal time for its administration given the risk of randomly using a broad-spectrum antibiotic like ertapenem (Citation2), and to assess a more convenient route of administration.
A multicenter retrospective cohort study was performed in 3 hospitals in the south of Spain (Andalusia) to evaluate the effectiveness and safety of treatment with ertapenem in patients with moderate to severe HS in the period between January 2017 and December 2022. Disease severity was assessed at baseline and follow-up at 6 weeks (end of treatment) and 12/16 weeks, using the International Hidradenitis Suppurativa Severity Score System (IHS4) and pain visual analogue scale (VAS) score. The Wilcoxon rank test was used to evaluate paired differences with respect to the baseline assessment, establishing the level of statistical significance at p < 0.05.
Fifteen patients with moderate to severe HS were included (20% Hurley II and 80% Hurley III). All included patients were male with a median age of 51 years. 40% of the patients had a family history of HS and 86.7% were smokers. Median body mass index was 30.1, median time of disease evolution was 19 years and median number of affected body areas was 5. The administered dose of ertapenem was 1 g for 6 weeks and the route was 60% intramuscular, 33.3% intravenous and 6.7% mixed. All patients except one had previously received biological treatment, mainly adalimumab (14/15). More than half of the patients (8/15) had undergone two or three biologics prior to treatment with ertapenem. The median IHS4 dropped from 28 to 18 (p = 0.008) and VAS from 9 to 2 (p = 0.004) after 6 weeks of ertapenem (). No patient discontinued treatment and no adverse effects were recorded. No patient underwent combined surgery. After treatment with ertapenem most patients (11/15) started a new biological treatment and the other 4 patients underwent antibiotic consolidation treatment with rifampicin/moxifloxacin/metronidazole combination. Between 12 and 16 weeks from baseline, these parameters were collected again: median IHS4 16 (p = 0.001) and median VAS 6.5 (p = 0.003), which shows a certain maintenance of the therapeutic response. On the other hand, we observed a better effectiveness for intramuscular vs. intravenous ertapenem (). Our sample size is limited but intramuscular administration appears to be at least as effective as intravenous with evidente advantages and convenience for the patient and a lower direct health related costs.
Figure 1. Box plot showing differences between basal time and 6 weeks of ertapenem (end of treatment). (A) Median IHS4 dropped from 28 to 18 (p = 0.008). (B) Median VAS changed from 9 to 2 (p = 0.004).
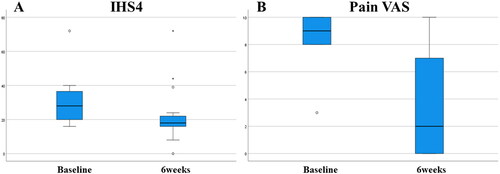
Figure 2. Box plot showing differences in favor of the intramuscular route in IHS4 and VAS. (A) Median IHS 4 dropped from 33 to 16 at 6 weeks (p = 0.018) and remained at 12/16 weeks (p = 0.011). (B) Median VAS changed from 10 to 0 at 6 weeks (p = 0.0014) and subsequently increased to 2 at 12/16 weeks (p = 0.020).
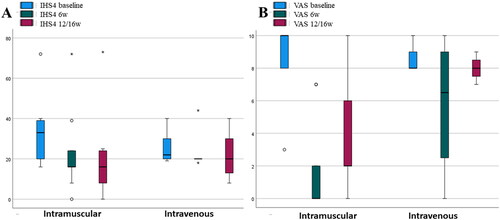
Parenteral administration of ertapenem to patients with HS may be a valid treatment option for seeking rapid improvement in cutaneous inflammation. Join-Lambert et al. reported a significant improvement in pain and drainage in patients treated with the daily administration of 1 g intravenous ertapenem (Citation3,Citation4). The efficacy of ertapenem is probably related to its broad spectrum of activity against aerobic and anaerobic bacteria associated with the microbiome of the HS patients although it also has immunomodulatory effects that have not yet been well determined (Citation5). The experience with our patients has been a significant decrease in the IHS4 and VAS score. As a novelty, the main route of drug administration was intramuscular, which has not been described to our knowledge to date in this type of patient.
Ertapenem could be reserved for severe patients with widespread disease who are not candidates for surgical treatment and as a cooling bridge therapy after biological treatment failure. Given the high bioavailability of intramuscular ertapenem (Citation6), its use should be encouraged for greater convenience and to avoid complications associated with intravenous use.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings are available from the corresponding author, APC, upon reasonable request.
Additional information
Funding
References
- Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization –systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019;33(1):19–31.
- Mendes-Bastos P, Martorell A, Magina S. Ertapenem for the treatment of hidradenitis suppurativa: how much do we need it? Actas Dermosifiliogr. 2018;109(7):582–583.
- Join-Lambert O, Coignard-Biehler H, Jais JP, et al. Efficacy of ertapenem in severe hidradenitis suppurativa: a pilot study in a cohort of 30 consecutive patients. J Antimicrob Chemother. 2016;71(2):513–520.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian hidradenitis suppurativa foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81(1):91–101.
- Braunberger TL, Nartker NT, Nicholson CL, et al. Ertapenem - a potent treatment for clinical and quality of life improvement in patients with hidradenitis suppurativa. Int J Dermatol. 2018;57(9):1088–1093.
- Musson DG, Majumdar A, Birk K, et al. Pharmacokinetics of intramuscularly administered ertapenem. Antimicrob Agents Chemother. 2003;47(5):1732–1735.

