Dear Editor,
Onychogryphosis is a form of nail deformity characterized by a distorted, yellowish-brown thickened nail plate that is skewed, grossly thickened, and partly curved like a ram’s horn. Onychogryphosis can be triggered by trauma, ichthyosis, psoriasis, the nail bed, and bony deformities like hallux valgus, and fungal infections (Citation1). The exact pathogenesis of the disease is not known very well but there are certain explanations. One of the possible reasons for the condition may be the deficiency of the nail matrix under the posterior fold to enforce a flattening effect so that one side of the matrix grows faster than the other one resulting in deformity to the side of the faster-growing part. In addition, it has been proposed that nail bed hyperplasia with hyperkeratinization may contribute to the deformity (Citation2). Although onychogryphosis has been described for more than one hundred years, there is no curative treatment for onychogryphosis. Nonsurgical conservative procedures and nail avulsion with or without matricectomy are the common treatment procedures (Citation3). The recurrence rate is high, so patients’ needs are not met in many cases (Citation4).
Pantothenic acid, a component of coenzyme A, is a cofactor for enzyme-catalyzed reactions. It has an important effect on the degradation of fatty acids, synthesis of sterols, and steroid hormones in the cells (Citation5). Dexpanthenol (DXP) is the alcohol form of pantothenic acid. DXP shows anti-inflammatory activities compared to class I corticosteroids (Citation6). Herein, we report for the first time the intramatrix injection of dexpanthenol in the treatment of onychogryphosis.
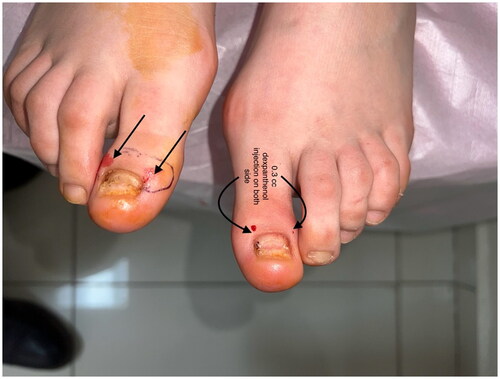
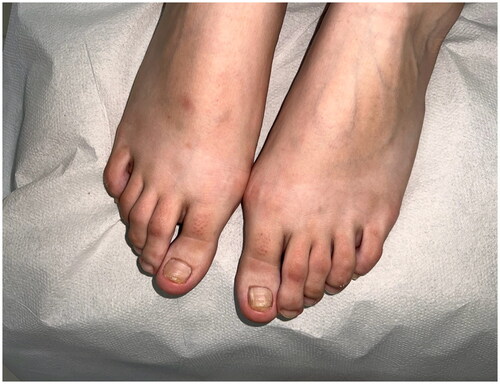
A 22-year-old female patient presented to the clinic with the complaint of acquired malalignment of the great toenails related onychogryphosis for 4 years. Although the patient’s nail previously had been removed 2 times, relapses occurred. Physical examination was notable for hallux valgus formation and thickening, lateral deviation of both first toenails (). The informed consent was obtained from the patient. We have performed nail avulsion without matricectomy (chemical or surgery). After nail avulsion, 0.3 ml 250 mg/ml dexpanthenol injection was perpendicularly applied to the 5–8 mm proximal-lateral site far from corner point where the proximal and lateral nail folds meet of both fingers toward the matrix. This procedure, intramatrix dexpanthenol injection, were performed at the 2nd week, 4th week, 8th week, 12th week (), and 24th week after nail plate avulsion. No side effects were observed during the treatment. The patient’s nails improved clinically significantly at 9 months () with high patient satisfaction. After a 5-month treatment-free period, the patient’s nails still were clinically highly improved ().
Figure 1. Onychogryphosis of the both great toenails. Thickening and lateral deviation of the toenails, resembling a ‘ram’s horn.’
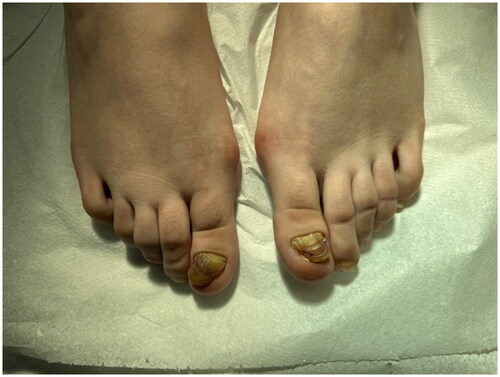
Figure 4. The clinical appearance of the nails 5 months after discontinuation of the dexpanthenol treatment.
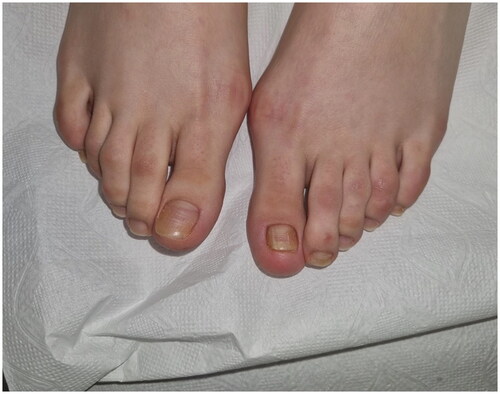
In the presented case, there was no improvement in onychogryphosis despite two times nail avulsions. The patient had hallux valgus, which leads to the occurrence of multiple minor traumas that could result in acquired malalignment of the great toenails. After multiple injections of the DXP, the patient’s toenails clinically improved significantly. We did not find any side effects of intramatrix DXP treatment. However, there are certain reports that emphasize allergic contact dermatitis can occur after DXP treatments (Citation7). In case of similar conditions, a few days of systemic steroid therapy may be sufficient if there is no regression in follow-up. Despite the discontinuation of the treatment for 5 months, the toenails clinically were still in good condition.
So far, numerous studies have been conducted on the effects of topical, intradermal and systemic DXP on the skin. Konus A and Aktas H reported the use of intradermal DXP on the onychodystrophy, but there are no reports about the use of intramatrical injection of DXP on the treatment of onychogryphosis (Citation8–11). DXP has hygroscopic features that moisturize skin, and repair skin elasticity. In a randomized, double-blind, placebo-control study, it has been shown that DXP increases stratum corneum hydration and reduces transepidermal water loss (Citation10). It has also been reported that there is an enhanced effect of DXP on the proliferation, migration, and attachments of the fibroblasts and collagen synthesis (Citation9).
The exact pathogenesis of the onychogryphosis still remains unclear. However, considering the result in our case, disturbed skin barrier function may contribute to onychogryphosis. Weimann et al. reported that turnover of coenzyme A in damaged skin is relatively high, hereby, higher amounts of DXP become necessary for wound healing and or tissue repair of damaged skin (Citation12). Furthermore, it has been reported that DXP upregulates IL-6, IL-1, CYP1B1, CXCL1, CCL18, and KAP 4–2 gene expression and downregulates psorasin mRNA and protein expression in the wound healing process (Citation13). In this context of the aforementioned results, we hypothesize that the decrease of the coenzyme A in the nail matrix and nail bed in onychogryphosis may contribute to the pathogenesis of onychogryphosis. Further studies are required in order to confirm this hypothesis and find the exact pathogenesis of onychogryphosis.
In conclusion, this is the first report about the effect of DXP on onychogryphosis with very good patient satisfaction. Our patient had been treated with surgery two times with recurrences. So far, there is no recurrence for fourteen months and she is still under follow-up.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data available on request from the corresponding author.
Additional information
Funding
References
- Ko D, Lipner SR. Onychogryphosis: case report and review of the literature. Skin Appendage Disord. 2018;4(4):1–3.
- Kouskoukis CE, Scher RK. Onychogryphosis. J Dermatol Surg Oncol. 1982;8(2):138–140.
- Haneke E. Nail surgery. Clin Dermatol. 2013;31(5):516–525.
- Greig J, Anderson J, Ireland A, et al. Simple avulsion of onychogryphotic toenails: a justifiable treatment? Postgrad Med J. 1989;65(768):741–742.
- Ebner F, Heller A, Rippke F, et al. Topical use of dexpanthenol in skin disorders. Am J Clin Dermatol. 2002;3(6):427–433.
- Wollina U, Nissen H-P, Kubicki J. Anti-inflammatory effects of dexpanthenol. Akt Dermatol. 2004;30:478–482.
- Fernandes RA, Santiago L, Gouveia M, et al. Allergic contact dermatitis caused by dexpanthenol-probably a frequent allergen. Contact Dermatitis. 2018;79(5):276–280.
- Kutlu Ö. Dexpanthenol may be a novel treatment for male androgenetic alopecia: analysis of nine cases. Dermatol Ther. 2020;33(3):e13381.
- Pugliese P, Farina J, Chautems Y. Efficacy of dexpanthenol in wound healing: double-blind assessment of excised wound tissue by ultrasound and histologic examination. Nouv Dermatol. 1995;14:130.
- Gehring W, Gloor M. Effect of topically applied dexpanthenol on epidermal barrier function and stratum corneum hydration. Arzneimittelforschung. 2000;50(07):659–663.
- Konus A, Aktas H. Idiopathic onychodystrophy improves with intradermal dexpanthenol injection: an extraordinary use. Rom J Clin Exp Dermatol. 2018;5(2):76–77.
- Weimann BI, Hermann D. Studies on wound healing: effects of calcium D-pantothenate on the migration, proliferation and protein synthesis of human dermal fibroblasts in culture. Int J Vitam Nutr Res. 1999;69(2):113–119.
- Heise R, Skazik C, Marquardt Y, et al. Dexpanthenol modulates gene expression in skin wound healing in vivo. Skin Pharmacol Physiol. 2012;25(5):241–248.