Abstract
Background
Dupilumab is prescribed in one dosage across adult atopic dermatitis patients. Differences in drug exposure may explain variation in treatment response.
Objective
Investigating the clinical relevance of dupilumab serum concentration in atopic dermatitis in real-world practice.
Methods
In two centers (Netherlands, UK), adults treated with dupilumab for atopic dermatitis were evaluated for effectiveness and safety pretreatment and at 2, 12, 24, and 48 weeks; trough serum samples were analyzed for dupilumab concentration at corresponding time points.
Results
In 149 patients, median dupilumab levels during follow-up ranged from 57.4 to 72.4 μg/mL. Levels showed high inter-patient and low intra-patient variability. No correlation was found between levels and ΔEASI. At 2 weeks, levels of ≥64.1 μg/mL predict EASI ≤7 at 24 weeks (specificity:100%, sensitivity:60%; p = .022). At 12 weeks, ≤32.7 μg/mL predicts EASI >7 at 24 weeks (sensitivity:95%, specificity:26%; p = .011). Inverse correlations were found between baseline EASI and levels at 2, 12, and 24 weeks (r = −0.25 to 0.36; p ≤ .023). Low levels were particularly observed in patients with adverse events, treatment interval deviation, and discontinuation.
Conclusion
At the on-label dosage, the measured range of dupilumab levels does not seem to yield differences in treatment effectiveness. However, disease activity does seem to influence dupilumab levels - higher baseline disease activity results in lower levels at follow-up.
Introduction
Dupilumab is a human monoclonal antibody against interleukin (IL)-4 receptor alpha that inhibits IL-4/IL-13 signaling (Citation1). In Europe, dupilumab is approved for the treatment of moderate-to-severe atopic dermatitis (AD) in patients aged ≥6 years. Dupilumab’s product assessment report by the European Medicines Agency (EMA) mentions that a dose-response study was conducted comparing different dosing schedules. A dose-dependent Eczema Area and Severity Index (EASI) reduction was observed from baseline to week 16, with the highest reductions in the 300 mg fortnightly and weekly groups (Citation2). In studies comparing weekly and fortnightly dosing schedules no differences were found regarding efficacy and safety (Citation3,Citation4). A fortnightly administration of 300 mg was defined as the licensed posology. Consistent with phase III trial data (Citation5), we have shown that this dosage delivers a sustained improvement of patient- and investigator-reported outcomes in real-world practice (Citation6–9).
Serum concentrations of therapeutics are determined by drug- and patient-related characteristics that affect absorption, distribution, and elimination (Citation10,Citation11). After a single subcutaneous injection of dupilumab, peak drug concentrations are achieved at approximately 7 d, followed by a slow decrease in concentration thereafter. Across clinical trials, steady-state concentrations resulting from fortnightly 300 mg injections are achieved by week 16 with mean trough concentrations ranging from 60.3 μg/mL to 80.2 μg/mL (Citation12,Citation13). After discontinuation, the median time to decrease below a non-detectable concentration is 10 weeks (Citation1,Citation14). At the population level, the on-label adult dosage was determined to achieve sufficient drug exposure with concentrations at the plateau of the exposure–effect relationship (Citation15). At an individual level, patient characteristics may influence drug exposure and therefore treatment response (i.e., both effectiveness and safety).
Four and 8-week dosage intervals were described to be associated with lower serum concentrations and decreased effectiveness (Citation4). Clinical trial patients with lower trough levels at week 16 were found to have less clinical improvement, but no exposure-response relationship was identified for adverse events (AEs) (Citation2). However, such data is limited, with published studies investigating the relationship between dupilumab levels and conjunctivitis as AE, and not on other aspects including effectiveness. Serum drug level data from the Phase 3 studies did suggest an inverse relationship whereby conjunctivitis incidence may decrease with higher week 16 trough concentrations of dupilumab (Citation16,Citation17). Local under-treatment (inadequate drug exposure), due to a higher target burden or lower tissue distribution, were suggested as potential contributing factors (Citation16). However, this was not replicated in adolescents where the incidence of conjunctivitis showed no relationship with concentrations at 16 weeks (Citation18). Conjunctivitis is a relevant adverse event in patients on dupilumab, but this diagnosis may capture a range of eye conditions including atopic eye disease and dupilumab-associated ocular surface disease, which likely differ in etiology which complicates the study of potential contributory factors such as drug level.
Variation in treatment response to dupilumab exists (Citation16). Differences in drug exposure may explain this variation. Further investigating the relationship between dupilumab levels and treatment response would give insight into the potential of therapeutic drug monitoring. The utility of therapeutic drug monitoring has been proven in the use of TNF-antagonists for psoriasis (Citation19). As currently a one-size-fits-all approach is applied when prescribing dupilumab, we performed an exploratory study to investigate this standard dosing. The aim of this study was to investigate the clinical relevance (i.e., the influence on both effectiveness and safety) of serum concentrations of dupilumab in AD in real-world practice.
Materials and methods
Study design and population
A prospective cohort study was conducted. Patients with AD based on the U.K. working party’s criteria (Citation20), receiving dupilumab treatment in the context of routine clinical care, were included from July 2018 to February 2021 at the Amsterdam University Medical Centers, The Netherlands (NL), and from July 2017 to November 2018 at the Guy’s and St Thomas’ NHS Foundation Trust, the United Kingdom (UK). All patients met the national reimbursement criteria for dupilumab, which stipulate a treatment episode with one or more conventional systemic therapies (Citation21,Citation22). The majority of participants (n = 103) were also participants of the TREAT NL (TREatment of ATopic eczema, the Netherlands) registry (Citation23,Citation24). In all patients, additional informed consent was obtained for participation in this study. Ethical approval has been obtained from the appropriate local ethics committee. Treatment discontinuation resulted in the discontinuation of study participation.
Study outcomes
Clinical outcomes included an investigator-assessed measure of effectiveness (EASI: 0–72 (Citation25)), patient-reported outcome measures (Numerical Rating Scale (NRS): 0–10, NRS peak pruritus past 24 h (NL); mean pruritus past 7 d (UK) (Citation26), Patient-Oriented Eczema Measure (POEM): 0-28 (Citation27) and Dermatology Life Quality Index (DLQI): 0–30 (Citation28)) and safety (AE definitions included in ) (Citation29), measured at baseline (NL + UK), 2 (UK), 12, 24 and 48 weeks (NL + UK).
Table 1. Adverse events.
Blood samples were collected at baseline (NL + UK), 2 (UK), 12 (NL + UK), 24 (NL), and 48 (NL) weeks. The time point of blood sampling was aspired to be at the trough level, just before a new dose administration. Samples were centrifuged, aliquoted into microtubes, and frozen at −20 °C (NL) and −80 °C (UK). Serum levels were measured using an enzyme-linked immunosorbent assay (ELISA). Maxisorp microtiter plates were coated overnight at room temperature (RT) with 1 μg/mL monoclonal anti-dupilumab (clone 1G11). This is a chimeric antibody of rabbit origin, with a mouse IgG2b Fc, recombinantly expressed as described before (Citation30). After five times washing with PBS/0.02% Tween (PT), plates were incubated for 1 h at RT with patient serum samples, diluted 100-fold, and 2000-fold in high-performance ELISA buffer (HPE, Sanquin). Subsequently, the plates were washed with PT and incubated for 1 h with 0.5 μg/mL mouse monoclonal antihuman IgG4 (clone MH164.4, Sanquin). After washing, the ELISA was developed with 1-step ultra TMB-ELISA Substrate Solution (thermoFischer) diluted with MQ (ratio 3:1). The reaction was stopped with 0.2 M HCl. Delta of the absorption at 450 and 540 nm was determined and compared to a titration curve of dupilumab in each plate. The lower limit of quantification is 0.3 μg/mL; accuracy and precision ranged from 87% to 102% and 4.4% to 12.2% CV (coefficient of variation).
Statistical analyses
Patient characteristics and outcomes were summarized using descriptive statistics. Furthermore, we predefined the following analyses of interest.
To investigate the relationship between concentration and clinical response, concentration-effect curves were established. Patients were ordered categorically based on dupilumab level into groups of 10 with corresponding ΔEASI (Citation19,Citation31). Sensitivity analysis included only patients with a moderate-to-severe baseline EASI (EASI ≥ 6.0) (Citation32). Both non-predictive (i.e., at individual time points) and predictive (i.e., levels predicting future response) analyses were performed. In addition, correlations between levels and (absolute) outcomes at each individual time point were explored using Spearman correlations, Chi-squared tests, and Mann–Whitney tests, as appropriate. Predictive analyses were also undertaken by assessing the correlation between baseline EASI and dupilumab levels.
To further examine whether early drug exposure predicted clinical response, we used internationally agreed criteria for response and stratified patients into a group that did and did not reach EASI ≤ 7 and ≥1 disease domain targets (EASI ≤ 7, NRS ≤ 4, POEM ≤ 7, DLQI ≤ 5) at 24 weeks, in accordance with the Treat-to-Target algorithm (Citation33). Subsequently, we investigated whether drug levels at 2, 12, and 24 weeks and baseline EASI predicted outcomes at 24 weeks using Mann–Whitney tests and receiver-operator characteristics (ROC) curves (Citation19,Citation31).
Statistical analysis of the data was performed using the software program SPSS (IBM SPSS Statistics 26). Missing data were excluded on a test-by-test basis. Results were considered statistically significant at p < .05.
Results
Patient characteristics
Of 178 consented to the study, 149 patients were included in the analyses. Twenty-nine patients were excluded based on a lack of serum sampling (n = 24), refraining from starting dupilumab (n = 3) or receiving dupilumab prior to participation (n = 2). Baseline characteristics are shown in . The majority was male (63.1%), white (75.2%), and had skin type II (42.3%). The median age was 43 years and BMI was 24.7. Dupilumab was prescribed according to the licensed posology (600 mg loading dose followed by 300 mg fortnightly). However, one patient received 300 mg at baseline due to a suspected adverse reaction to dupilumab. In 17 patients the dosing schedule was adjusted during follow-up (range: 10 d–5 weeks; see results section on treatment regimen deviations). Eighteen patients concomitantly used prednisone at baseline. Four patients continued prednisone treatment during follow-up and 14 (NL) patients used prednisone in a tapering schedule for a median of 24.5 d. The other 131 patients were treated with dupilumab monotherapy often in combination with oral antihistamines and topical treatments. The median follow-up duration was 48 weeks for the outcome measures (range: 0–58 weeks) and 24 weeks for the blood samples (range: −4 to 59 weeks). Median EASI, NRS, POEM, and DLQI scores at baseline and during follow-up are described in .
Table 2. Patient characteristics at baseline (n = 149).
Table 3. Outcome measures and dupilumab serum concentrations at baseline and during follow-up.
Dupilumab trough drug levels
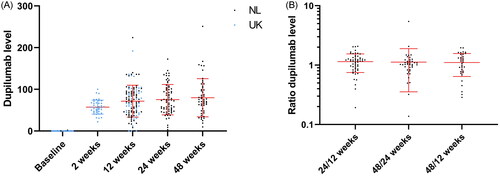
At baseline, 2, 12, 24, and 48 weeks, respectively, serum samples were obtained from 115, 41, 115, 71, and 53 patients. Dupilumab levels ranged from being undetectable at baseline to a level of 251.0 μg/mL at 48 weeks. The minimum dupilumab concentration measured during the follow-up was 22.2 μg/mL. The median concentrations per time point are displayed in . shows that dupilumab drug levels seem to reach a plateau by 12 weeks and to be stable over time when assessing the ratios between time points (i.e., low intra-patient variability).
Figure 1. (A) Dupilumab serum levels over time. Dot plot of the dupilumab levels at different time points (with mean and SDs in red). (B) The ratio of dupilumab serum levels. Dot plot of the ratios of the dupilumab levels at different time points (with mean and SDs in red), which approach 1 (i.e., the concentrations are similar over time).
Correlation between dupilumab levels and ΔEASI
There was no correlation between serum dupilumab at 12 and 24 weeks and change in EASI from baseline (ΔEASI) at respectively 12 and 24 weeks (); this was also true for the subpopulation with moderate-to-severe baseline EASI (≥6.0) at 24 weeks and for the subpopulation on monotherapy dupilumab at 12 weeks (Supplementary Figure 1 and 2). All curves showed large inter-patient variability in dupilumab levels.
Figure 2. (A) The concentration-effect curve for dupilumab level and ΔEASI at 24 weeks. A concentration-effect curve showing the dupilumab serum level in μg/mL at 24 weeks on the x-axis and correlating ΔEASI at 24 weeks (versus baseline) on the y-axis. All patients were sorted from low to high drug concentration, with each dot representing the mean concentration with SDs and correlating ΔEASI for 10 patients (last group 9 patients). (B) The concentration-effect curve for dupilumab level and ΔEASI at 12 weeks. The concentration-effect curve showing the dupilumab serum level in μg/mL at 12 weeks on the x-axis and correlating ΔEASI at 12 weeks (versus baseline) on the y-axis. All patients were sorted from low to high drug concentration, with each dot representing the mean concentration with SDs and correlating ΔEASI for 10 patients (last group 11 patients).
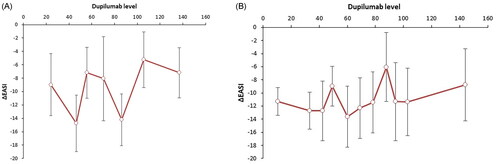
As for predictive analyses, Supplementary Figure 3 shows that serum levels at 12 weeks also do not seem to correlate with ΔEASI at 48 weeks. In additional curves, we also found no correlation between serum levels at 2 and 12 weeks and ΔEASI at 24 weeks.
Early dupilumab levels, baseline severity and further prediction of response
At 24 weeks, 64.6% (n = 73/113) patients reached EASI ≤ 7 and 86.1% (n = 105/122) patients reached ≥1 disease domain targets. We observed a higher dupilumab level at 2 and 12 weeks in patients that reached EASI ≤ 7, compared to patients who did not (p = .022 (median: 66.5 vs. 50.2 μg/mL) and p = .011 (76.0 vs. 57.62 μg/mL), respectively).
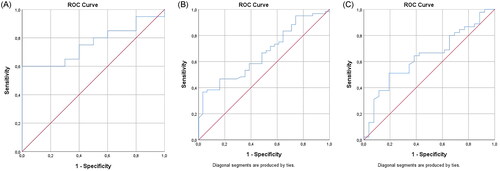
ROC curves in show an area under the curve (AUC) of 0.760 (95% CI: 0.591–0.929, p = .022) and 0.664 (95% CI: 0.552–0.777, p = .011) for dupilumab levels at respectively 2 and 12 weeks. AUCs were significantly different from 0.5, indicating that dupilumab levels at 2 and 12 weeks have the ability to distinguish between the group that did and did not reach EASI ≤ 7 at 24 weeks. At 2 weeks, a sensitivity of 60% and a specificity of 100% was found for 64.1 μg/mL. At 12 weeks, a sensitivity of 95% and a specificity of 26% was found for a concentration of 32.7 μg/mL. No difference was found for levels at 24 weeks (p = .053, ); and for reaching ≥ 1 disease domain targets at 24 weeks and levels at any of the time points.
Figure 3. (A) ROC curve for patients reaching EASI ≤ 7 at 24 weeks of treatment and dupilumab serum level at 2 weeks. The AUC in the ROC curve is not significantly different from 0.5, indicating that dupilumab concentration does not have the ability to distinguish between the group that reached EASI ≤ 7 (AUC 0.760, 95% CI: 0.591–0.929, p = .022). At 64.1 μg/mL, a sensitivity of 60% and a specificity of 100% was found. (B) ROC curve for patients reaching EASI ≤ 7 at 24 weeks of treatment and dupilumab serum level at 12 weeks. The AUC in the ROC curve is significantly different from 0.5, indicating that the dupilumab concentration has the ability to distinguish the group that reached EASI ≤ 7 (AUC 0.664, 95% CI: 0.552–0.777, p = .011). At 32.7μg/mL, a sensitivity of 95% and a specificity of 26% was found. (C) ROC curve for patients reaching EASI ≤ 7 at 24 weeks of treatment and dupilumab serum level at 24 weeks. The AUC in the ROC curve is not significantly different from 0.5, indicating that dupilumab concentration does not have the ability to distinguish between the group that reached EASI ≤ 7 (AUC 0.638, p = .053, borderline significant). At 59.6μg/mL, a sensitivity of 67% and a specificity of 58% was found for EASI ≤ 7.
When investigating the predictive value of baseline severity, we found a lower baseline EASI score in patients reaching EASI ≤ 7 at 24 weeks (p < .001 (12.5 vs. 23.6)).
Correlation between dupilumab levels and absolute outcomes
Analyzing data at the same time point, we found a weak negative correlation between dupilumab levels and absolute EASI at 24 (r = −0.31, p = .009) and 12 weeks (r = −0.33, p < .001). Correspondingly, when dividing patients into high and low-level groups (based on the median dupilumab level), we found higher EASI scores at 2 and 12 weeks in patients with low serum levels at respectively 2 and 12 weeks (p = .030 and p = .015).
When analyzing predictive relationships between baseline EASI and dupilumab levels at follow-up, a correlation was found between baseline EASI and levels at 2, 12, and 24 weeks of follow-up (r = −0.25 to 0.36, p ≤ .023).
Correlation between dupilumab levels and adverse events
In total, 126 AEs were registered in 72 patients (n = 72/149, 48.3%; ). The median number of days from the start dupilumab until the event presentation was 59 d. Eye disorders were most frequently reported (n = 49), starting after a median of 70 d. Twelve serious AEs were reported, of which one (arthralgia) was considered possibly related to dupilumab.
At 24 weeks, 59% of patients with a low serum level (based on the median) has experienced at least one AE, in contrast to 28% of patients with a high level (p = .009). At 48 weeks, 68% of patients with low levels has experienced at least one AE, in contrast to 26% of patients with high levels (p = .002). No associations were found for 2 and 12 weeks.
Correlation between dupilumab levels and treatment regimen deviations
In 17 patients (n = 17/149, 11.4%) the dosing schedule was adjusted without resulting in treatment discontinuation, either by prolonging or shortening the injection interval. Eleven patients prolonged, all due to AEs. Five of these increased the interval to once every 3 weeks, one to once every 4 weeks, and five to different intervals ranging from 2 to 5 weeks across the study. In six patients the interval was shortened to a 10 d interval due to ineffectiveness. One of these switched back to on-label use.
We found an association between low concentrations at 48 and 24 weeks and the presence of treatment interval deviations during the study. At 48 weeks, 36.0% of patients with low levels has experienced interval deviations, in contrast to 7.4% of patients with high levels (p = .012). Similar results were found for levels at 24 weeks (p = .023). No association was found for 12 weeks.
Correlation between dupilumab levels and treatment discontinuation
Seventeen patients (n = 17/149, 11.4%) discontinued dupilumab. In five patients discontinuation occurred due to ineffectiveness, after 218 d on average. Two of these patients applied a 10 d interval prior to discontinuation. One patient discontinued due to combined ineffectiveness and AEs (eye complaints). Three patients discontinued resulting from non-adherence, one because of a child wish, one because of elective surgery, and six due to AEs: eye complaints (n = 4), facial redness (n = 1), and panniculitis of unknown origin (n = 1). The 17 patients who discontinued treatment with dupilumab, simultaneously stopped study participation (i.e., there are no patients that underwent serum sampling after discontinuation of dupilumab).
We found an association between low dupilumab levels at 48 weeks and treatment discontinuation during the study. Of patients with low levels at 48 weeks, 16.0% has discontinued treatment, in contrast to 0.0% of patients with high levels (p = .031). No associations were found between discontinuation and levels at 2, 12, and 24 weeks.
Discussion
This study gives an overview of dupilumab levels in real-world AD patients and how these levels affect treatment response. We found median levels consistent with published pharmacokinetics data from clinical trials (Citation12,Citation13). In the available literature, treatment duration, gender, and age have shown not to affect dupilumab levels and the impact of weight appears to be negligible (Citation10,Citation13). No literature was available on the role of other potential factors, such as (baseline) disease activity. In accordance with other IgG antibodies, dupilumab has a low volume of distribution and a slow rate of elimination (Citation11). Available pharmacokinetics data shows that a strong non-linear clearance is present, in particular with lower concentrations (below 10 μg/mL) (Citation10,Citation11,Citation15). This may have been the reason for ascertaining a high dosage, as a strategy to achieve maximum drug exposure. The lower bound of dupilumab concentration during follow-up in this study was 22.2 μg/mL, which is high in comparison to other cytokine-targeting biologics, such as ustekinumab or TNF-inhibitors (Citation19,Citation34). Besides preventing a strong clearance rate, a high dosage could be aspired to overrule immunogenicity. A therapeutic window for dupilumab concentration has not yet been defined.
In the context of effectiveness, drug levels did not seem to correlate with ΔEASI and therefore we are not able to define a therapeutic window. A large inter-patient variability in concentrations was observed, in which all measured concentrations seem to yield sufficient responses, as the group with the lowest dosages also reaches the minimal clinically important difference of 6.6 for EASI (Citation35). If patients are dosed much higher than required, high drug levels have the potential to impair the ability to determine relevant clinical differences based on these levels, as few if any patient will have suboptimal concentrations. However, we did observe a weak negative correlation between dupilumab levels and absolute EASI at 12 and 24 weeks. No correlations were found for the other outcome measures and time points, illustrating that higher drug levels do not necessarily correspond to lower EASI, NRS, POEM, and DLQI. When dividing patients into high and low serum levels, we did find higher EASI in patients with low levels at 2 and 12 weeks.
How to interpret correlations between EASI and drug levels may be difficult. A concentration-response relationship may exist in either direction. An association between serum concentration and subsequently (Δ)EASI (as outcome) may be expected at optimal dosages within the therapeutic window and at the current dosage we did not find this association, potentially because dupilumab is highly dosed. This is in accordance with previous findings, where concentrations at the plateau of the exposure-effect relationship were observed (Citation15). However, a directional association between EASI and concentration (as outcome) could also be observed, corresponding with the results of this study.
Baseline EASI was shown to be subsequently negatively correlated with dupilumab levels, suggesting that disease activity has an influence on drug levels. As baseline EASI cannot be affected by exposure to dupilumab (i.e., at baseline patients were not exposed to dupilumab yet), the direction of this effect can only exist in one way (i.e., with drug level as the outcome and not the other way around). We hypothesize that higher disease activity leads to a higher clearance rate of dupilumab (e.g., because more target (IL4R) is available) and thereby lower drug levels of dupilumab (i.e., target-mediation disposition).
Interestingly, we found that dupilumab serum levels at 2 and 12 weeks have the ability to predict treatment response at 24 weeks. At 2 weeks, a serum level of ≥64.1 μg/mL predicts an EASI ≤ 7 at 24 weeks. At 12 weeks, a serum level of ≤32.7 μg/mL predicts an EASI > 7 at 24 weeks. However, these correlations may also be determined by the relation between drug levels and disease activity, as a correlation was also found between baseline EASI and EASI ≤ 7 at 24 weeks.
As for safety and other treatment aspects, AEs were particularly observed in patients with low drug levels. This corresponds with trial data showing a trend for an inverse relationship between concentrations and conjunctivitis (Citation16,Citation17). Given this is a real-world study, dosing intervals may have been amended to mitigate AEs like eye complaints, which makes this difficult to interpret (i.e., another example of a bidirectional correlation). We did not investigate the direction of this effect in this scoping study. Furthermore, we found more interval deviations and discontinuation in patients with low dupilumab serum levels.
Limitations
As a consequence of a real-world setting, no randomization or blinding was performed. No washout periods were applied, resulting in relatively low baseline severity scores, influencing ΔEASI analyses. All available measurements were included. Despite efforts to collect all data, protocol deviations were present. COVID-19 has resulted in missing data at random. In a small subset, serum samples were not obtained at a trough level. Potentially, multiple testing could have increased the risk of false-positive results. Furthermore, dosing interval deviations, concomitant treatment, treatment non-adherence and anti-drug antibodies (ADA) may have influenced our findings. A more comprehensive assessment of total drug exposure would enable a more accurate evaluation of the relationship between dose, exposure, and outcome. To do this accurately would require formal pharmacokinetic/pharmacodynamic (PK/PD) modeling, which is beyond the scope of this exploratory work. We did not evaluate ADA. In theory, ADA could decrease circulating functional drug levels. Based on trial data, ADA development to dupilumab can be considered low and not clinically relevant (Citation5). Lastly, a few differences in data collection were present between the centers.
Implications for clinical practice and future research
Currently, therapeutic decision-making is not influenced by dupilumab levels. We have found that there could be added value of measuring dupilumab levels in clinical practice. As dupilumab is administered in high dosages, it would be interesting to investigate whether dosage interval prolongation yields sufficient treatment responses. One study has already shown that effectiveness remains after increasing administration intervals (Citation36). Increasing dosage intervals and thereby lowering dosages could not only positively affect costs, but also safety aspects. Dose reduction on an individualized basis using proactive (based on levels predicting response) and reactive (based on current levels correlating with response) therapeutic drug monitoring should be the subject of further investigation. A bidirectional relationship between serum levels and both effectiveness (including disease activity) and safety should be taken into consideration.
Conclusions
High inter-patient and low intra-patient variability of dupilumab levels was observed. No correlations were found between dupilumab serum levels and ΔEASI. Serum levels at 2 and 12 weeks were found to have the ability to predict EASI ≤ 7 at 24 weeks. A correlation was found between baseline EASI and dupilumab drug levels at 2, 12, and 24 weeks. Low levels were particularly observed in patients with the presence of AEs, treatment interval deviation, and discontinuation. The interpretation of correlations with a bidirectional nature can be difficult.
All in all, at the current on-label dosage, the measured broad range of dupilumab levels does not seem to yield differences in treatment effectiveness. No relationship between serum drug concentration and effectiveness was identified. However, baseline disease activity influences dupilumab serum concentrations. Higher baseline disease activity results in lower dupilumab levels.
Supplemental Material
Download Zip (46.1 KB)Acknowledgments
The authors would like to thank Ariënna Hyseni and Bryan van den Broek for their assistance.
Disclosure statement
RW: Principal or co-investigator in clinical trials – Abbvie, Amgen, Anaptys Bio, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Galderma, Janssen-Cilag, Kymab, Leo Pharma, Pfizer, Sanofi and UCB. Honoraria from and consultancy work for Abbvie, Eli Lilly, Janssen-Cilag, Leo Pharma, Novartis, Sandoz, Sanofi and UCB. Honoraria from NICE (clinical expert).
PS: receives departmental independent research grants for TREAT NL registry, for which she is Chief Investigator (CI), from pharma companies since December 2019, is involved in performing clinical trials with many pharmaceutical industries that manufacture drugs used for the treatment of e.g., psoriasis and atopic dermatitis, for which financial compensation is paid to the department/hospital.
AB, CS and PS: Investigators on IMI-EU funded research consortium to identify biomarkers in atopic dermatitis; multiple industry partners including Sanofi (biomap-imi.eu). All financial compensation is paid to the department/hospital.
No other conflicts of interest were reported.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Additional information
Funding
References
- Dupixent. Summary of product characteristics. 2017 [cited 2021 Feb 26]. Available from: https://www.ema.europa.eu/en/documents/product-information/dupixent-epar-product-information_en.pdf
- Dupixent. European public assessment report. 2017 [cited 2020 Sep 29]. Available from: https://www.ema.europa.eu/en/documents/assessment-report/dupixent-epar-public-assessment-report_en.pdf
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):1–9.
- Worm M, Simpson EL, Thaci D, et al. Efficacy and safety of multiple dupilumab dose regimens after initial successful treatment in patients With atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(2):131–143.
- Deleuran M, Thaçi D, Beck LA, et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol. 2020;82(2):377–388.
- Bosma AL, de Wijs LEM, Hof MH, et al. Long-term effectiveness and safety of treatment with dupilumab in patients with atopic dermatitis: results of the TREAT NL (TREatment of ATopic eczema, The Netherlands) registry. J Am Acad Dermatol. 2020;83(5):1375–1384.
- Bosma AL, Ouwerkerk W, Günal M, et al. Work ability and quality of working life in atopic dermatitis patients treated with dupilumab. J Dermatol. 2021;48(9):1305–1314.
- Bosma AL, Ouwerkerk W, Heidema MJ, et al. Comparison of real-world treatment outcomes of systemic immunomodulating therapy in atopic dermatitis patients with dark and light skin types. JAAD Int. 2023;10:14–24.
- Sears AV, Woolf RT, Gribaleva E, et al. Real-world effectiveness and tolerability of dupilumab in adult atopic dermatitis: a single-centre, prospective 1-year observational cohort study of the first 100 patients treated. Br J Dermatol. 2021;184(4):755–757.
- Kovalenko P, Davis JD, Li M, et al. Base and covariate population pharmacokinetic analyses of dupilumab using phase 3 data. Clin Pharmacol Drug Dev. 2020;9(6):756–767.
- Li Z, Radin A, Li M, et al. Pharmacokinetics, pharmacodynamics, safety, and tolerability of dupilumab in healthy adult subjects. Clin Pharmacol Drug Dev. 2020;9(6):742–755.
- Dupixent. Dupixent (dupilumab)) injection for subcutaneous use highlights of prescribing information by Regeneron Pharmaceuticals. 2022 [cited 2011 Dec 30]. Available from: https://www.regeneron.com/downloads/dupixent_fpi.pdf.
- Matera MG, Calzetta L, Rogliani P, et al. Monoclonal antibodies for severe asthma: pharmacokinetic profiles. Respir Med. 2019;153:3–13.
- Eshtiaghi P, Gooderham MJ. Dupilumab: an evidence-based review of its potential in the treatment of atopic dermatitis. Core Evid. 2018;13:13–20.
- Kamal MA, Davis JD, Kovalenko P, et al. Pharmacokinetics, pharmacodynamics, and exposure-efficacy of dupilumab in adults with atopic dermatitis. Clin Transl Sci. 2022;15(10):2342–2354.
- Simpson EL, Akinlade B, Ardeleanu M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017;376(11):1090–1091.
- Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol. 2019;181(3):459–473.
- Bansal A, Simpson EL, Paller AS, et al. Conjunctivitis in dupilumab clinical trials for adolescents with atopic dermatitis or asthma. Am J Clin Dermatol. 2021;22(1):101–115.
- Menting SP, Coussens E, Pouw MF, et al. Developing a therapeutic range of adalimumab serum concentrations in management of psoriasis: a step Toward personalized treatment. JAMA Dermatol. 2015;151(6):616–622.
- Williams HC, Burney PG, Pembroke AC, et al. The U.K. Working party’s diagnostic criteria for atopic dermatitis. III. Independent hospital validation. Br J Dermatol. 1994;131(3):406–416.
- Schuttelaar MLA, De Bruin-Weller M, Oosting AJ, et al. Introduction of dupilumab for severe constitutional eczema. Ned Tijdschr Dermatol Venereol. 2018;28:56–57.
- NICE. Dupilumab for treating moderate to severe atopic dermatitis: technology appraisal guidance. 2018 [cited 2022 Oct 13]. Available from: https://www.nice.org.uk/guidance/ta534/chapter/1-Recommendations
- Gerbens LAA, Apfelbacher CJ, Irvine AD, et al. TREatment of ATopic eczema (TREAT) registry taskforce: an international delphi exercise to identify a core set of domains and domain items for national atopic eczema photo- and systemic therapy registries. Br J Dermatol. 2019;180(4):790–801.
- Vermeulen FM, Gerbens LAA, Bosma AL, et al. TREatment of ATopic eczema (TREAT) registry taskforce: consensus on how and when to measure the core dataset for atopic eczema treatment research registries. Br J Dermatol. 2019;181(3):492–504.
- Hanifin JM, Thurston M, Omoto M, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI evaluator group. Exp Dermatol. 2001;10(1):11–18.
- Yosipovitch G, Reaney M, Mastey V, et al. Peak pruritus numerical rating scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181(4):761–769.
- Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol. 2004;140(12):1513–1519.
- Finlay AY, Khan GK. Dermatology life quality index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216.
- International Conference of Harmonization. Harmonised tripartite guideline. Clinical safety data management: definitions and standards for expedited reporting E2A. 2014 [cited 2020 March 13]. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2A/Step4/E2A_Guideline.pdf
- Großerichter-Wagener C, Kos D, van Leeuwen A, et al. Biased anti-idiotype response in rabbits leads to high-affinity monoclonal antibodies to biologics. MAbs. 2020;12(1):1814661.
- Pouw MF, Krieckaert CL, Nurmohamed MT, et al. Key findings towards optimising adalimumab treatment: the concentration-effect curve. Ann Rheum Dis. 2015;74(3):513–518.
- Chopra R, Vakharia PP, Sacotte R, et al. Severity strata for eczema area and severity index (EASI), modified EASI, scoring atopic dermatitis (SCORAD), objective SCORAD, atopic dermatitis severity index and body surface area in adolescents and adults with atopic dermatitis. Br J Dermatol. 2017;177(5):1316–1321.
- De Bruin-Weller M, Biedermann T, Bissonnette R, et al. Treat-to-Target in atopic dermatitis: an international consensus on a set of core decision points for systemic therapies. Acta Derm Venereol. 2021;101(2):adv00402.
- Menting SP, van den Reek JM, Baerveldt EM, et al. The correlation of clinical efficacy, serum trough levels and antidrug antibodies in ustekinumab-treated patients with psoriasis in a clinical-practice setting. Br J Dermatol. 2015;173(3):855–857.
- Schram ME, Spuls PI, Leeflang MM, et al. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy. 2012;67(1):99–106.
- Spekhorst LS, Bakker D, Drylewicz J, et al. Patient-centered dupilumab dosing regimen leads to successful dose reduction in persistently controlled atopic dermatitis. Allergy. 2022;77(11):3398–3407.

