Abstract
Background
Patients who completed the originating studies, BREEZE-AD1 (NCT03334396), BREEZE-AD2(NCT03334422), and BREEZE-AD7 (NCT03733301), were eligible for enrollment in the multicenter,phase-3, long-term extension study BREEZE-AD3 (NCT03334435).
Methods
At week 52, responders and partial responders to baricitinib 4 mg were re-randomized (1:1) into the sub-study to dose continuation (4 mg, N = 84), or dose down-titration (2 mg, N = 84). Maintenance of response was assessed from week 52 to 104 of BREEZE-AD3. Physician-rated outcomes included vIGA-AD (0,1), EASI75, and mean change from baseline in EASI. Patient-reported outcomes included DLQI, P OEM total score, HADS, and from baseline: WPAI (presenteeism, absenteeism, overall work impairment, daily activity impairment) and change from baseline in SCORAD itch and sleep loss.
Results
With continuous treatment with baricitinib 4 mg, efficacy was maintained up to week 104 in vIGA-AD (0,1), EASI75, EASI mean change from baseline, SCORAD itch, SCORAD sleep loss, DLQI, P OEM, HADS, and WPAI (all scores). Patients down-titrated to 2 mg maintained most of their improvements in each of these measures.
Conclusion
The sub-study of BREEZE AD3 supports flexibility in baricitinib dosing regimens. Patients who continued treatment with baricitinib 4 mg and down-titrated to 2 mg maintained improvements in skin, itch, sleep, and quality of life for up to 104 weeks.
Introduction
Atopic dermatitis (AD) is a common, chronic, relapsing, heterogenous, highly symptomatic inflammatory skin disease. AD is often associated with significant itch, sleep disturbance (Citation1), secondary infections (Citation2), and skin pain (e.g., discomfort or soreness) (Citation3).
A high proportion of patients with AD report poor quality of life (QoL) (Citation4). The global prevalence of AD is 15% to 20% in children and 7% to 10% in adults (Citation4,Citation5), with clinical heterogeneity regarding the age of onset, lesion morphology, severity and distribution of skin involvement, and long-term persistence. AD results from an interaction between the dysfunction of the epidermal barrier, immune system, genetic predisposition, and environmental factors (Citation6–10). Due to AD's chronic and relapsing nature, with seasonal variation, a rapid response is required to achieve optimum physician- and patient-reported outcomes and reduce the risk of adverse events through the flexibility of dosage through down-titration while maintaining disease control.
Patients with moderate-to-severe AD are initially treated with topical corticosteroids (TCS) (Citation11,Citation12); however, some patients do not respond to these treatments (Citation13). In addition, alternative treatments such as emollients, phototherapy, and conventional systemic therapies are available, but these can result in adverse effects or may be ineffective due to the severity of the disease (Citation6,Citation12,Citation14). For patients with moderate-to-severe AD, there is still an unmet need for a tolerable treatment option with long-term efficacy to curb inflammation, itch, and sleep disturbances due to itch, associated with a negative impact on QoL (Citation4,Citation6,Citation12).
Janus kinase (JAK) inhibitors are fast-acting, tolerable, and efficacious drugs licensed for a number of different inflammatory conditions, including dermatologic conditions of AD and alopecia areata (Citation15–17). JAK1 and JAK2 mediate signaling of multiple cytokines involved in the pathophysiology of AD (Citation12,Citation18,Citation19). Baricitinib is an oral cytokine (primarily selective of JAK1 and 2) inhibitor approved in the many countries for treating moderate-to-severe AD in adults who are candidates for systemic therapy and is in late-stage development for pediatric patients with moderate-to-severe AD (Citation16,Citation20). In three phase-3 trials, baricitinib significantly improved the clinical signs and symptoms of AD in adults following 16 weeks of treatment as a monotherapy (BREEZE-AD1 [NCT03334396] and BREEZE-AD2 [NCT03334422]) and when combined with TCS (BREEZE-AD7 [NCT03733301]) (Citation21).
BREEZE-AD3 (NCT03334435) is an ongoing phase-3, double-blind, long-term extension study evaluating the maintenance of efficacy of baricitinib primarily in patients who had completed the 16-week studies BREEZE-AD1, BREEZE-AD2, or BREEZE-AD7. Maintenance of efficacy was previously demonstrated in BREEZE-AD3 through 52 weeks in patients who were responders (achieved clear or almost clear skin) or partial responders (achieved mild AD) at week 16 of the originating studies. Results, which represented 68 weeks of continuous treatment, were published separately for the cohort of patients who originated from the monotherapy studies (BREEZE-AD1 and AD2 pooled) (Citation22) and the cohort originating from the BREEZE-AD7 TCS combination therapy study (Citation23).
Here, we report 104-week physician- and patient-rated assessment endpoints for patients with moderate-to-severe AD who were responders and partial responders to baricitinib 4 mg, the approved starting dose for most patients, at week 52 of BREEZE-AD3 and were subsequently re-randomized to dose continuation (4 mg to 4 mg) or dose down-titration (4 mg to 2 mg).
Material and methods
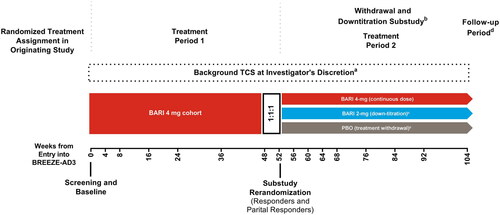
Patients who participated and completed the final active treatment visit at week 16 in the originating studies, BREEZE-AD1, BREEZE-AD2, and BREEZE-AD7, could enroll in the multicenter, phase 3, long-term extension study (); ). The data cutoff for the current analysis is 03 November 2021 (BREEZE-AD3). Studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and approved by the individual institutional review boards at each participating study center. All patients provided written informed consent.
Figure 1. Study design diagram for patients who entered BREEZE-AD3 as responders or partial responders. BARI: baricitinib; TCS: topical corticosteroids. aBackground TCS may have been initiated or reinitiated at any time during the study and were to be provided as part of rescue or retreatment any time patient’s IGA score became ≥3. bEligible patients were re-randomized in the withdrawal and down-titration sub-study. Patients who did not enroll in the sub-study remained on their treatment. cPatients enrolled in the sub-study were automatically retreated if their IGA score became ≥3. dA post-treatment follow-up visit was conducted approximately 28 days after the last dose of the investigational product, either at the end of the study or following early termination.
Study design and eligibility criteria
To be eligible to enroll in BREEZE-AD3, patients must have completed the final active treatment visit at week 16 from one of the originating studies, BREEZE-AD1, BREEZE-AD2, or BREEZE-AD7. Outcomes through Week 52 of BREEZE-AD3 have been reported previously (Citation22,Citation23). Patients with moderate-to-severe AD treated with once-daily baricitinib 4 mg in BREEZE-AD3 who, at week 52, were responders (validated Investigator Global Assessment (vIGA-AD) score 0 or 1) or partial responders (vIGA-AD score 2) were re-randomized (1:1:1) to dose continuation (4 mg to 4 mg), dose down-titration (4 mg to 2 mg), or placebo (baricitinib 4 mg to placebo). Patients enrolled in the substudy were automatically retreated with their original baricitinib dose if their IGA score became ≥3. TCS use was allowed at the investigators’ discretion and provided as a part of retreatment. The maintenance of improvements in efficacy measures was assessed from week 52 up to week 104 in the baricitinib cohorts. This analysis focuses on patients who continued treatment with baricitinib 4 mg or who were down-titrated to baricitinib 2 mg (); patients re-randomized to PBO are not included, as it was previously shown that patients who withdrew from active treatment and lost response generally did so within the first 4 weeks (Citation24).
Outcomes
Efficacy outcomes include the proportion of patients with a response of Eczema Area and Severity Index 75% (EASI75) improvement from baseline of originating study assessed at week 16 after re-randomization (week 68) and week 104. Other key outcomes included the proportion of responders (vIGA 0 or 1), assessed at week 16 after re-randomization (week 68) and week 104. In addition, the change from baseline (CFB) for the following measures: EASI total score, SCORing Atopic Dermatitis (SCORAD) itch and sleep loss score, and Work Productivity and Activity Impairment (WPAI) at week 16 after re-randomization (week 68) and week 104. The proportion of patients who achieved at least 4-point improvement in Patient-Oriented Eczema Measure (POEM), Dermatology Life Quality Index (DLQI) 0/1, and Hospital Anxiety and Depression Scale (HADS) <8 for anxiety and depression domains were also evaluated at week 104.
Statistical analysis
Data collected after permanent treatment discontinuation or after retreatment were considered as missing. Last observation carried forward was used for missing data imputation. Logistic regression and ANCOVA model were used for analysis. All results are reported descriptively over time as there was no multiplicity control. Analyses were performed using SAS, version 9.4 or higher (SAS Institute Inc).
Results
Patient disposition and baseline characteristics
In this substudy of BREEZE-AD3, a total of 168 patients who achieved a vIGA-AD of 0, 1, or 2 at week 52 (responders or partial responders at entry into the substudy) were entered into the sub-study of BREEZE-AD3 (). The overall population of patients (N = 1373) who entered BREEZE-AD3 at baseline and were analyzed through weeks 0–52 was reported previously (Citation22,Citation23). Patients in this substudy who were previously taking baricitinib 4 mg were re-randomized at week 52 to remain on baricitinib 4 mg or down-titrate to baricitinib 2 mg (). Of these, 84 patients maintained their study treatment of baricitinib 4 mg, and 84 were down-titrated to baricitinib 2 mg. Baseline demographics and disease characteristics were similar among the treatment groups (). Study discontinuation rates were numerically lower in the down-titration versus continuation cohort (17.9% vs. 26.2%), with Withdrawal by Subject, Lack of Efficacy, and Adverse Event cited as the most common reason (4.8% and 8.3%; 10.7% and 8.3%; 1.2% and 7.1%, respectively) (). For patients who experienced a relapse (vIGA >3) between week 52 to 104 after down-titration to baricitinib 2 mg, (n/N = 35/41) 85.4% re-achieved vIGA [0, 1, 2] and (n/N = 24/41) 58.5% achieved EASI75 within 4 weeks of follow-up retreatment. To maintain the study blind, patients on continuous baricitinib 4 mg who experienced a relapse were also “retreated” with their original dose (i.e., baricitinib 4 mg), and (n/N = 24/32) 75.0% of these achieved vIGA [0,1,2], and (n/N = 23/32) 71.9% achieved EASI75 within 4 weeks of ‘retreatment’.
Table 1. Summary of demographics and disease characteristics at baseline at week 52 in randomized patients with atopic dermatitis treated with baricitinib continuation and down-titration substudy.
Clinician reported outcomes
Among patients continuing on baricitinib 4 mg, the proportion who achieved or maintained vIGA-AD (0,1) was stable from week 52 to 104 (week 52 [51.2%], 68 [51.2%], 104 [47.6%]) (). Patients who continued on BARI 4-mg also largely maintained EASI-75 response (week 52 [82.1%], 68 [79.8%], 104 [73.8%]) (). The reduction of severity based on EASI total score CFB was maintained from week 52 to 104 (LSM, week 52, [−27.77], 68 [−26.97], 104 [−26.56]) ().
Figure 2. Skin response over time: (A) vIGA-AD (0,1), (B) EASI75, and changes from baseline over time for (C) EASI. *p ≤ .05, **p ≤ .01, and ***p ≤ .001 denote significant differences between treatment groups
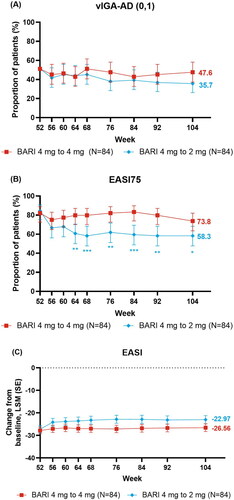
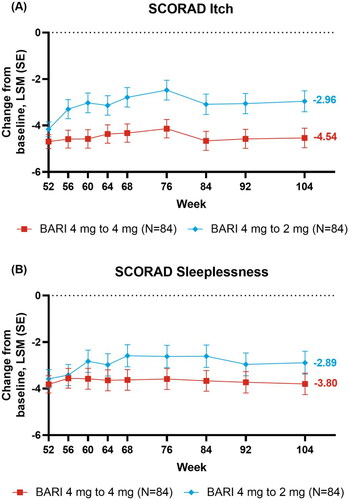
Most patients who were down-titrated to baricitinib 2 mg also maintained skin response through to week 104. Most patients who down-titrated maintained vIGA-AD (0,1) response (week 52 [51.2%], 68 [45.2%], 104 [35.7%]) (). Further, the EASI75 response was maintained in the baricitinib continuation cohort beginning at week 56 (week 52 [84.5%], 68 [58.3%], 104 [58.3%]) and for EASI total score at week 56 (LSM, week 52 [−27.21], 68 [−23.21], 76 [−22.81], 104 [−22.97]) ().
Patient-reported outcomes
Responses to baricitinib remained relatively stable throughout BREEZE-AD3 for patient-reported outcomes in the continuation cohort (). The response for DLQI (0/1), indicating no impact on patient’s life, was maintained from week 52 to 104 (week 52 [38.1%], 68 [34.5%], 104 [34.5%]) (). Likewise, POEM ≥4 point improvement was also maintained (week 52 [83.3%], 68 [76.2%], 104 [77.4%]) (). Similar to the other patient-reported results, responses were maintained from week 52 to 104 for HADS-Anxiety <8 (week 52 [50.0%], 104 [55.9%]) and HADS-Depression <8 (week 52 [61.5%], 104 [61.5%]) () with patients presenting no borderline or abnormal severity scores for anxiety or depression (Citation25). Improvements in mean WPAI CFB were generally stable from week 52 to 104 for presenteeism (LSM week 52 [−26.8], 68 [−28.2], 104 [−22.5]) and absenteeism (LSM week 52 [−2.7], 68, [−3.7], 104 [−2.1]), and in patients employed for pay for overall work impairment (Nx = 48, LSM, week 52 [−26.7], 68 [−27.0], 104 [−22.0]), as well as daily activity impairment scores in all patients (Nx = 84, LSM, week 52 [−27.8], 68 [−26.0], 104 [−24.5]) (). From week 52 to 104, improvements in mean CFB in SCORAD itch (LSM, week 52 [−4.69], 68 [−4.33], 104 [−4.54]) and sleep loss (LSM, week 52 [−3.82], 68 [−3.63], 104 [−3.80]) were maintained in the 4 mg continuation cohort ().
Figure 3. Patient-reported responses over time: (A) DLQI (0,1), (B) POEM ≥4 point improvement, (C) HADS-A < 8, and (D) HADS-D < 8. aIn patients with borderline or abnormal severity scores at baseline (HADS-A ≥8 or HADS-D ≥8)
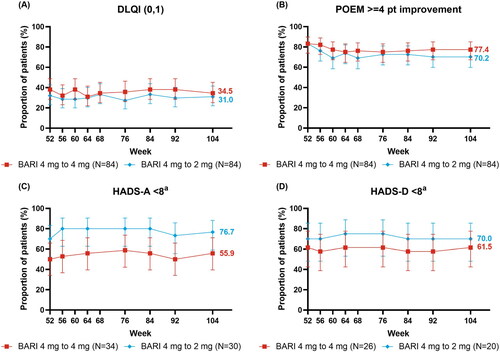
Figure 4. Changes from baseline over time 4-mg: (A) Presenteeism, (B) Absenteeism, (C) Overall Work Impairment, (D) Daily Activity§. aEmployed patients only, bEmployed for pay patients only, §Nx is the number of patients with presenteeism, absenteeism, daily activity impairment, and overall work impairment evaluated at week 52.
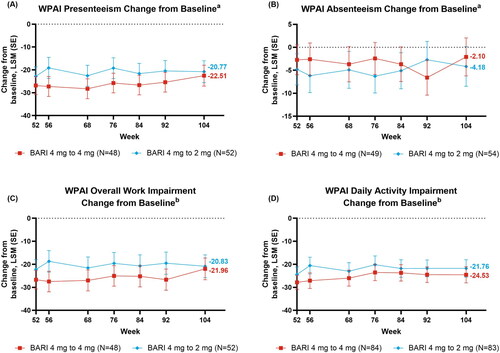
Table 2. Outcomes for physician- and patient rated assessments at week 52, 68, and 104.
Improvements in patient-reported outcomes for the down-titration cohort were also consistently maintained, with no meaningful reductions in the maintenance of response (). From week 52 to 104, the response was maintained for DLQI (0/1) (week 52 [32.1%], 68 [33.3%], 104 [31.0%]) () and POEM ≥4 point improvement (week 52 [83.3%], 68 [69.0%], 104 [70.2%]) (). As in baricitinib 4 mg, for baricitinib 2 mg responses were maintained from week 52 to 104 for HADS-Anxiety <8 (week 52 [70.0%], 104 [76.7%]) and HADS-Depression <8 (week 52 [70.0%], 104 [70.0%]) (). The WPAI scores were maintained from week 52 to 104 for presenteeism (LSM, week 52 [−22.8], 68 [−22.6], 104 [−20.8]) and absenteeism (LSM, week 52 [−4.8], 68 [−4.9], 104 [−4.2]), and in patients employed for pay for overall work impairment (Nx = 52, LSM, week 52 [−22.2], 68 [−21.5], 104 [−20.8]) and daily activity impairment in all patients (Nx = 83, LSM, week 52 [−24.4], 68 [−23.0], 104 [−21.8]) (). Patients down-tritiated to baricitinib 2 mg did experience numerically lower SCORAD itch scores from week 56 to 104 (LSM, week 52 [−4.2], 68 [−2.8], 104 [−3.0]) (). Patients down-titrated to baricitinib 2 mg maintained improvement for SCORAD sleep loss (LSM, week 52 [−3.6], 68 [−2.6], 104 [−2.9]) ().
Discussion
In BREEZE-AD3, patients with moderate-to-severe AD who were responders and partial responders to baricitinib 4 mg were re-randomized at week 52 to either continue on baricitinib 4 mg or down-titrate to 2 mg. Baricitinib demonstrated flexibility of dose down-titration and maintenance of benefit over the signs and symptoms of AD up to 104 weeks of treatment. This complements several recent publications of phase 3 clinical trials, including BREEZE-AD3, which have investigated baricitinib in adults with moderate-to-severe AD, establishing baricitinib 4 mg and 2 mg as effective and well-tolerated to 104 weeks (Citation22,Citation26–28).
When examining the maintenance of response across time, patients treated with both baricitinib doses continued to experience improvement in skin inflammation, skin pain, sleep disturbance, and QoL through to week 104. Maintenance of improvements in physician- (vIGA-AD [0,1], EASI75, and EASI total score CFB) and patient-reported outcomes (DLQI, POEM, HADS-A, HADS-D, SCORAD itch and sleep loss, WPAI scores) were monitored through 52 weeks of continuous treatment, with TCS used at investigators discretion.
In line with previous findings for long-term treatment with baricitinib for moderate-to-severe AD (Citation22, Citation23), improvements in physician-reported outcomes were generally maintained from week 52 to 104 in baricitinib continuous treatment with 4 mg or down-titration to 2 mg. Clinically meaningful improvements in the signs and symptoms of patients with AD are reflected by EASI75 (Citation29). The population of patients treated with baricitinib 4 mg in this study demonstrated maintenance in the proportion of patients achieving EASI75 up to week 104. Most patients who were down-titrated to 2 mg also maintained improvement in EASI75, although small numerical decreases were observed early on. These decreases could be related to the absolute threshold of EASI75, where as little as a 1-point difference in EASI total score could contribute to the likelihood of achieving the threshold. Itch is highlighted as a primary contributor to AD morbidity (Citation30,Citation31), and ratings of itch intensity significantly correlate with QoL (Citation32, Citation33). As there is reduced maintenance of effect in the down-titrated cohort for SCORAD itch from week 56, itch could directly influence other efficacy outcomes examined here, for which itch is a contributing factor and reflects findings from previous studies (Citation34,Citation35). However, this reduction in response in baricitinib 2 mg was not observed in other patient-reported outcomes, with the maintenance of improvement for both baricitinib continuation and down-titration cohorts. This is consistent with the flaring nature of the disease, with varying symptoms across different thresholds and changing patients’ perception of symptoms of itch and skin pain severity over time (Citation36).
Notably, patients in both cohorts experienced maintenance of over 30% improvement in DLQI (0,1), one of the commonly used scales in clinical trials to assess QoL (Citation37). This was replicated, with over 69.0% response maintained for POEM 4-point improvement, in both cohorts, throughout the 52 weeks of the study. Further, a reduction in daily activity impairment in this study, assessed by WPAI, which is significantly correlated with disease severity (Citation38), supports the maintained improvements in QoL. Symptoms of anxiety and depression are major comorbidities in patients with AD, correlated to the disease’s painful and psychosocial symptoms (Citation39,Citation40) and inflammatory cytokines, which may be linked to depression (Citation41). The maintenance of HADS-Anxiety and depression subscale scores reported here for both baricitinib continuation on 4 mg and down-titration to 2 mg indicate that baricitinib treatment offers a long-term reduction to the psychosocial dimensions of AD.
Baricitinib provides an option for patients with AD who require a flexible dose to reflect fluctuating disease activity and risk of adverse events. Previous results from the initial 16 weeks following re-randomization on the efficacy of down-titration, treatment withdrawal, and retreatment have been disclosed for BREEZE-AD3, with a sustained clinical response in groups down-titrated to baricitinib 2 mg from 4 mg (vIGA-AD of 0,1,2) (Citation42). For those patients who do lose response after down-titration to baricitinib 2 mg, retreatment with baricitinib 4 mg results in the recapture of response in the majority of patients within 4 weeks of retreatment. Interestingly, even among patients in the baricitinib 4 mg continuation group who relapsed during the 52- to 104-week period, the majority of those also regained response within 4 weeks of ‘retreatment’ with the same dose. Results presented here confirm the maintenance of response up to 104 weeks and indicate that baricitinib can be used as an oral treatment option for both short-term moderate flaring AD to long-term chronic AD.
This study is limited by the majority of patients being Asian or White, with no representation for Black or African American patients. As race is suggested as a factor in the phenotypic expression of AD (Citation43–45), the results presented here can only be generalized to Asian and White populations. TCS use was also not assessed, however, patients who were not experiencing sufficient clinical benefit from baricitinib treatment and required higher-potency TCS or systemic therapies were encouraged to discontinue. The previous combination of baricitinib with low- or moderate-potency TCS improved the signs and symptoms of moderate-to-severe atopic dermatitis (Citation46). This study did not follow the maintenance of responses in individual patients but instead tracked responses over time for the population as a whole who had achieved response or partial response at week 52.
Overall, the results presented here align with growing evidence for long-term efficacy of baricitinib for the treatment of AD (Citation23, Citation26, Citation28), with similar maintenance of improvements over 52 weeks for both continuation of baricitinib 4 mg and a down-titration to 2 mg. Patients who continued on baricitinib 4 mg from week 52 to 104 maintained greater control in some outcome measures relative to patients who were re-randomized to baricitinib 2 mg, with somewhat greater maintenance of EASI75 and SCORAD itch. Improvement in patient-rated outcomes was sustained in both treatment arms.
Supplemental Material
Download PDF (60.4 KB)Acknowledgment
The authors thank the patients, investigators, and study staff who were involved in these studies. The patients in this manuscript have given written informed consent to publication of their casedetails.
The authors would also like to acknowledge Sarah Ryan, of Eli Lilly and Company, who provided editing support.
Disclosure statement
AW: Has received personal fees for lectures or advisory boards, grants, or nonfinancial support from AbbVie, Almirall, Arena, Beiersdorf, Galderma, Leo Pharma, Eli Lilly, L’Oreal, Maruho, MedImmune, Novartis, Pfizer, Pierre Fabre, Regeneron, and Sanofi-Aventis.
SB: Is an investigator or speaker for Almirall, Sanofi-Genzyme, AbbVie, Novartis, Janssen, Leo Pharma, Pfizer, Eli Lilly, UCB Pharma, Chiesi.
HH: Has received: grant funding from Pfizer, Janssen, and Merck Serono; honoraria and/or consultancy fees and/or travel bursaries from La Roche-Posay, Janssen, Abbvie, UCB, Sanofi-Genzyme, Regeneron, Novartis, Almirall, Leo, Eli Lilly DICE Therapeutics and UNION Therapeutics and has acted as an investigator for Janssen, Abbvie, UCB, Sanofi-Genzyme, Novartis, Almirall, Leo, Eli Lilly, DICE Therapeutics, UNION Therapeutics, and Evelo Biosciences Inc.
TW: Has received personal fees from AbbVie, Almirall, Eli Lilly, Galderma, Janssen/JNJ, Leo Pharma, Novartis, Pfizer, Regeneron/Sanofi for lectures or advisory boards.
JPT: An advisor for AbbVie, Almirall, Arena Pharmaceuticals, Coloplast, OM Pharma, Aslan Pharmaceuticals, Union Therapeutics, Eli Lilly & Co, LEO Pharma, Pfizer, Regeneron, and Sanofi-Genzyme, a speaker for AbbVie, Almirall, Eli Lilly & Co, LEO Pharma, Pfizer, Regeneron, and Sanofi-Genzyme, and received research grants from Pfizer, Regeneron, and Sanofi-Genzyme.
AB, LC, LS, EP: Employees and stockholders at Eli Lilly and Company.
NL: is an employee of Precision Statistics Consulting.
Data availability statement
Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and the European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Additional information
Funding
References
- Yano C, Saeki H, Ishiji T, et al. Impact of disease severity on sleep quality in japanese patients with atopic dermatitis. J Dermatol Sci. 2013;72(2):1–9.
- Farag AK, Roesner LM, Wieschowski S, et al. Specific T cells targeting Staphylococcus aureus fibronectin-binding protein 1 induce a type 2/type 1 inflammatory response in sensitized atopic dermatitis patients. Allergy. 2022;77(4):1245–1253. eng.
- Vakharia PP, Chopra R, Sacotte R, et al. Burden of skin pain in atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119(6):548–552.e3. eng.
- Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123(2):144–151.
- Sacotte R, Silverberg JI. Epidemiology of adult atopic dermatitis. Clin Dermatol. 2018;36(5):595–605.
- Puar N, Chovatiya R, Paller AS. New treatments in atopic dermatitis. Ann Allergy Asthma Immunol. 2021;126(1):21–31.
- Guttman-Yassky E, Waldman A, Ahluwalia J, et al. Atopic dermatitis: pathogenesis. Sem Cutan Med Surg. 2017;36(3):100–103.
- Brown SJ, Elias MS, Bradley M. Genetics in atopic dermatitis: historical perspective and future prospects. Acta Derm Venereol. 2020;100(12):adv00163.
- Narla S, Silverberg JI. The role of environmental exposures in atopic dermatitis. Curr Allergy Asthma Rep. 2020;20(12):74.
- Thyssen JP, Rinnov MR, Vestergaard C. Disease mechanisms in atopic dermatitis: a review of aetiological factors. Acta Derm Venereol. 2020;100(12):adv00162.
- Wollenberg A, Barbarot S, Bieber T, the European Dermatology Forum (EDF), the European Academy of Dermatology and Venereology (EADV), the European Academy of Allergy and Clinical Immunology (EAACI), the European Task Force on Atopic Dermatitis (ETFAD), European Federation of Allergy and Airways Diseases Patients’ Associations (EFA), the European Society for Dermatology and Psychiatry (ESDaP), the European Society of Pediatric Dermatology (ESPD), Global Allergy and Asthma European Network (GA2LEN) and the European Union of Medical Specialists, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32(5):657–682.
- Wollenberg A, Christen-Zäch S, Taieb A, for the European Task Force on Atopic Dermatitis/EADV Eczema Task Force, et al. ETFAD/EADV eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34(12):2717–2744.
- Johnson BB, Franco AI, Beck LA, et al. Treatment-resistant atopic dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:181-192.
- Abeck F, Booken N, Schneider S. [Inappropriate systemic therapy in severe atopic dermatitis-severe long-term damage]. Dermatologie (Heidelb). 2022; 73(8):638–640.
- OLUMIANT prescribing information: ©lilly USA. LLC. 2022. Available from: https://www.olumiant.com/
- Agency EM. Olumiant - baricitinib - An overview of Olumiant and why it is authorised in the EU 2022. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant.
- FDA. FDA Approves First Systemic Treatment for Alopecia Areata 2022. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-systemic-treatment-alopecia-areata.
- Bieber T, Paller AS, Kabashima K, et al. Atopic Dermatitis: pathomechanisms and lessons learned from novel systemic therapeutic options. J Eur Acad Dermatol Venereol. 2022;36(9):1432–1449.
- Wang F, Kim BS. Itch: a paradigm of neuroimmune crosstalk. Immunity. 2020;52(5):753–766. eng.
- Nakashima C, Yanagihara S, Otsuka A. Innovation in the treatment of atopic dermatitis: emerging topical and oral Janus kinase inhibitors. (1440-1592 (Electronic)).
- Thyssen JP, Buhl T, Fernández-Peñas P, et al. Baricitinib rapidly improves skin pain resulting in improved quality of life for patients with atopic dermatitis: analyses from BREEZE-AD1, 2, and 7. Dermatol Ther (Heidelb). 2021;11(5):1599–1611.
- Silverberg JI, Simpson EL, Wollenberg A, et al. Long-term efficacy of baricitinib in adults with moderate to severe atopic dermatitis who were treatment responders or partial responders: an extension study of 2 randomized clinical trials. JAMA Dermatol. 2021;157(6):691–699.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Long-Term efficacy (up to 68 weeks) of baricitinib in combination with topical corticosteroids in adult patients with moderate-to-Severe atopic dermatitis: analysis of treatment responders, partial responders and nonresponders originating from study BREEZE-AD7. J Eur Acad Dermatol Venereol. 2022. DOI:10.1111/jdv.18816
- Reich K, Simpson E, Wollenberg A, et al. Efficacy of downtitration or treatment withdrawal compared to continuous dosing, after successful treatment with baricitinib in patients with moderate-to-severe atopic dermatitis in a randomised substudy from the long-term extension study, BREEZE-AD3. Br J Dermatol. 2021;188(2):208–217.
- Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes. 2003;1:29.
- Bieber T, Thyssen JP, Reich K, et al. Pooled safety analysis of baricitinib in adult patients with atopic dermatitis from 8 randomized clinical trials. J Eur Acad Dermatol Venereol. 2021;35(2):476–485. eng.
- Simpson EL, Lacour JP, Spelman LA-O, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183(2):242–255.
- King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22(3):395–405. eng.
- Hanifin JM, Baghoomian W, Grinich E, et al. The eczema area and severity Index-A practical guide. Dermatitis. 2022;33(3):187–192.
- Hon KLE, Leung TF, Wong KY, et al. Does age or gender influence quality of life in children with atopic dermatitis? Clin Exp Dermatol. 2008;33(6):705–709. eng.
- Dawn A, Pa D, Chan YH, et al. Itch characteristics in atopic dermatitis: results of a web-based questionnaire. Br J Dermatol. 2009;160(3):642–644. eng.
- Weisshaar E, Diepgen T, Bruckner T, et al. Itch intensity evaluated in the german atopic dermatitis intervention study (GADIS): correlations with quality of life, coping behaviour and SCORAD severity in 823 children. Acta Derm Venereol. 2008;88(3):234–239.
- Chamlin SL, Mc L, Fi J, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. J Am Acad Dermatol. 2008;58(3):415–420.
- Chrostowska-Plak D, Reich A, Fau- Szepietowski JC, et al. Relationship between itch and psychological status of patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2013;27(2):e239–e242.
- Cork MJ, Eckert L, Simpson EL, et al. Dupilumab improves patient-reported symptoms of atopic dermatitis, symptoms of anxiety and depression, and health-related quality of life in moderate-to-severe atopic dermatitis: analysis of pooled data from the randomized trials SOLO 1 and SOLO 2. J Dermatolog Treat. 2020;31(6):606–614.
- Newton L, DeLozier AM, Griffiths PC, et al. Exploring content and psychometric validity of newly developed assessment tools for itch and skin pain in atopic dermatitis. J Patient Rep Outcomes. 2019;3(1):42.
- Rehal B, Armstrong AW. Health outcome measures in atopic dermatitis: a systematic review of trends in disease severity and quality-of-life instruments 1985-2010. PLoS One. 2011;6(4):e17520.
- Yano C, Saeki H, Ishiji T, et al. Impact of disease severity on work productivity and activity impairment in japanese patients with atopic dermatitis. J Dermatol. 2013;40(9):736–739.
- Gochnauer H, Valdes-Rodriguez R, Cardwell L, et al. The psychosocial impact of atopic dermatitis. Adv Exp Med Biol. 2017;1027:57–69.
- von Kobyletzki LB, Thomas KS, Schmitt J, et al. What factors are important to patients when assessing treatment response: an international cross-sectional survey. Acta Derm Venereol. 2017;97(1):86–90.
- Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229.
- Reich K, Simpson E, Wollenberg A, et al. Efficacy with continuous dosing, down-titration, or treatment withdrawal after successful treatment with baricitinib in patients with moderate-to-severe atopic dermatitis. Journal of Investigative Dermatology - Clinical Research and Epidemiology - Abstracts. 2021;141(041):S155.
- Nomura T, Wu J, Kabashima K, et al. Endophenotypic variations of atopic dermatitis by age, race, and ethnicity. J Allergy Clin Immunol Pract. 2020;8(6):1840–1852.
- Kaufman BP, Guttman-Yassky E, AF A. Atopic dermatitis in diverse racial and ethnic groups-Variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27(4):340–357.
- Sanyal RD, Pavel AB, Glickman J, et al. Atopic dermatitis in african American patients is T(H)2/T(H)22-skewed with T(H)1/T(H)17 attenuation. Ann Allergy Asthma Immunol. 2019;122(1):99–110.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(12):1333–1343.