Dear Editor,
Atopic dermatitis (AD) is a common inflammatory cutaneous disease that can affect people of all age groups, presented by the age of five years old in nearly 85% of the patients. Recently, elderly AD has been considered a distinct clinical type of AD (Citation1). However, the treatment of these patients can be challenging because of the higher prevalence of comorbidities and the side effects of conventional systemic drugs (i.e. cyclosporin). Moreover, data on the effectiveness and safety of innovative drugs, such as dupilumab and JAK inhibitors, are still very limited (Citation2,Citation3).
Dupilumab is a monoclonal antibody that targets the interleukin (IL)-4 receptor, approved for treating moderate-to-severe AD in children ranging from 6 to 11 years old and both adolescents and adults (Citation4,Citation5). However, few elderly subjects have been included in clinical trials and real-life studies evaluating dupilumab (Citation6).
Here, we report our experience on 40 patients aged ≥ 65 years who were all affected by AD and treated with dupilumab. All patients completed at least 16 weeks of therapy, with 23 of them reaching week 52. Additionally, eight patients were followed up for more than two years.
Twenty-one patients were male (52.5%). Their mean age was 75.9 ± 6.5 years, and 75% of the patients experienced the first onset of AD starting from the age of 65. Five patients (12.5%) reported a new onset of AD after receiving COVID-19 vaccination. Only eight patients had at least one atopic comorbidity, such as rhinitis, asthma, or conjunctivitis. Interestingly, ten patients (25%) had known contact allergies (with positive patch test results), and six patients had a previous history of drug allergies (15%). Almost all patients had been previously treated with topical and/or oral corticosteroids (38 patients, 95%). Most patients did not receive cyclosporine due to comorbidities, predominantly arterial hypertension. The complete demographic and clinical characteristics of the patients are shown in .
Table 1. Demographic and clinical characteristics of the patients.
During the follow-up period, the mean EASI (Eczema Area and Severity Index) decreased from 25.33 ± 1.83 at baseline to 3.20 ± 4.35 at week 16. The mean EASI was lower at week 32 (1.76 ± 2.63), week 52 (1.56 ± 2.38), and week 104 (0.87 ± 1.25). At weeks 16 and 52, 57.5% and 73.9% of our patients achieved a 90% reduction in the EASI score (EASI90), respectively. In addition, complete skin clearance (EASI100) was observed in 27.5% and 56.5% of patients at weeks 16 and 52, respectively. The improvement in the pruritus-NRS (Numerical Rating Scale) score was significant at week 16, as 93% of our patients experienced a decrease of at least 4 points from baseline. Additional effectiveness endpoints are shown in . No serious adverse events (AEs) or AEs leading to discontinuation were reported throughout the study period.
Figure 1. Percentages of patients that reached EASI 75, EASI 90, EASI 100, Validated Investigator Global Assessment scale for Atopic Dermatitis (vIGA-AD) score 0 or 1 (left); percentages of mean delta EASI variation from baseline (top right); percentages of patients that reached a difference of pruritus-NRS of four points from baseline (bottom right).
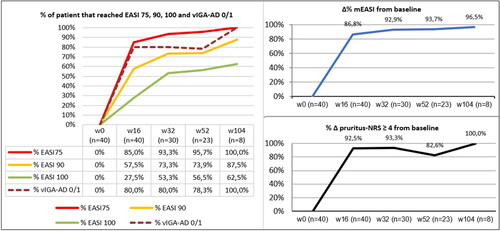
People over 65 years of age are under-represented in clinical trials evaluating drugs for AD because of the higher rates of comorbidities and multiple concomitant medications. Few data are available regarding the role of dupilumab in elderly patients with AD. Our study found a slightly better response in terms of mean EASI reduction after one year compared with the study from Patruno et al. (93.7% versus 87.2%) (Citation7). These data are consistent with those reported in the long-term registration trial, CHRONOS, which was conducted mainly in younger patients. Dupilumab demonstrated a rapid effect on pruritus in our cohort, with a higher proportion of patients experiencing a reduction of at least 4 points in the pruritus-NRS compared with the clinical trials SOLO 1 and SOLO 2 (Citation5). Dupilumab was well-tolerated in our study, as no new safety findings were reported.
Our experience supports the effectiveness and safety of dupilumab in a cohort of elderly patients, most of them having contraindications to conventional systemic treatments. However, additional studies, possibly with longitudinal designs, are needed to further assess the role of dupilumab in these patients.
Compliance with ethics guidelines
Institutional review board approval was exempted as the study protocol did not deviate from standard clinical practice. All patients received dupilumab as in good clinical practice, in accordance with European guidelines. All included patients had provided written consent for retrospective study of data collected during routine clinical practice (demographics, clinical scores). The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Data collection and handling complied with applicable laws, regulations, and guidance regarding patient protection, including patient privacy.
Disclosure statement
L. Gargiulo has received research grants from Almirall. M. Valenti has been a consultant and/or speaker for Sanofi, Leo Pharma, Eli Lilly and Boehringer Ingelhei. A. Costanzo has served as an advisory board member, consultant and has received fees and speaker’s honoraria or has participated in clinical trials for Abbvie, Almirall, Biogen, LEO Pharma, Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB-Pharma. A. Narcisi has served on advisory boards, received honoraria for lectures and research grants from Almirall, Abbvie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi-Genzyme, Amgen and Boehringer Ingelheim. The other authors have nothing to disclose.
Data availability statement
Data available on request from the authors.
References
- Williams HC, Strachan DP. The natural history of childhood eczema: observations from the british 1958 birth cohort study. Br J Dermatol. 1998;139(5):1–2.
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema: part I - systemic therapy. J Eur Acad Dermatol Venereol. 2022;36(9):1409–1431.
- Gargiulo L, Ibba L, Cortese A, et al. Real-Life effectiveness and safety of upadacitinib in adults and adolescents with moderate-to-Severe atopic dermatitis: a Single-Center 16-Week study. Dermatol Ther (Heidelb). 2023;13(2):651–660.
- Costanzo A, Amerio P, Asero R, Italian AD Study Group, et al. Long-term management of moderate-to-severe adult atopic dermatitis: a consensus by the italian society of dermatology and venereology (SIDeMaST), the association of italian territorial and hospital allergists and immunologists (AAIITO), the italian association of hospital dermatologists (ADOI), the italian society of allergological, environmental and occupational dermatology (SIDAPA), and the italian society of allergy, asthma and clinical immunology (SIAAIC). Ital J Dermatol Venerol. 2022;157(1):1–12.
- Simpson EL, Bieber T, Guttman-Yassky E, SOLO 1 and SOLO 2 Investigators, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348.
- Worm M, Simpson EL, Thaçi D, et al. Efficacy and safety of multiple dupilumab dose regimens after initial successful treatment in patients with atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:131–143.
- Patruno C, Fabbrocini G, Longo G, Dupilumab for Atopic Dermatitis of the Elderly (DADE) Study Group, et al. Effectiveness and safety of Long-Term dupilumab treatment in elderly patients with atopic dermatitis: a multicenter Real-Life observational study. Am J Clin Dermatol. 2021;22(4):581–586.

