Abstract
Psoriasis in pediatric patients is uncommon and the management of moderate-to-severe cases can be challenging. We report the case of a 17-year-old girl who presented with severe plaque psoriasis unresponsive to UVB phototherapy. The Psoriasis Area Severity Index (PASI) was 18 and the Dermatology Life Quality Index was 24. We decided to prescribe ixekizumab, observing complete skin clearance after only 8 weeks. The patient is still on treatment with no reported adverse events after two years.
Keywords:
Dear Editor,
Psoriasis is an inflammatory disease affecting up to 3% of the population worldwide (Citation1). Pediatric psoriasis is not very common, and moderate-to-severe cases can be challenging to treat (Citation1–2). In fact, the long-term use of conventional immunosuppressive systemic drugs in these patients is not approved (Citation2), and phototherapy is not always feasible because it can be time-consuming (Citation3). Among biological therapies, only etanercept, ustekinumab, secukinumab and ixekizumab are currently reimbursed by the Italian healthcare system for patients aged 6 years or older, while adalimumab is approved for patients aged ≥ 4 years (Citation4). In particular, ixekizumab is a monoclonal antibody that selectively targets interleukin (IL)-17A and has recently been approved for the treatment of moderate-to-severe plaque psoriasis also in pediatric patients (Citation4–5). However, real-world experience with ixekizumab in this population is still limited (Citation3), with very little long-term data.
We report the case of a 17-year-old girl affected by plaque psoriasis since the age of 11. In 2018, she underwent 40 sessions of narrowband UVB phototherapy with no response. She was subsequently referred to another hospital, where she received topical and systemic corticosteroids with only a partial response. The patient also had a previous diagnosis of anxiety-depressive disorder with recurrent panic attacks and reported suicidal ideations and was on treatment with aripiprazole 5 mg/day and fluoxetine 5 mg/day.
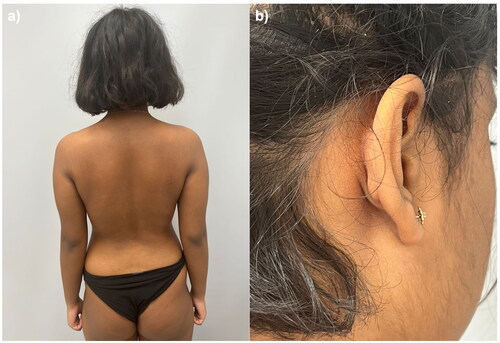
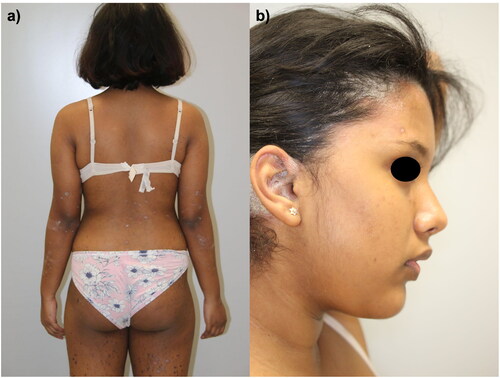
She came to our attention in January 2021 with several erythematous-desquamative plaques, which involved the upper and lower limbs, the trunk, and the head (). The involvement of the scalp and retro-auricular regions was particularly severe, with a scalp-PGA (Psoriasis Global Assessment) of 4. The Psoriasis Area and Severity Index (PASI) was 18, and the Dermatology Life Quality Index (DLQI) of the patient was 24. A rheumatological examination did not evidence any joint involvement. Given the psychiatric comorbidities, concomitant medications, and previous failure of both topical and systemic therapies, we suggested to the patient and her family treatment with ixekizumab. As screening blood exams were all within normal ranges, we started therapy with ixekizumab 80 mg according to the summary of product characteristics. The patient returned after only eight weeks showing already complete skin clearance with a PASI of 0 (). To date, the patient is still on treatment and has completed two years of follow-up with no reported relapses or adverse events. The patient did not report any worsening of her anxiety-depressive disorder, and she is still undergoing psychiatric follow-up visits.
Figure 1. Several erythematous-desquamative patches and plaques spread at the back, lower limbs (a) and scalp (b) of a 17-year-old girl.
Limited evidence is available in the literature regarding the use of ixekizumab in pediatric patients. The Phase-3 clinical trial IXORA-PEDS showed a 75% reduction in PASI (PASI75) compared with baseline in 89% of patients after 12 weeks, with sustained or improved clinical responses through week 48 (Citation5). An update at week 108 of the IXORA-PEDS study has been recently published with a sustained response rate throughout two years of treatment (Citation6). In the aforementioned clinical trial, no significant safety findings were reported. Real-life experiences with ixekizumab in pediatric psoriatic patients are very limited, with only one case reported by Megna et al. with 24 weeks of follow-up (Citation3).
We decided to prescribe ixekizumab to our patient for several reasons, including its favorable safety profile, the strength of data from clinical trials on rapid and sustained efficacy (Citation5,Citation6), and our previous positive experience with the drug, even in complex patients (Citation7). Our experience, although limited to a single case, suggests that ixekizumab may represent a valid therapeutic option for pediatric patients with plaque psoriasis also in the long-term. Large real-life studies are strongly needed to evaluate further the role of IL-17 inhibitors in pediatric psoriasis in the long term.
Compliance with ethics guidelines
Institutional review board approval was exempted as the study protocol did not deviate from standard clinical practice. The patient received biologics as in good clinical practice, in accordance with European guidelines. The patient and her parents had provided written consent for retrospective study of data collected during routine clinical practice (demographics, clinical scores) and for the publication of clinical pictures. The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Data collection and handling complied with applicable laws, regulations, and guidance regarding patient protection, including patient privacy.
Disclosure statement
A. Narcisi has served on advisory boards, received honoraria for lectures and research grants from Almirall, Abbvie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi‐Genzyme, Amgen and Boehringer Ingelheim. M. Valenti has been a consultant and/or speaker for Sanofi, Leo Pharma, Eli Lilly and Boehringer Ingelheim. A. Costanzo has served as an advisory board member, consultant and has received fees and speaker’s honoraria or has participated in clinical trials for Abbvie, Almirall, Biogen, LEO Pharma, Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB-Pharma. L. Gargiulo has been a consultant for Almirall. The other authors have nothing to disclose.
Data availability statement
Data available on request from the authors.
Additional information
Funding
References
- Peris K, Fortina AB, Bianchi L, et al. Update on the management of pediatric psoriasis: an Italian consensus. Dermatol Ther. 2022;12(8):1–3.
- Gargiulo L, Ibba L, Pavia G, et al. Upadacitinib for the treatment of concomitant psoriasis and atopic dermatitis: a case series. J Dermatolog Treat. 2023;34(1):2183729.
- Megna M, Fornaro L, De Lucia M, et al. A case of pediatric psoriasis successfully and rapidly treated with ixekizumab. Dermatol Ther. 2021;34(6):e15108.
- Sun HY, Phan K, Paller AS, et al. Biologics for pediatric psoriasis: a systematic review and meta-analysis. Pediatr Dermatol. 2022;39(1):42–48.
- Paller AS, Seyger MMB, Alejandro Magariños G, et al. Efficacy and safety of ixekizumab in a phase III, randomized, double‐blind, placebo‐controlled study in paediatric patients with moderate‐to‐severe plaque psoriasis (IXORA‐PEDS). Br J Dermatol. 2020;183(2):231–241.
- Paller AS, Seyger MMB, Magariños GA, et al. Long-term efficacy and safety of up to 108 weeks of ixekizumab in pediatric patients with moderate to severe plaque psoriasis: the IXORA-PEDS randomized clinical trial. JAMA Dermatol. 2022;158(5):533–541.
- Gargiulo L, Pavia G, Valenti M, et al. Safety of biologic therapies in patients with moderate-to-severe plaque psoriasis and concomitant viral hepatitis: a monocentric retrospective study. Dermatol Ther. 2022;12(5):1263–1270.