Abstract
Introduction
The injectable skin fillers available for soft tissue augmentation are constantly growing, providing esthetic surgeons with more options in the treatment of scars, lines, and wrinkles. Hyaluronic acid (HA)-derived injectable fillers are ideal to reduce the appearance of nasolabial folding. This study investigated the efficacy and safety of the commercially available HA filler from Maxigen Biotech Inc. (MBI-FD) in the treatment of nasolabial folds (NLFs).
Methods
We analyzed 1,4-butanediol diglycidyl ether (BDDE) residues and injection force test and observed the protein content in MBI-FD, and then was cultured in fibroblast L929 cells and examined for cytotoxicity. Finally, 95 healthy participants underwent dermal filler injection therapy to evaluate the efficacy and safety for 24 and 52 weeks, respectively.
Results
BDDE residues in MBI-FD was <0.125 µg/mL. MBI-FD was fitted using 27- and 30-G injection needles with an average pushing force of 14.30 ± 2.07 and 36.43 ± 3.11 N, respectively. Sodium hyaluronate protein in MBI-FD was 7.19 µg/g. The cell viabilities of 1× and 0.5× MBI-FD were 83.25% ± 3.58% and 82.23% ± 1.85%, respectively, indicating MBI-FD had no cytotoxicity, and decreased NLF wrinkles with no serious adverse events.
Conclusion
MBI-FD is an effective filler for tissue augmentation of the NLFs and may be a suitable candidate as an injectable dermal filler for tissue augmentation in humans in the future.
Introduction
In recent years, due to the improvement in the quality of life, individuals gradually have begun to pay attention to their maintenance, particularly facial wrinkles. Therefore, several wrinkle removal methods are available on the market; however, subcutaneous injection to treat wrinkles on the face is the most direct treatment method (Citation1). Filler materials are often used with autografts, allografts, synthetic materials, and biosynthetic fillers, and some have been approved by the U.S. Food and Drug Administration (Citation2). However, the currently used filling materials have considerable shortcomings. The skin structure can be divided into three layers, including the epidermis, dermis, and subcutaneous fat layers, in order from the outside to the inside (Citation3). The main function of the epidermis is defense; the dermis is mostly composed of connective tissue, including collagen, elastic tissue, and ground substance, of which collagen is the main component (Citation4). Of the connective tissue collagens in the human body, the proportion of type I collagen is 80% in young individuals. Furthermore, the dermis is rich in glycosaminoglycans, which are mainly composed of hyaluronic acid (HA), whose main function is to retain moisture (Citation5).
HA is a linear polysaccharide composed of repeating disaccharide units of N-acetyl-D-glucosamine and D-glucuronic acid (Citation6). HA is a versatile glycosaminoglycan that is present in all biological fluids and tissues and is involved in several biochemical processes, including cell signaling, wound repair, and regeneration (Citation7). The skin on the face gradually ages, showing fine lines and wrinkles (Citation8). In recent years, HA fillers can locally improve skin aging (Citation9). Therefore, subcutaneous injection of HA has rapidly developed in the medical beauty market (Citation10). The biocompatibility, biodegradability, and high water absorption of HA make it the main material for dermal fillers (Citation11). However, uncross-linked HA is easily decomposed by enzymes in the physiological environment and subsequently metabolized by the human body, which reduces its tolerance in the skin tissue, thereby limiting the application of HA as a dermal filler (Citation12). The cross-linked HA can effectively stimulate collagen proliferation; moreover, they pointed out calcium phosphate-modified cross-linked HA particles can be used as long-acting hypodermic fillers (Citation13). Studies have indicated there are several factors affecting HA fillers, including concentration, percentage of crosslinking, type of crosslinking, and injection technique (Citation14). To improve efficiency, several manufacturers use a variety of agents and techniques for crosslinking HA. Formaderm from Maxigen Biotech, Inc. (MBI-FD) is an HA filler that can be injected into the dermis of the facial skin for wrinkle correction and lip augmentation (Citation15). MBI-FD uses the ECHA™ balanced crosslinking technology to flatten the entangled HA molecular chain, add a small amount of 1,4-butanediol diglycidyl ether (BDDE) crosslinking agent, evenly distribute around HA, and evenly distribute HA particle size, with special temperature control for a long time. It is made to achieve two-phase crosslinking of HA. Impurities are removed through purification, so that the residue of crosslinking agent is close to zero, ensuring extremely high product safety. First, using MBI-FD, we conducted a three-stage study to analyze BDDE residues and injection force test and subsequently observed the protein content. Second, MBI-FD was cultured in L929 cells and examined for cytotoxicity. Third, subjects were recruited to evaluate the efficacy and safety of MBI-FD for 24 and 52 weeks, respectively.
Methods
Formaderm dermal filler injection (MBI-FD)
First, the HA solution was prepared by dissolving sodium hyaluronate (bacterial fermentation, purity of >95%, and the average molecular weight is approximately 1500 kDa) in 0.1-N NaOH. Subsequently, the HA solution was cross-linked with BDDE to prepare the cross-linked HA (cHA). After cross-linking, the cHA was mixed with uncross-linked sodium hyaluronate solution with a volume ratio of 80:20 to prepare the product formulation with 2% sodium hyaluronate content. The product formulation was then filled into a syringe by using the aseptic filling process by ISO 13408. Finally, the pre-filled syringe was sterilized by using moist heat by ISO 17665.
BDDE residues
BDDE residues can be tested by detecting the fluorescence intensity of the substance produced by BDDE and nicotinamide, which have strong fluorescent and excitation, and emission wavelengths located at 370 and 430 nm, respectively. Then, 200 μL of 16.0, 8.0, 4.0, 2.0, 1.0, 0.5, 0.25, 0.125, and 0.0625 µg/mL BDDE solution and 200 μL of 125 mmol/L nicotinamide solution were respectively mixed in tubes and incubated for 2 h at 37 °C. Five milliliters of formic acid were added, heated for 5 min in a 60 °C water bath, and subsequently chilled on ice for 5 min. The solution was left at room temperature for 10–15 min. The fluorescence values were determined using a multifunctional microplate reader SpectraMax M5 (Thermo Fisher Science, CA, USA) with excitation and emission wavelengths located at 370 nm. BDDE concentration and fluorescence were used as the abscissa and ordinate to make the standard curve.
Pushing force determination
The physical and mechanical aspects of the relevant powers and forces were assessed, and two combinations of HA fillers and needles were selected. The machine was programmed to push and displace the syringe plunger at a constant speed. The injection force was determined by analyzing the results corresponding to the speed (30 mm/min). Plunger displacement and injection force measurements were recorded at 0.1-s intervals using a data acquisition system.
Protein content determination
Two grams of cross-linked sodium hyaluronate gel were weighed and put in a 20-ml headspace bottle to avoid contacting the bottle wall and affecting acidolysis. Next, 3-ml 0.5 mol/l sulfuric acid solution was added without shaking, and the bottle cap was pressed tightly and placed in a constant temperature drying oven at (95 ± 5)°C for 45 min to completely dissolve it. After cooling at room temperature, the solution was transferred in the headspace vial to a 10-ml quantitative bottle, the headspace vial was rinsed three times using 3 ml of 1 mol/l sodium hydroxide solution, the rinse solution was transferred to a volumetric flask, and finally fixed with water. From the 10-ml solution, 1 ml was aspirated and placed in a test tube. The repeat test number for the protein content of the product was n = 3. The standard protein solution was prepared by using bovine serum albumin with 2, 4, 6, and 10 µg/mL, and the water for injection was used as the blank control. After serial dilution, 5.0 ml of Coomassie brilliant blue G-250 was added to the test tube and mixed well. After 5 min at room temperature, the absorbance was measured at a wavelength of 595 nm. The regression equation was calculated with the concentration of bovine albumin on the corresponding absorbance, and the protein content of the test solution was calculated from the regression equation.
MTT assay for cell viability
L929 cells (1 × 104 cells/well) were seeded into 96-well plates in the minimum essential medium at 37 °C with 5% CO2 and maintained in culture for 24 h. They were subsequently exposed to the test sample over a range of concentrations, including 0.5 − 1× of polar vehicle extract (L929 culture medium without 10% serum) and 0.5 − 1× of additional vehicle extract (L929 culture medium). Negative control (culture medium) and positive control (5% DMSO). Next, the cell viability was assessed using a tetrazolium bromide reduction (MTT) assay for mitochondrial activities in all of the test and control groups. After 24 h treatment, 10 µL of the 5-mg/mL MTT solution was added to each well. After 4 h of incubation at 37 °C, the formazan precipitates were solubilized by the addition of 100 µL of an acid solution of SDS (10% SDS in 0.01-M HCl), and the plate was incubated overnight at 37 °C. Absorbance at 570 nm was determined using a spectrophotometric microplate reader (SpectraMAX Plus, Molecular Devices) and observed using a microscope.
Clinical trial
A randomized, double-blind (assessor and participant), with a control group (self and control for the same period), non-inferior, and single-center trial of MBI-FD was performed in Tri-Service General Hospital (IRB Number: 2-102-05-113), and the study was registered on ClinicalTrials.gov Identifier: NCT05822778. Q-Med “Restylane®” was used as the control to evaluate the efficacy and safety of the trial product. MBI-FD was injected into 95 participants aged 30–65 years old whose nasolabial fold (NLF) wrinkle baseline was 3–4 points and was symmetrical. The following were the major inclusion criteria: participants aged 30–65 years old of both sexes; those with a wrinkle severity rating scale (WSRS) baseline measurement of 3–4 points, and the left and right sides were symmetrical; and those with healthy facial skin, without any disease that possibly interfered with skin aging status assessment. The following were the major exclusion criteria: female participants who were pregnant, breastfeeding, and planning to become pregnant during the trial period; those who have severe skin disease, inflammation, or related symptoms, such as infections, psoriasis, and herpes; those who underwent laser treatment or dermabrasion and facial wrinkle augmentation surgery, such as Botox injections, within the past 12 months; those who underwent chemical peels treatment within the last 3 months; and those with a medical history of cosmetic filling agent allergy or any type of HA implants. Following recruitment and providing informed consent, the participant’s control and experimental group’s injection site was determined through randomized coding. The rating physician and the physician administering injections had to be different individuals. The injection volume was limited to 2 ml per injection site. Two weeks after injection, the participants had to enter the postoperative log to record their postoperative condition. They were re-visited on weeks 2, 4, 12, 24, 36, and 52 for the injection, and the rating physician performed the assessment and recorded the results in the case report. The safety tracking period was 52 weeks, and the validity was 24 weeks. The primary efficacy assessment indicator was the effective rate of trial treatment, which is the relative baseline (V0) WSRS improvement ratio at week 24 (V5); the secondary efficacy assessment indicators were the participant’s WSRS improvement score and Global Esthetic Improvement Scale class. Conversely, safety assessment was the observation of adverse reactions/events.
Statistical analysis
Data were expressed as means ± SEM. Statistical significance among multiple groups was assessed using one-way ANOVA. p values <0.05 were considered statistically significant.
Results
MBI-FD had very low BDDE residues
According to safety data sheets provided for BDDE as a raw material, exposure to BDDE may induce irritation and allergic reactions at greater concentrations than the safety threshold of 2 ppm. BDDE residues in MBI-FD were <0.125 µg/mL (0.125 ppm) (). The results indicated that MBI-FD uses the crosslinking agent BDDE with a low residual content, which can effectively reduce the risk of adverse reactions in patients postoperatively.
Table 1. The residues of BDDE and protein in MBI-FD.
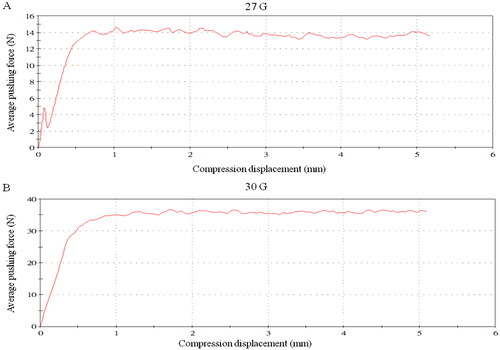
MBI-FD had stable injection force and reduced protein content
By applying force to the push rod of the product syringe, the operation of the product in clinical use was simulated at a speed of 30 mm/min. The results showed that MBI-FD was fitted with a 27-G injection needle, with an average pushing force of 14.30 ± 2.07 N. When the 30-G injection needle was placed, the average pushing force was 36.43 ± 3.11 N, which had a stable injection force (). During the extrusion process of the sample, the feedback stress was stable, indicating the HA gel in the product was uniformly dispersed without aggregation and concentration. Moreover, the average protein content in MBI-FD was 7.19 ± 0.34 µg/g, which complies with the requirement of YYT 0962-2021 that the protein content of cross-linked sodium hyaluronate gel for plastic surgery should be <20 µg/g (). Therefore, the low protein content of MBI-FD can effectively reduce the risk of adverse reactions in patients postoperatively.
MBI-FD had no cytotoxicity in vitro
Subsequently, the cytotoxicity of MBI-FD was examined. We used MBI-FD to culture with L929 cells for 24 h and then examined the viability using MTT. The negative control group (without the test sample) was 100% ± 2.94%, the positive control (5% DMSO) was 30.67% ± 1.84%, and 1× and 0.5× MBI-FD (without serum) were 83.25% ± 3.58% and 82.23% ± 1.85%, respectively. Additionally, 1× and 0.5 × MBI-FD (with serum) were 100.36% ± 2.71% and 105.74% ± 2.19%, respectively (). Cell images are shown in . According to ISO 10993-5:2009 “Biological evaluation of medical devices–Test for in vitro cytotoxicity,” the cell viability was >70%, indicating MBI-FD had no cytotoxicity to L929 cells.
Figure 2. Cell toxicity of MBI-FD. Morphologic evaluation of L929 cells exposed to different MBI-FD by MTT and observed using a microscope. Magnification 100×.
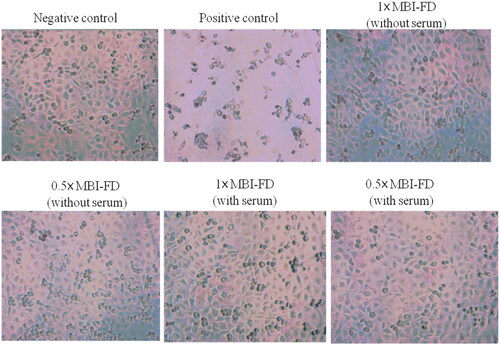
Table 2. The cytotoxicity of MBI-FD by MTT assay.
MBI-FD decreased wrinkles and had no serious adverse events
Next, we evaluated the efficacy and safety of MBI-FD on the face. A total of 95 participants (aged 30–65 years old) were recruited to examine the efficacy (24 weeks) and safety (52 weeks) of the MBI-FD therapy. The primary outcome of this trial was the rate of improvement in the WSRS at week 24 after injection by professional physicians. The treatment effective rate of the MBI-FD group was 71%, whereas that of the control group was 72% in the full analysis set. Furthermore, the treatment-effective rate of the MBI-FD group was 68%, while the treatment-effective rate of the control group was 70% per protocol set (PPS) analysis (). Therefore, the efficacy of MBI-FD was equivalent to that of the control product. Regarding safety assessment, the evaluation of adverse events, includes erythema, lumps, scabs, pain, pigmentation, and facial swelling. Among the 92 participants at 24 weeks after injection, 1 (1%), 3 (3%), and 1 (1%) developed erythema, lumps, and scabs in the MBI-FD group, respectively, whereas only 1 (1%) developed erythema in the control group. No adverse events were noted in the 90 and 93 participants at weeks 36 and 52 post-injection, respectively ().
Table 3. Improvement rate in the wrinkle severity rating scale.
Table 4. MBI-FD adverse Event evaluation.
Discussion
In recent years, with the continuous emergence of new materials, drugs, and technologies, injection cosmetic procedures and injection filling technology have rapidly developed, and clinical applications have become increasingly extensive. An ideal filling material for injection should have the following conditions: good histocompatibility; non-sensitizing, non-carcinogenic, and non-teratogenic; has a certain binding ability with human tissues; not causes inflammation and foreign body reactions; and not causes immune- and tissue-related diseases (Citation16). After the filling material is placed in the human body, it is easy to form, shape, and fix, and it is not easily absorbed. Moreover, the effect is lasting, and it is easy to disinfect and store (Citation17). Currently, commonly used filler materials include collagen, HA, botulinum toxin, hydroxyapatite, autologous fibroblasts, and autologous fat (Citation18). Among them, HA is widely used and can be used as filler in medical cosmetology. Additionally, it exists in the connective tissue and skin of the human body (Citation5). In this study, we found that MBI-FD was safe and effective. Regarding safety, MBI-FD had very low residues of BDDE, protein content, and no cytotoxicity. Regarding efficacy, it had stable thrust, biocompatibility, and reduced NLF wrinkle.
Natural HA has a loose structure, is easily decomposed by metabolism, is not easy to shape, and cannot be directly used for filling (Citation12). It requires to be artificially modified by a crosslinking agent so that natural HA molecules can produce more effective connections, which can resist the effects of biological enzymes and physical external forces (Citation7). Therefore, it is not easily metabolized and decomposed, which enables it to be maintained for a longer time. BDDE is metabolized by cytochrome P450 enzymes in the human body, and the main metabolic intermediates are glycerol and butanediol, most of which are excreted in urine or further degraded into water and carbon dioxide (Citation19). The unreacted BDDE content in FDA-approved HA fillers must be <2 ppm (Citation19,Citation20). For example, the sterile pyrogen-free physiological solution of cross-linked HA that is not of animal origin, with a BDDE content of <2 ppm (Citation14). Additionally, another brand of hyaluronic acid has a BDDE content of 0.249 ppm (Citation21). In this study, MBI-FD had very low BDDE residues of <0.125 ppm.
The application of HA injection in cosmetic medicine is mainly for the treatment of static lines and facial modification and sculpture (Citation22). Subcutaneous injection is a commonly used method. Therefore, the stability of the injection will be affected by the smoothness of its thrust. According to the Hagen–Poiseuille law, under a constant flow rate and fluid viscosity, the loss of pressure through the needle is inversely proportional to the square of the radius (inner diameter) of the needle and directly proportional to the length of the needle (Citation23). A previous study reported that 25-, 27-, and 30-G injection needles had an average pushing force of 9.5, 13.9, and 33.2 N, respectively (Citation24). Consistent with our results, MBI-FD using 27- and 30-G injection needles had an average pushing force of 14.3 and 36.4 N, respectively. The MBI-FD exhibited a constant elastic modulus. The viscosity of the filler did not induce any variations in the injection force with respect to the needle dimensions due to the manufacturer’s control of the filler properties of this series. According to the UNI EN ISO 10993 regulation, the L929 cell line was chosen for cytotoxicity assay, cell proliferation test, and cell morphology evaluation by H&E staining (Citation25). The study showed none of the HA fillers analyzed had cytotoxic effects on L929 cells at 24, 48, and 72 h, with viability values of >70% (Citation24,Citation26). Consistent with our results, the cell viabilities of 1× and 0.5 × MBI-FD were 83.25% ± 3.58% and 82.23% ± 1.85%, respectively.
The process of HA-based materials degradation and integration are key factors (Citation27). Degradation studies in vitro should help to establish the proper time points for in vivo evaluation (Citation28). During the material degradation process, chronic inflammation can also be observed. Shortly after implantation, the reaction due to the surgical procedure itself is difficult to distinguish from the reaction caused by the implant (Citation29). Finally, a homeostatic state of the tissue is expected after complete absorption of the material. Some HA fillers can take 12–18 months or 2–5 years to begin biodegrading without any cytotoxic response to the intact material and its degradation products (Citation27). Specific brands in the market showed complete clinical biodegradation was observed within 52 weeks of the last treatment (Citation21). Consistent with our results, MBI-FD can take 1 year to begin biodegrading, and no abnormal signs were observed. In the clinical case of dermal fillers, FDA-approved brands are used. Some studies have shown that WSRS can improve by approximately 38–75% after 6 months of using dermal fillers. However, adverse events still occur (Citation30). In this study, we used a specific brand in the market as the control group and found MBI-FD improved WSRS by approximately 68%–75% and had no serious adverse events, suggesting MBI-FD is equal to or better than a specific brand in the market. Overall, MBI-FD showed excellent safety and efficacy.
Conclusion
Based on the results of the present study, MBI-FD had low residual and protein content, no cytotoxicity, and good biocompatibility. In clinical application, it showed better stability of the injection and effectively decreased NLF wrinkles. Notably, no adverse serious reactions occurred in the safety assessment. Therefore, MBI-FD may be a suitable candidate as an injectable dermal filler for tissue augmentation in humans in the future.
Ethical approval
All the procedures were approved by the Tri-Service General Hospital approved this study (institutional review board approval number: 2-102-05-113), and the study was registered on ClinicalTrials.gov Identifier: NCT05822778
Acknowledgements
Thanks to the Tri-Service General Hospital team and the subjects who participated in the trial.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data is available on request from the corresponding author.
Additional information
Funding
References
- Ganceviciene R, Liakou AI, Theodoridis A, et al. Skin anti-aging strategies. Dermatoendocrinol. 2012;4(3):1–7. doi:10.4161/derm.22804.
- Arlette JP, Trotter MJ. Anatomic location of hyaluronic acid filler material injected into the nasolabial fold: a histologic study. Dermatol Surg. 2008;34 Suppl 1:S56–S62. discussion S-3. doi:10.1111/j.1524-4725.2008.34244.x.
- Abdo JM, Sopko NA, Milner SM. The applied anatomy of human skin: a model for regeneration. Wound Med. 2020;28:100179. doi:10.1016/j.wndm.2020.100179.
- Brodell LA, Rosenthal KS. Skin structure and function: the body’s primary defense against infection. Infect Dis Clin Pract. 2008;16:113–117. doi:10.1097/IPC.0b013e3181660bf4.
- Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinol. 2012;4(3):253–258. doi:10.4161/derm.21923.
- Kogan G, Soltes L, Stern R, et al. Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett. 2007;29(1):17–25. doi:10.1007/s10529-006-9219-z.
- Fallacara A, Baldini E, Manfredini S, et al. Hyaluronic acid in the third millennium. Polymers. 2018;10(7):701. doi:10.3390/polym10070701.
- Swift A, Liew S, Weinkle S, et al. The facial aging process from the "inside Out. Aesthet Surg J. 2021;41(10):1107–1119. doi:10.1093/asj/sjaa339.
- Gold MH. Use of hyaluronic acid fillers for the treatment of the aging face. Clin Interv Aging. 2007;2(3):369–376. doi:10.2147/cia.s1244.
- Juncan AM, Moisă DG, Santini A, et al. Advantages of hyaluronic acid and its combination with other bioactive ingredients in cosmeceuticals. Molecules. 2021;26(15):4429. doi:10.3390/molecules26154429.
- Brandt FS, Cazzaniga A. Hyaluronic acid gel fillers in the management of facial aging. Clin Interv Aging. 2008;3(1):153–159. doi:10.2147/cia.s2135.
- Fakhari A, Berkland C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater. 2013;9(7):7081–7092. doi:10.1016/j.actbio.2013.03.005.
- Fan Y, Choi T-H, Chung J-H, et al. Hyaluronic Acid-Crosslinked filler stimulates collagen type 1 and elastic fiber synthesis in skin through the TGF-β/smad signaling pathway in a nude mouse model. J Plast Reconstr Aesthet Surg. 2019;72(8):1355–1362. doi:10.1016/j.bjps.2019.03.032.
- Allemann I, Baumann L. Hyaluronic acid gel (juvéderm™) preparations in the treatment of facial wrinkles and folds. Clin Interv Aging. 2008;3(4):629–634. doi:10.2147/cia.s3118.
- Zhao P, Zhao W, Zhang K, et al. Polymeric injectable fillers for cosmetology: current status, future trends, and regulatory perspectives. J Appl Polym Sci. 2020;137:48515.
- Prasetyo AD, Prager W, Rubin MG, et al. Hyaluronic acid fillers with cohesive polydensified matrix for soft-tissue augmentation and rejuvenation: a literature review. Clin Cosmet Investig Dermatol. 2016;9:257–280. doi:10.2147/CCID.S106551.
- Bago I, Plotino G, Katic M, et al. Evaluation of filling material remnants after basic preparation, apical enlargement and final irrigation in retreatment of severely curved root canals in extracted teeth. Int Endod J. 2020;53(7):962–973. doi:10.1111/iej.13287.
- Li K, Meng F, Li YR, et al. Application of nonsurgical modalities in improving facial aging. Int J Dent. 2022;2022:8332631–8332618. doi:10.1155/2022/8332631.
- De Boulle K, Glogau R, Kono T, et al. A review of the metabolism of 1,4-butanediol diglycidyl ether-crosslinked hyaluronic acid dermal fillers. Dermatol Surg. 2013;39(12):1758–1766. doi:10.1111/dsu.12301.
- Guarise C, Barbera C, Pavan M, et al. HA-based dermal filler: downstream process comparison, impurity quantitation by validated HPLC-MS analysis, and in vivo residence time study. J Appl Biomater Funct Mater. 2019;17(3):2280800019867075. doi:10.1177/2280800019867075.
- Xie Y, Wu S, Li S, et al. Evaluation of the long-term safety and biodegradability of hyaluronic acid dermal fillers (YVOIRE®) for the correction of nasolabial folds: two multicenter, prospective, observational cohort studies. J Cosmet Dermatol. 2022;21(6):2387–2397. doi:10.1111/jocd.14952.
- Trévidic P, Kaufman-Janette J, Weinkle S, et al. Injection guidelines for treating midface volume deficiency with hyaluronic acid fillers: the ATP approach (anatomy, techniques, products). Aesthet Surg J. 2022;42(8):920–934. doi:10.1093/asj/sjac007.
- Ginosar Y, Smith Y, Ben-Hur T, et al. Novel pulsatile cerebrospinal fluid model to assess pressure manometry and fluid sampling through spinal needles of different gauge: support for the use of a 22 G spinal needle with a tapered 27 G pencil-point tip. Br J Anaesth. 2012;108(2):308–315. doi:10.1093/bja/aer372.
- Vo A, Doumit M, Rockwell G. The biomechanics and optimization of the needle-syringe system for injecting triamcinolone acetonide into keloids. J Med Eng. 2016;2016:5162394–5162398. doi:10.1155/2016/5162394.
- Cannella V, Altomare R, Chiaramonte G, et al. Cytotoxicity evaluation of endodontic pins on L929 cell line. Biomed Res Int. 2019;2019:3469525–3469525. doi:10.1155/2019/3469525.
- Cannella V, Altomare R, Leonardi V, et al. In vitro biocompatibility evaluation of nine dermal fillers on L929 cell line. Biomed Res Int. 2020;2020:8676343.
- Huerta-Angeles G, Nesporova K, Ambrozova G, et al. An effective translation: the development of Hyaluronan-based medical products from the physicochemical, and preclinical aspects. Front Bioeng Biotechnol. 2018;6:62. doi:10.3389/fbioe.2018.00062.
- Freier T, Kunze C, Nischan C, et al. In vitro and in vivo degradation studies for development of a biodegradable patch based on poly(3-hydroxybutyrate). Biomaterials. 2002;23(13):2649–2657. doi:10.1016/s0142-9612(01)00405-7.
- Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86–100. doi:10.1016/j.smim.2007.11.004.
- Carruthers A, Carey W, De Lorenzi C, et al. Randomized, double-blind comparison of the efficacy of two hyaluronic acid derivatives, restylane perlane and hylaform, in the treatment of nasolabial folds. Dermatol Surg. 2005;31(11 Pt 2):1591–1598; discussion 1598. doi:10.2310/6350.2005.31246.