Abstract
Background
Platelet-rich plasma (PRP) is an adjunctive treatment in androgenetic alopecia (AGA). Its role as a monotherapy, when compared to FDA-approved therapies in moderate grades of androgenetic alopecia is not established.
Objectives
We sought to study the efficacy and safety of standardized non-activated PRP preparation against topical minoxidil in AGA.
Methods
Men aged 20–50 with Grade III and IV (Modified Hamilton-Norwood) AGA were randomized to receive 5% Minoxidil (×6 months) or PRP injections (monthly ×3). The primary endpoints were global photographic assessment at week 24, change in target area hair counts, density, and anagen hair at week 12. Other outcomes were subjects’ satisfaction and adverse events.
Results
In total, 64 participants were randomized. At week 24, 56% responded to Minoxidil arm and 38% to PRP (p = 0.124). There was a significant increase in target area hair count and density at week 12 within the groups. The difference between the groups was not statistically significant. Adverse events occurred in 53% and 37% of the PRP and minoxidil groups, respectively. Patient satisfaction was better with Minoxidil.
Conclusion
PRP is effective in the treatment of moderate grades of androgenetic alopecia in men, although perhaps not different from minoxidil. Side effects occur more frequently with PRP.
Introduction
Androgenetic alopecia (AGA) is the most common alopecia, affecting up to 80% of men during their lifetime. The current standard of treatment for AGA in males includes oral finasteride and topical minoxidil. Unfortunately, current therapies are not effective for all subjects with AGA. On the one hand, patients with AGA need medications indefinitely but adhere poorly to them. Autologous platelet-rich plasma therapy has captured attention as a safe and easy method for treating AGA associated with limited side effects. Growth factors in PRP promote hair regrowth by inducing the proliferative phase of the hair follicle.
The effectiveness of PRP treatment for AGA has been summarized in recent reviews and meta-analyses (Citation1–4). It effectively increases hair density and hair thickness in AGA. The outcome assessments were not objective and the efficacy of PRP was compared against a placebo in most of the previous trials. The combination of PRP with minoxidil has resulted in increased efficacy of treatment. Studies have shown it to be less effective in advanced stages of AGA, where surgical options are considered with maintenance using medical therapy. would be the only solution. As an upcoming treatment option, many opportunities exist for future research. There remains a need for clinical trials performing an evaluator-blinded, direct comparison between FDA-approved therapies and the PRP treatment in moderate grades of AGA. Such a direct comparison may help elucidate the actual role of PRP in managing AGA.
We thus aimed to study the efficacy of a standardized non-activated PRP preparation against topical minoxidil in treating males with moderate grades of AGA. The primary objective was to compare the proportion of responders, the changes in hair count, hair density, and anagen proportion. Secondary objectives included patient satisfaction score, safety, and tolerance.
Materials and methods
This was a randomized, open-label, outcome assessor-blinded, a 24-week trial conducted at a tertiary care centre in south India. The trial was registered in the clinical trial registry of India before commencement (CTRI/2020/05/025389). The study had Institutional ethics approval (JIP/IEC/2019/465), and the participants provided written informed consent before enrollment.
Study participants
Eligible participants were men 20 to 50 years with androgenetic alopecia classified as type III, III vertex, or IV using the Modified Hamilton-Norwood classification. Two dermatologists confirmed the diagnosis of AGA on the basis of clinical and trichoscopy features (more than 20% variability in hair diameter between affected and uninvolved areas). Subjects had to maintain the same hairstyle and colour for the study duration. Exclusion criteria were subjects with known platelet disorders, known hypersensitivity to minoxidil, antiplatelet and anticoagulant therapy, and any form of medical/surgical treatment for AGA for the last 6 months. Additionally, those with other disorders of the scalp, a tendency to form keloid, uncompensated diabetes mellitus, acute infections, cancer, immunosuppression, and allergy to anaesthetics were excluded from the study. Patients were randomized to PRP or Minoxidil arm using block randomization (block size of 4 and 6) with a computer-generated randomization table. Allocations were sealed in opaque envelopes. Randomization and allocation concealment was performed by a physician not involved in the trial.
PRP preparation
Our institution’s PRP preparation protocol is standardized and has consistently resulted in a platelet yield of 3–5 times the baseline value. The platelet concentration in the PRP ranged from 9 to 10.2 lakhs/µL. The yield is a pure PRP, i.e. the percentage of platelets in the PRP compared with RBC and leucocytes ranges from 70 to 90%. We collected whole blood (19 ml) and transferred it to 2 Acid citrate dextrose-containing vacutainer tubes, 8.5 ml each. The sample was centrifuged at 400 g for 10 min at room temperature. At the end of the first spin, we transferred the upper and intermediate layers to plain 8.5 ml tubes, without disturbing the bottom layer of RBCs. We performed the second centrifugation at 900 g for 10 min to concentrate the platelets and WBCs in the PRP. At the end of the second spin, we discarded two-thirds of the supernatant and gently suspended the lower third (PRP). At the end, we obtained almost 2 ml of PRP for injection.
Treatment arms
Minoxidil-treated arm received topical minoxidil 5% alone 1 ml twice daily for six months. This was a supervised application at inclusion and at each follow-up visit. The subjects produced empty containers at each follow-up visit to ensure compliance. Subjects used diaries or phones (as convenient) to record any adverse event or missed application. In the PRP-treated arm, the non-activated PRP was injected at a depth of 3–4mm, delivering 0.1–0.2 ml per injection, approximately 1 cm apart in the interfollicular areas. The area planned for injection was anaesthetized using topical anaesthetic cream available as a Eutectic mixture before the procedure at a maximal dose of 20 g/200cm2. Subjects were advised not to wash their hair for four hours following treatment. There were three treatment sessions at monthly intervals with a final follow-up visit three months after the last treatment.
Assessments
The primary outcome measure was a panel (two dermatologists blinded to allocation and treatment arms) global photographic assessment of improvement at the vertex and frontal views (from baseline to week 24). This was graded on a 7-point scale as dense, moderate or minimal growth, no change, and minimal, moderate or dense loss. The first three grades were considered ‘responders’ and the last four ‘non-responders’ (4). The other primary endpoints were change from baseline in total and terminal hair count, total hair density and proportion of anagen hairs within a 2-cm diameter target minizone delineated using a semi-permanent tattoo at the vertex at 12 weeks, using phototrichogram (Dermlite DL4) (Citation5). Secondary endpoints included: Subjects’ satisfaction with hair density and texture on a scale of 1–10 (week 12) and adverse events to therapy.
Statistical analysis
A sample size of 29 was obtained in each group using mean change in terminal hair count as 18.6 ± 25.4 with minoxidil (Olsen et al. (Citation6)) and 33.6 ± 12.8 with PRP (Gentile et al. (Citation7)) using sample size calculation for mean difference. With a drop-out of 20%, 35 subjects in each group would be needed at randomization, providing 80% power and 95% confidence interval. This sample size of 70 in total provided 80% power and 95% confidence level to demonstrate greater responders among PRP-treated arm using global photography by 30% than Minoxidil arm.
A statistician blinded to the intervention performed the intent-to-treat analysis of all randomized subjects. Chi-square test was used to compare the proportion of responders using global photography between the two treatment groups at 24 weeks. The change in hair counts hair density, and change in anagen hairs at 12 weeks within the individual groups were analyzed using paired sample t-test, and between-group comparisons for the parameters were done using independent t-test or Mann-Whitney test based on the normality of the data. Analysis was carried out at 5% level of significance and p < 0.05 was considered statistically significant.
Results
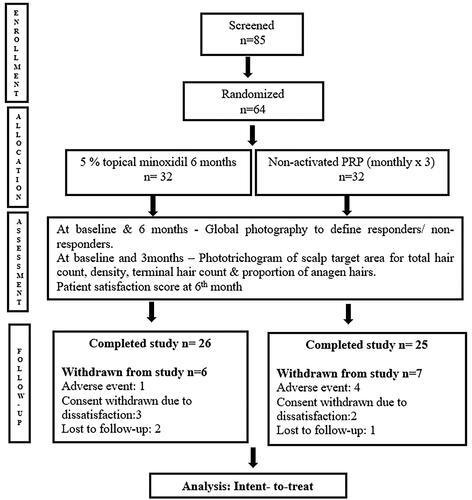
shows subject disposition during the study period from January 2020 to February 2022. The intent-to-treat population contained 64 participants of which 51 completed the study. Subject demographics and baseline characteristics were similar across treatment groups ().
Table 1. Baseline parameters of treatment groups.
Global photographic assessment
There was a fair to moderate agreement on the assignment of scores for Global photographic assessments at week 24 by 2 dermatologists (kappa coefficients ranging from 0.39 to 0.61, highest for observing no change in hair improvement). The proportion of subjects who responded as per the median of 2 dermatologist scores of Global photographic assessments at week 24 was 56% for Minoxidil and 38% for PRP (p = 0.124) ( and ). The proportion of subjects with any improvement in panel assessment scores at week 24 was higher for PRP group than Minoxidil group (). The global photographic assessment correlated positively with the patient satisfaction score for hair density at the end of treatment (R 0.824, p < 0.00001).
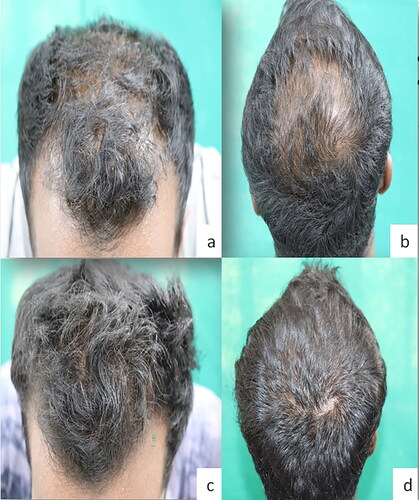
Figure 2. Baseline (a,b) and week 24 (c,d) global photographs of a responder with AGA in Minoxidil group.
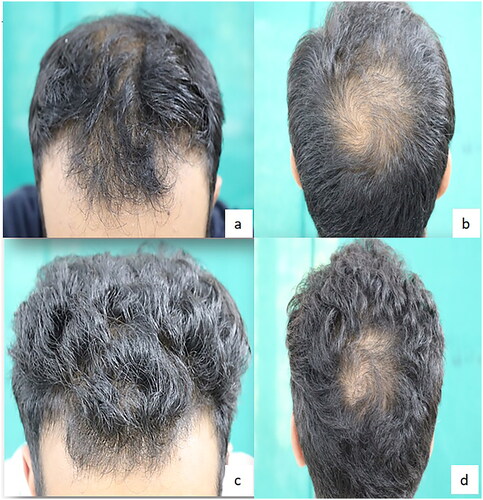
Figure 4. Mean change from baseline in target area total hair count, density and terminal hair count at week 12 for minoxidil and PRP groups.
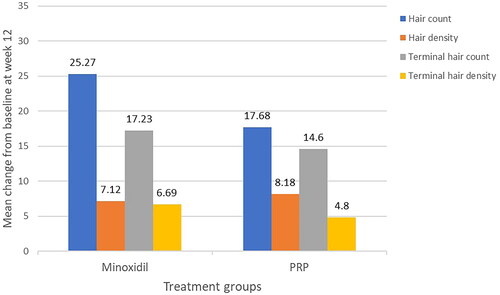
Table 2. Panel global photographic assessment at week 24 in hair growth across the treatment groups.
Phototrichogram parameters in the scalp target area (2 cm)
The increase in basal hair count, hair density, terminal hair count and density was significant in the Minoxidil group at week 12 (all p < 0.001). These parameters were also significant in the PRP group at week 12 compared to baseline (p = 0.014, 0.001, 0.029, and 0.046 respectively) (). On comparing the treatment groups, there was no statistical difference in the propensity to increase total hair count, terminal hair count and density at week 12 (). However, minoxidil seemed superior to PRP (p = 0.407, 0.763 and 0.509 respectively). PRP increased the total hair density at week 12 better than minoxidil, though not significantly (p = 0.713).
The proportion of anagen hairs at baseline and week 12 in Minoxidil group was 0.87 ± 0.07 and 0.89 ± 0.04, respectively; in the PRP group, 0.86 ± 0.04 and 0.87 ± 0.04, respectively. There was no significant increase in anagen proportion through 3 months within the groups (p = 0.94 and p = 0.19).
Patient satisfaction score
The median patient satisfaction score for hair texture at week 24 was better for Minoxidil than PRP (p = 0.029), but not for hair density (p = 0.123).
Adverse events
The overall incidence of adverse events was similar between treatment groups, 37% in Minoxidil arm and 53% in PRP arm (p = 0.21). Most of the adverse events were mild in Minoxidil arm. The common side effects were mild headache (n = 4) and scalp pruritus (n = 4). Three of the subjects experienced dryness of the scalp following minoxidil use. One of them experienced allergic contact dermatitis to the vehicle, presenting as erythema and a burning sensation over the hairline after one week of use. The episode resolved with topical steroid application in a week. He withdrew from the study, despite the advice to change the formulation. The pain was the most common side effect (n = 17) associated with PRP injection. This usually lasted for up to 4–5 h post-procedure. Five of them needed a painkiller for relief, while the remaining could tolerate it. Four patients refused further sessions after the second sitting, as they could not tolerate the pain post-injection. All of them presented for assessment at week 12.
Discussion
The results of the study suggest that PRP is effective in treating moderate grades of androgenetic alopecia in men, although perhaps not different in efficacy from minoxidil. Both treatments are well tolerated; however, side effects occur more frequently with PRP. Patient satisfaction is better with Minoxidil therapy.
There were four other studies resembling ours where the researchers compared the efficacy of PRP as monotherapy with the standard of care, Minoxidil. The results of our study are comparable with three of them, where PRP failed to demonstrate superior efficacy over minoxidil in AGA. Bruce et al. reported PRP as an effective option in the treatment of AGA, but not better than minoxidil based on quantitative parameters. In their study, quality of life responses was better in the PRP arm than minoxidil (Citation8). Farid et al.’s study reported a faster and superior mean rise in hair count after treatment with minoxidil than the combinational PRP and micro-needling group (16 hairs vs 5 hairs/cm2) (Citation9). The investigator-rated assessment in the study showed a greater improvement with minoxidil than with PRP (65 vs. 45%, respectively). In the study by Singh SK et al. the efficacy of four treatment arms, a combination of PRP and minoxidil, PRP alone, and topical minoxidil alone versus placebo saline injection were compared in moderate grades of AGA using trichoscopic hair density assessment and patient perception. The authors observed increased hair density and patient satisfaction better with the combined PRP/minoxidil arm. There was no significant difference between PRP and topical minoxidil alone groups (Citation10).
The findings by Verma et al. are contrary to the results of this study and the above. Accordingly, four sessions of PRP showed a superior benefit in hair growth than six months of minoxidil use as per global photography, hair pull test, and standardized hair growth questionnaire. The study, however, had a significant number of patients belonging to milder disease i.e. Grade 2 disease and did not employ objective ways to study the outcome (Citation11).
A study evaluating the efficacy of PRP versus topical minoxidil 5% in the treatment of alopecia areata by clinical and trichoscopic evaluation, showed both have significant hair growth than placebo at the end of 3 months of treatment. Patients treated with PRP had an earlier response in the form of hair regrowth, reduction in short vellus hair and dystrophic hair. PRP was effective in treatment of patchy alopecia areata better than the generalized types (Citation12).
The side effects of PRP are generally mild and pain is the most common event reported (Citation13). Rarely, it is a reason for therapy discontinuation. The subdermal injection technique of PRP is associated with less pain and could be considered for future use (Citation14). The proportion of those who reported adverse events to therapy in the study, however, does not include those who lost to follow-up due to therapy dissatisfaction or poor adherence.
PRP preparation employed standardized parameters in this study that yielded good platelet concentration. Considering the published literature on the success of PRP in hair growth in less severe grades of AGA, the choice of subjects in this study is appropriate (Citation15). The number and frequency of injections of PRP used in this study were as per literature, where three months is the time point of maximal benefit (Citation16). Our outcome assessments included investigator-based, patient-based as well as quantitative parameters. Our study’s results are reliable, showing that both PRP and topical minoxidil are effective in therapy for AGA; however, PRP is not superior to minoxidil in treating men with moderate grades of AGA.
We could have performed phototrichogram assessment at six months for the subjects in this study. Patients considered this inconvenient because of visits on day 0 and day 2 for the procedure. Longer follow-up post-procedure may be considered in future, as non-activated PRP allows a sustained long-term response compared to activated PRP.
Ethics approval
The study was approved by the Institutional scientific and ethics committee (JIP/IEC/2019/465) on 11-02-2020.
Patient consent
The researchers obtained patient consent for scientific publication.
Author contributions
Mithinkumar was involved in protocol drafting, running the treatment, and assisting in drafting the manuscript. Rashmi Kumari screened subjects of AGA for recruitment and provided inputs for the protocol and final draft. Sivaranjini was involved in protocol refining, screened subjects for recruitment, lead the draft of the manuscript and overall supervised. Dermatosurgery fellow in the department handled the random table and allocation. Two other experienced dermatologists in the department, blinded to the allocation, did the outcome assessment. A blinded statistician in the institution did data analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author [SR], upon reasonable request.
Additional information
Funding
References
- Evans AG, Mwangi JM, Pope RW, et al. Platelet-rich plasma as a therapy for androgenic alopecia: a systematic review and meta-analysis. J Dermatolog Treat. 2022;33(1):498–511.
- Roohaninasab M, Goodarzi A, Ghassemi M, et al. N. Systematic review of platelet-rich plasma in treating alopecia: focusing on efficacy, safety, and therapeutic durability. Dermatol Ther. 2021;34(2):e14768.
- Gentile P, Garcovich S. Systematic review of platelet-rich plasma use in androgenetic alopecia compared with minoxidil®, finasteride®, and adult stem cell-based therapy. IJMS. 2020;21(8):2702.
- Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42(4):491–497.
- Reygagne P. Phototrichogram. In: humbert P, Fanian F, Maibach HI, Agache P, editors. Agache’s measuring the skin: non-invasive investigations, physiology. Normal Constants. Cham: Springer International Publishing; 2017. p. 813–825.
- Olsen EA, Dunlap FE, Funicella T, et al. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2002;47(3):377–385.
- Gentile P, Garcovich S, Bielli A, et al. The effect of platelet-rich plasma in hair regrowth: a randomized placebo-controlled trial. Stem Cells Transl Med. 2015;4(11):1317–1323.
- Bruce AJ, Pincelli TP, Heckman MG, et al. A randomized, controlled pilot trial comparing platelet-rich plasma to topical minoxidil foam for treatment of androgenic alopecia in women. Dermatol Surg. 2020;46(6):826–832.
- Farid CI, Abdelmaksoud RA. Platelet-rich plasma microneedling versus 5% topical minoxidil in the treatment of patterned hair loss. J. Egypt Women's Dermatologic Soc. . 2016;13:29–36.
- Singh S, Kumar V, Rai T. Comparison of efficacy of platelet-rich plasma therapy with or without topical 5% minoxidil in male-type baldness: a randomized, double-blind placebo control trial. Indian J Dermatol Venereol Leprol. 2020;86(2):150.
- Verma K, Tegta G, Verma G, et al. A study to compare the efficacy of platelet-rich plasma and minoxidil therapy for the treatment of androgenetic alopecia. Int J Trichol. 2019;11(2):68.
- El Taieb MA, Ibrahim H, Nada EA, et al. Platelets rich plasma versus minoxidil 5% in treatment of alopecia areata: a trichoscopic evaluation. Dermatol Ther. 2017;30(1):e12437.
- Mao G, Zhang G, Fan W. Platelet-rich plasma for treating androgenic alopecia: a systematic review. Aesth Plast Surg. 2019;43(5):1326–1336.
- Hausauer AK, Jones DH. Evaluating the efficacy of different platelet-rich plasma regimens for management of androgenetic alopecia: a single-center, blinded, randomized clinical trial. Dermatol Surg. 2018;44(9):1191–1200.
- Gupta AK, Carviel JL. Meta-analysis of efficacy of platelet-rich plasma therapy for androgenetic alopecia. J Dermatolog Treat. 2017;28(1):55–58.
- Gkini M-A, Kouskoukis A-E, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7(4):213–219.