Abstract
Purpose: The overlap of psoriasis and atopic dermatitis (AD) is rare and treating moderate-to-severe cases can be challenging. Conventional immune-suppressive drugs cannot be used long-term, and no biological drugs are currently approved for treating both conditions.
Method: We report the cases of four patients with overlapping features of both psoriasis and AD.
Result: After being treated with several systemic drugs, including gold-standard treatments for both psoriasis and AD, they received upadacitinib 15 or 30 mg, achieving complete remission. Upadacitinib is an inhibitor of Janus Kinase 1, currently approved for treating moderate-to-severe AD.
Conclusion: To date, very limited data are available regarding the efficacy of upadacitinib in psoriasis. In a phase-3 trial on the efficacy of upadacitinib 15 mg in patients affected by psoriatic arthritis, 52.3% of patients achieved a 75% improvement in Psoriasis Area and Severity Index (PASI75) after one year. Currently, no clinical trials are evaluating the efficacy of upadacitinib in plaque psoriasis.
Keywords:
Introduction
Psoriasis and atopic dermatitis (AD) are the two most common inflammatory skin diseases (Citation1). Despite being considered two opposite diseases, it is now well known that they can coexist in the same individual (Citation2). Diagnosing this peculiar clinical picture is complex and treating moderate-to-severe cases can be challenging.
Upadacitinib is a selective JAK-inhibitor, which predominantly targets JAK1 [3], approved for treating moderate-to-severe AD in adults and adolescents across two different dosages (15 and 30 mg) after being evaluated in multiple Phase-3 trials (Citation3). Upadacitinib has also been approved for treating psoriatic arthritis in patients with an inadequate response and/or intolerance to anti-TNF medications (Citation4).
We report four cases of patients affected by plaque psoriasis and/or psoriatic arthritis who received upadacitinib because of concomitant AD, achieving excellent clinical responses.
Report
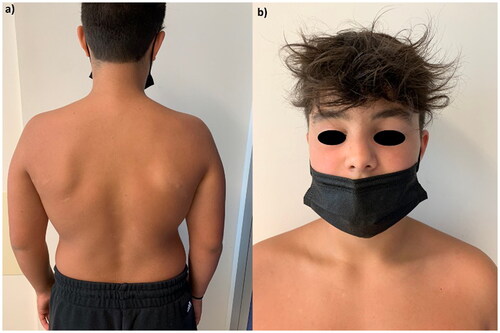
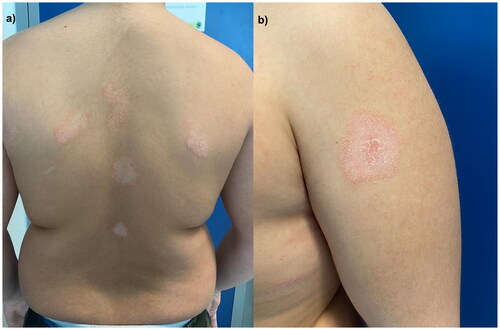
The first patient is a 12-year-old boy affected by psoriasis since early childhood involving the trunk, upper limbs, eyelids () and genitals () with a Psoriasis Area and Severity Index (PASI) of 10. The patient and his parents reported a severe impact of the cutaneous disease on his quality of life as Dermatology Life Quality Index (DLQI) was 16. After receiving ustekinumab 45 mg for 18 months, at the clinical examination, we observed erythematous and scaly patches on the back (), left shoulder (), with persistent psoriatic onychopathy and genital psoriasis. Given the unsatisfying response to ustekinumab, as the clinical picture was inconclusive for psoriasis, we interrupted the treatment and prescribed a biopsy of one back’s patch. Histopathology revealed psoriasiform hyperplasia with focal spongiosis, parakeratotic hyperkeratosis and small scales with a perivascular lymphocytic infiltrate. We performed tape stripping on a trunk lesion and subjected the strips to RNA sequencing to identify biomarkers of psoriasis and/or AD (Citation5). We observed high expression of both Th2– and Th17-related products, along with nitric oxide synthase2/inducible nitric oxide synthase (NOS2/iNOS). Subsequently, given the predominance of AD lesions with an Eczema Area and Severity Index (EASI) of 12 and an itch-NRS (Numerical Rating Scale) of 8/10, we started dupilumab 300 mg. The patient returned after four months, showing improvement in AD but experiencing a psoriasis relapse on nails and soles. His DLQI was again > 10. Hence, we discontinued dupilumab and prescribed upadacitinib 15 mg/day. After 16 weeks, the patient experienced complete remission of both diseases ().
Figure 1: Psoriasis involving the face () and genitals () of a 12-year-old boy before the start of ustekinumab.
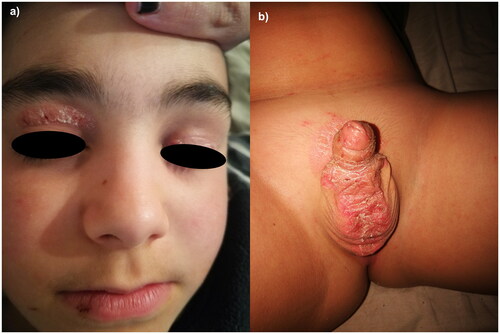
Figure 2. Erythematous follicular patches on the back () and left shoulder () after 18 months of treatment with ustekinumab.
The second patient is a 39-year-old man diagnosed with psoriasis since childhood with an anamnesis of allergies to several inhalants. He had erythematous plaques and patches on both elbows and palms with severe onychopathy. After failing cyclosporin, he received brodalumab. After six months, he showed persistence of the onychopathy and palmar psoriasis and an eczematous patch on the suprapubic area. As the clinical picture was suspicious for an overlap of psoriasis and AD, we performed two biopsies on the lesions of the palms and pubic area. The histological examination of the pubic region showed ortho-parakeratosis with psoriasiform epidermis hyperplasia, while the histopathology of the right palm showed focal spongiosis and micro-pustulation. At the following visit, we observed the spreading of eczematous patches on both legs with the persistence of palmar psoriasis. As the patient referred an itch-NRS of 9/10 and his quality of life was severely affected (DLQI 15), we prescribed upadacitinib 15 mg/day. After 16 weeks, the patient achieved complete skin clearance.
The third patient is a 50-year-old woman affected by psoriatic spondyloarthropathy and palmar psoriasis, previously treated with salazopyrin, methotrexate, ustekinumab, secukinumab and apremilast, unsuccessfully. She presented with palmoplantar hyperkeratosis and some fissures on the palms/soles and fingers. Histopathology of a right sole’s biopsy showed ortho-parakeratosis, epidermal hyperplasia and concomitant spongiosis with a mixed dermal infiltrate. The patient reported an itch-NRS of 10/10 and a pain-VAS (Visual Analogue Scale) of 80/100. Patch tests revealed a delayed hypersensitivity for carbamates. In accordance with the Rheumatologist, we prescribed upadacitinib 15 mg/day. After four weeks, the patient reported a subjective improvement in symptoms and a reduction of hyperkeratosis.
Our fourth patient is a 42-year-old woman diagnosed with palmoplantar psoriasis in 2015, previously treated with acitretin, methotrexate, adalimumab and risankizumab unsuccessfully. She came to our attention showing erythematous plaques with scales-crusts on the soles and lower limbs. She also presented eczematous patches on the back. After a skin biopsy of the right leg showed irregular acanthosis, mild hyperkeratosis, focal parakeratosis and spongiosis, given the evidence of chronic spongiotic and superficial perivascular dermatitis, she started dupilumab. Four months later, as her plantar psoriasis worsened, we stopped dupilumab and started upadacitinib 30 mg/day. The patient achieved complete skin clearance after 16 weeks with a resolution of itch.
All four patients are still on treatment without experiencing any adverse events, and they have completed at least 32 weeks of follow-up ().
Table 1. Characteristics of our four patients with concomitant psoriasis and atopic dermatitis receiving upadacitinib.
Discussion
The overlap of AD and psoriasis is rare. The pathogenesis of plaque psoriasis is primarily due to a Th17 inflammation, while Type 2 inflammation is predominant in AD (Citation6). Multiple skin biopsies can be required to differentiate AD from psoriasis (Citation1,Citation2). He H. et al. (Citation5) proposed using tape strips to distinguish different immune profiles in psoriasis and AD. The high expression of NOS2/iNOS, IL-17 A/F and interferon-γ is specific for psoriasis while, in AD lesions, a preferential expression of Th2-related cytokines (IL-13) is observed (Citation5). The analysis of tape strips in our first patient confirmed the overlap of the two diseases, identifying the high expression of cytokines of both psoriasis and AD (Citation5).
Regarding the clinical presentation, it has been reported that most patients with overlapping psoriasis and AD show involvement of palms and soles, usually recalcitrant to topical therapies1. All our patients had severe involvement of palms and soles. Regarding systemic treatment, conventional immunosuppressants (cyclosporine, methotrexate) cannot be prescribed long-term because of safety concerns. All biologics approved for psoriasis did not show high efficacy in AD trials (Citation1), while dupilumab is ineffective on psoriasis (Citation7,Citation8). The combination of biologics has yet to be fully investigated, and it could present an unsustainable cost for the healthcare systems (Citation8). Upadacitinib has demonstrated efficacy in clinical trials and real-life studies in patients with AD (Citation3,Citation9,Citation10). Phase-3 clinical trials are ongoing for patients affected by psoriatic arthritis: in the SELECT-PsA 2 study, skin outcomes were also evaluated with 52.3/40.8/26.9% of patients achieving 75/90/100% improvement in PASI at week 56 with upadacitinib 15 mg (Citation4). Currently, no data are available from clinical trials regarding the role of upadacitinib in cutaneous psoriasis.
We presented four cases of patients with overlapping features of psoriasis and AD. Despite the failure of several biologic drugs, including gold-standard therapies for psoriasis and AD, our patients achieved complete remission of both diseases with upadacitinib. Upadacitinib in our patients did not show new safety findings compared with published data. However, our experience is limited, and the follow-up is short. Further reports and longitudinal and retrospective studies are needed to assess the exact role of upadacitinib in managing psoriasis.
Ethical approval
Institutional review board approval was exempted as the study protocol did not deviate from standard clinical practice. All patients received upadacitinib as in good clinical practice, in accordance with European guidelines. All included patients had provided written consent for retrospective study of data collected during routine clinical practice (demographics, clinical scores). The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Data collection and handling complied with applicable laws, regulations, and guidance regarding patient protection, including patient privacy.
Disclosure statement
L. Gargiulo has received research grants from Almirall. A. Narcisi has served on advisory boards, received honoraria for lectures and research grants from Almirall, Abbvie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi‐Genzyme, Amgen and Boehringer Ingelheim. A. Costanzo has served as an advisory board member, consultant and has received fees and speaker’s honoraria or has participated in clinical trials for Abbvie, Almirall, Biogen, LEO Pharma, Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB-Pharma. The other authors have nothing to declare. No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Barry K, Zancanaro P, Casseres R, et al. Concomitant atopic dermatitis and psoriasis - a retrospective review. J Dermatolog Treat. 2021;32(7):716–720.
- Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68–73.
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157(9):1047–1055.
- Mease PJ, Lertratanakul A, Papp KA, et al. Upadacitinib in patients with psoriatic arthritis and inadequate response to biologics: 56-week data from the randomized controlled phase 3 SELECT-PsA 2 study. Rheumatol Ther. 2021;8(2):903–919.
- He H, Bissonnette R, Wu J, et al. Tape strips detect distinct immune and barrier profiles in atopic dermatitis and psoriasis. J Allergy Clin Immunol. 2021;147(1):199–212.
- Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139(4S):S65–S76.
- Guttman-Yassky E, Krueger JG, Lebwohl MG. Systemic immune mechanisms in atopic dermatitis and psoriasis with implications for treatment. Exp Dermatol. 2018;27(4):409–417. Epub 2017 May 9. PMID: 28266782.
- Barry K, Zancanaro P, Casseres R, et al. A retrospective review of dupilumab and psoriasis biologic combination therapy. J Dermatolog Treat. 2021;32(4):438–439.
- Chiricozzi A, Gori N, Narcisi A, ACCURATE Group, et al. Effectiveness and safety of upadacitinib in the treatment of moderate-severe atopic dermatitis: a multicentric, prospective, real-world, cohort study. Drugs R D. 2022;22(3):245–252.
- Gargiulo L, Ibba L, Cortese A, et al. Real-life effectiveness and safety of upadacitinib in adults and adolescents with moderate-to-severe atopic dermatitis: a single-center 16-week study. Dermatol Ther. 2023;13(2):651–660.