Abstract
Aim
This study aimed to evaluate the efficacy and safety of bimekizumab for psoriasis.
Methods
The PubMed, Web of Science, Cochrane Library, and Embase databases were systematically searched until November 20, 2022, to identify randomized controlled trials (RCTs) reporting the efficacy and safety of bimekizumab. The identified studies were screened according to inclusion and exclusion criteria, and a meta-analysis was performed on the selected studies using the Stata (version 17.0) software to investigate the efficacy and safety of bimekizumab.
Results
Six studies involving 1252 participants were considered. Compared with the control group which received placebo, the bimekizumab group had a larger number of patients with improvement in Psoriasis Area and Severity Index (PASI) of at least 75% (PASI75) (RR: 20.54, 95%CI: 12.41–33.99; p = .000), at least 90% (PASI90) (RR:16.99, 95%CI: 7.09–40.68; p = .000) and 100%(PASI100) (RR:14.57; 95%CI: 5.26–40.35; p = .000) and a larger number with improvement in Investigator Global Assessment (IGA) response (RR:22.57; 95%CI: 12.74–39.98; p = .000). There was no obvious difference between the bimekizumab and placebo groups in treatment of emergent adverse events (TEAEs) (RR:1.17; 95%CI: 0.93–1.47; p > .05) and serious TEAEs (RR: 0.67; 95%CI: 0.28–1.61; p > .05).
Conclusions
Bimekizumab shows promising efficacy for the treatment of psoriasis with favorable safety records.
Introduction
Psoriasis is a chronic and debilitating immune-mediated inflammatory skin disease with a high recurrence rate, which affects over 60 million adults and children worldwide (Citation1). In 2014, WHO defined it as chronic, non-communicable, painful, disfiguring, and disabling disease for which there is no cure (Citation1). In addition, psoriasis also affects multiple systems and multiple organs (Citation2,Citation3). Studies have reported that psoriasis is associated with significant comorbidities including chronic pulmonary disease, diabetes, liver disease, cardiovascular disease, chronic kidney disease, and rheumatologic disease (Citation4,Citation5). Psoriasis is an autoimmune disease characterized by over-activation and dysfunction of T lymphocytes, in which Th17 cells specifically express interleukin (IL-17A) and IL-17F (Citation6,Citation7). IL-17A has strong inflammatory activity, which can promote the production of chemokines in the body, drive the rapid increase of monocytes and neutrophils and stimulate cells to produce inflammatory factors and enhance local inflammatory response (Citation8). IL-17F is structurally like IL-17A, has the same receptor, and can produce powerful inflammatory effects in vivo and in vitro (Citation9,Citation10).
Bimekizumab, a monoclonal IgG1 antibody, specifically inhibits IL-17F and IL-17A in psoriasis pathogenesis, which can strongly inhibit the inflammatory response of psoriasis. IL-17F and IL-17A have superimposed biological characteristics and independently drive the inflammatory response (Citation10). Therefore, bimekizumab with bidirectional inhibition of two key factors has more therapeutic potential. A few clinical trials of bimekizumab in psoriasis have been conducted and shown to be effective with a favorable safety profile (Citation11–16). Considering the rapid developments in this field, we performed this systemic review and meta-analysis of randomized controlled trials (RCTs) to pool and quantify the overall efficacy and safety.
Methods
Search strategy
We reported and undertook this review in line with the criteria published in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A systematic search was conducted for studies published until November 20, 2022, using the following four electronic databases: PubMed, Excerpta Medica Database (EMBASE), Cochrane Library, and Web of Science. We used a search strategy combining MeSH heading and text with the following terms: ‘bimekizumab’ or ‘UCB4940’ and ‘Psoriasis’, ‘Psoriases’, ‘Pustulosis of Palms and Soles’, ‘Pustulosis Palmaris et Plantaris’, ‘Palmoplantaris Pustulosis’ or ‘Pustular Psoriasis of Palms and Soles.’ The reference lists of the included papers were also screened.
Selection criteria
In this study we included patients who meet the diagnostic criteria (Citation16) of psoriasis and receive bimekizumab or placebo as an intervention; we used a randomized controlled trial (RCT) design and set no restrictions on the dose of intervention. Data of the placebo group was extracted for comparison. As for outcome measures, studies reporting changes from baseline in the Psoriasis Area and Severity Index (PASI) were eligible. For safety, qualitative reports of treatment-emergent adverse events (TEAEs) were accepted.
We excluded studies for the following reasons: inappropriate design; lack of related data; animal trials; not written in English; or were case reports, letter to editors, reviews, conference abstracts, duplications, or full text were unavailable.
Data extraction
Publications that met the inclusion criteria were independently searched by two reviewers (YY.Q. and QL.L.). Subsequently, the two investigators collected data of the eligible trials and recorded them in the information extraction table. The extracted information included the year of publication, first author’s name, study locations, NCT number, sample size, sex and age, intervention, and outcome measures. A third author resolved any disagreements.
Efficacy outcomes were evaluated considering the proportion of patients achieving the following endpoints: (1) PASI75 (defined as 75% reduction in PASI score); (2) PASI90 (defined as 90% reduction in PASI score); (3) PASI100 (defined as 100% reduction in PASI score); (4) IGA response (defined as achieving a vIGA-AD score of 0 (clear) or 1 (almost clear) with a 2-point or bigger improvement from baseline); (5) P-SIM responses for pain, itch, and scaling items (defined as the proportion of patients with a prespecified point improvement), the threshold response for pain was 1.98, for itch 2.39, and for scaling 2.86, and the analysis was limited to the patients with a baseline response at or above the threshold scores; and (6) the American College of Rheumatology (ACR)20 (defined as at least 20% improvement in the ACR response criteria), ACR50 (defined as at least 50% improvement in the ACR response criteria) and ACR70(defined as at least 70% improvement in the ACR response criteria). Regarding the safety outcome, the development of treatment-emergent adverse events (TEAEs), and serious TEAEs were evaluated.
Quality assessment
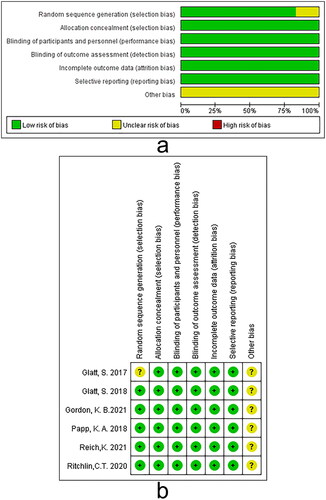
To examine the risk of bias, the Cochrane risk of bias assessment tool was applied for every included RCT, which refers to the following aspects: randomizing sequence generation, allocation concealment, blinding for patients, intervention givers, outcome measures, incomplete data, selective reporting, and other sources of bias. The assessment risk of bias for each aspect was categorized as low, high, or unclear (Citation17).
Statistical analysis
Data were analyzed using Stata 17.0 (StataCorp, College Station, TX, USA). Risk ratios (RR) and 95% confidence intervals (CIs) were used to describe the overall effects. The overall effect was calculated using a Z-test, and statistical significance was set at p < .05 (two-tailed). Potential heterogeneity was evaluated using Cochran’s Q and I2 statistic; when heterogeneity was low (p ≥ .05, I2 ≤ 50%), a fixed-effects model was applied. If high heterogeneity occurred (p < .05, I2 > 50%), its potential sources and applied corresponding measures were analyzed (Citation18). First, a sensitivity analysis was performed if clinical heterogeneity was evident, and a random-effect model was used if statistical heterogeneity was being considered. Egger’s test was used to assess publication bias in which a value of p > .05 indicated a low chance of publication bias.
Results
Literature search and screening
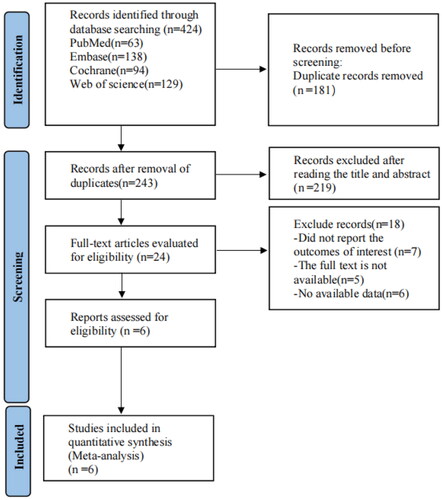
A total of 424 articles requiring further evaluation were retrieved from four foreign databases (63 in PubMed, 94 in Cochrane Library, 138 in Embase, and 129 in Web of Science); 181 of them were deleted because of duplicate records. After screening the titles and abstracts, 219 papers were excluded for several reasons. Finally, after screening the full text, only six studies met the inclusion criteria. presents the detailed flow of the selection process.
Study characteristics and quality assessment
This study included six RCTs of suitable quality involving 1252 participants. All trials used bimekizumab as the experimental group (986 participants) and placebo as the control group (266 participants). Mean age of participants ranged from 37.4 to 50.4 years across studies. Of studies that reported gender, 72.8% (912/1252) were male. The key features of the included studies are illustrated in . The Cochrane risk of bias assessment tool was used to evaluate the risk of bias in the included studies. All the included studies were of suitable quality, as shown in .
Table 1. Summary of clinical studies included in the meta-analysis.
PASI75
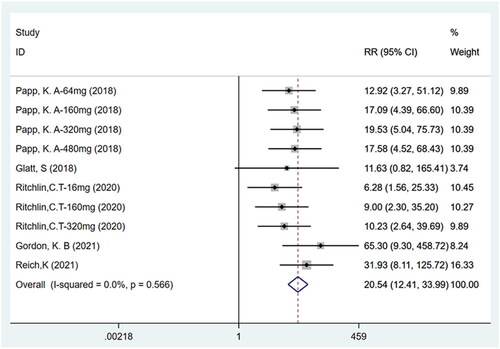
Five studies (Citation11,Citation12, Citation14–16) including ten trials with a total of 1186 participants have reported PASI75. Fixed effects model analyses indicated that the number of patients achieving PASI75 (75% improvement over the baseline score) was higher for bimekizumab than for the placebo (RR: 20.54, 95% CI: 12.41–33.99, p = .000, I2 = 0.0%, P-heterogeneity = 0.566, fixed-effects model, ). Thus, bimekizumab showed significantly higher PASI75 than placebo.
PASI90
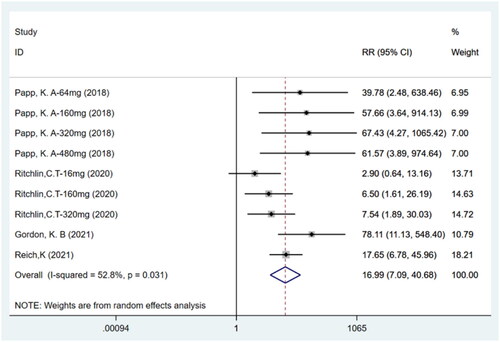
Four studies (Citation11,Citation14–16) including nine trials with a total of 1133 participants have reported that the number of patients achieving PASI90 was higher for bimekizumab than for the placebo (RR: 16.99, 95% CI: 7.09–40.68, p = .000, I2 = 52.8%, P-heterogeneity = 0.031, random-effects model, ). These results indicate that bimekizumab group showed significantly higher PASI90 than placebo group.
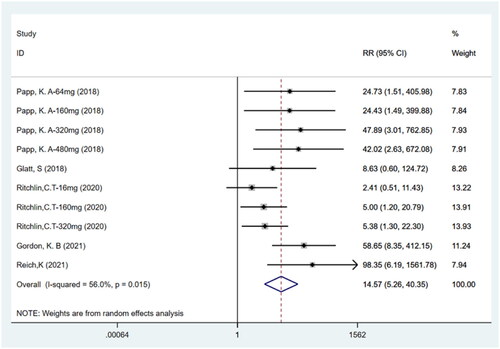
PASI100
Five studies (Citation11,Citation12,Citation14–16) including ten trials with a total of 1186 participants reported PASI100. The meta-analysis showed that patients treated with bimekizumab achieved a higher rate of PASI100 (total clearance) compared to the placebo group (RR: 14.57; 95% CI: 5.26–40.35; p = .000; I2 = 56.0%; P-heterogeneity = 0.015; random-effects model; ). This result indicates that bimekizumab was more effective than placebo.
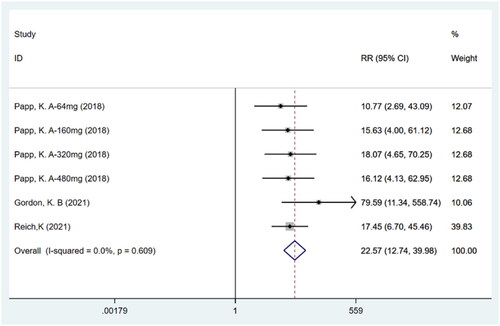
IGA response
Meta-analysis of three studies (Citation14–16) including six trials with a total of 1049 participants showed that patients in the bimekizumab group achieved a higher IGA response compared to those in the placebo group (RR: 22.57; 95% CI: 12.74–39.98; p = .000; I2 = 0.0%; P-heterogeneity = 0.609; fixed-effects model; ).
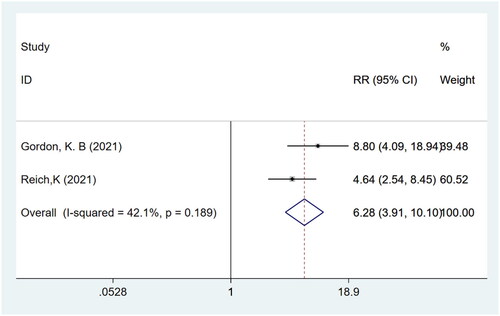
P-SIM pain response
Meta-analysis of two studies (Citation15,Citation16) with a total of 839 participants showed that patients in the bimekizumab group achieved a higher P-SIM pain response compared to those in the placebo group (RR: 6.28; 95% CI: 3.91–10.10; p = .000; I2 = 42.1%; P-heterogeneity = 0.189; fixed-effects model; ).
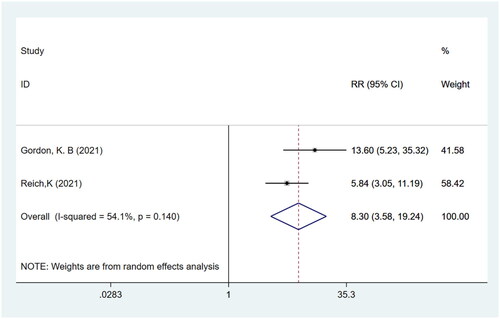
P-SIM itch response
Meta-analysis of two studies (Citation15,Citation16) with a total of 839 participants showed that patients in the bimekizumab group achieved a higher P-SIM itch response compared to those in the placebo group (RR: 8.30; 95% CI: 3.58–19.24; p = .000; I2 = 54.1%; P-heterogeneity = 0.140; random-effects model; )
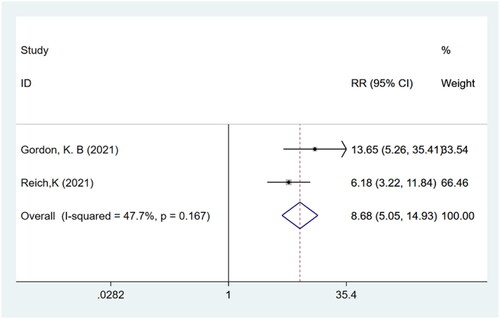
P-SIM scaling response
Meta-analysis of two studies (Citation15,Citation16) with a total of 839 participants showed that patients in the bimekizumab group achieved a higher P-SIM scaling response compared to those in the placebo group (RR: 8.68; 95% CI: 5.05–14.93; p = .000; I2 = 47.7%; P-heterogeneity = 0.167; fixed-effects model; ).
ACR20, ACR50 and ACR70
Two studies (Citation11,Citation12) with a total of 164 participants with psoriatic arthritis(PsA) reported ACR20, ACR50 and ACR70. The meta-analysis showed that patients treated with bimekizumab achieved a higher rate of ACR20 (RR: 2.65; 95% CI:1.91–3.68; p = .000; I2 = 49.8%; P-heterogeneity = 0.113; fixed-effects model; Figure S1), ACR50 (RR: 4.67; 95% CI: 2.44–8.94; p = .000; I2 = 0.0%; P-heterogeneity = 0.881; fixed-effects model; Figure S2) and ACR70 (RR: 3.24; 95% CI: 1.34–7.85; p = .009; I2 = 0.0%; P-heterogeneity = 0.912; fixed-effects model; Figure S3) compared to the placebo group.
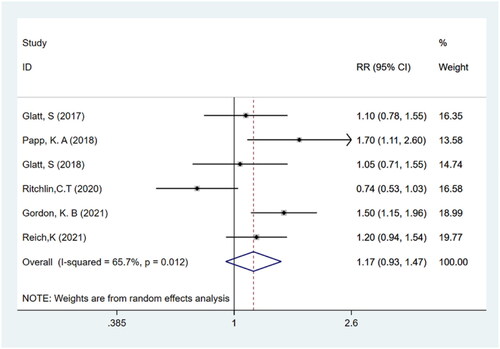
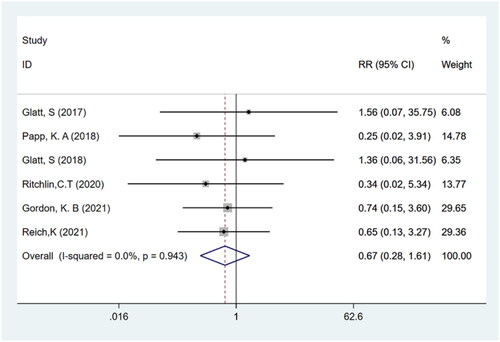
TEAEs and serious TEAEs
Six studies (Citation11–16) with a total of 1252 participants reported TEAEs and serious TEAEs. It was found that the frequency of adverse events did not differ significantly between bimekizumab and placebo groups (RR: 1.17; 95% CI: 0.93–1.47; p > .05; I2 = 65.7%; P-heterogeneity = 0.012; random-effects model; ). In addition, the results showed that there was no significant difference in the incidence of serious TEAEs between bimekizumab group and placebo group (RR: 0.67; 95% CI: 0.28–1.61; p > .05; I2 = 0.0%; P-heterogeneity = 0.943; fixed-effects model; ).
Publication bias analysis and sensitivity analysis
Egger’s test was applied to the assessment of publication bias. For PASI75, PASI90, IGA response, TEAEs and serious TEAEs in the present study, Egger’s tests demonstrated that there was no publication bias (p > .05, Figure S4-S8). However, Egger’s test indicated potential publication bias for the relationship between bimekizumab and PASI100 (p = .007 for Egger’s test; Figure S9). To evaluate the stability of the meta-analysis, sensitivity analysis was performed if clinical heterogeneity was evident, including PASI90, PASI100 and TEAEs. As indicated by sensitivity analysis, no single study significantly affected the overall pooled estimate (Figure S10–S12). Thus, the research results can be deemed reliable.
Discussion
To the best of our knowledge, this was the first meta-analysis to explore efficacy and safety of bimekizumab for the treatment of psoriasis. This meta-analysis of six RCTs representing 1252 psoriasis patients presented the efficacy and safety of bimekizumab compared to placebo. The pooled estimate yielded a statistically significant improvement in PASI75, PASI90, PASI100, IGA response, P-SIM pain response, P-SIM itch response and P-SIM scaling response of psoriasis treated with bimekizumab compared with placebo. Moreover, the meta-analysis revealed that patients in the bimekizumab group had significantly higher rates achieving ACR20, ACR50 and ACR70, which shows that bimekizumab has been found effective in treating PsA as well in addition to the improvement of plaque lesions identified in the present study. In terms of adverse event rate, no significant difference was observed between bimekizumab and placebo.
Bimekizumab proved to be superior to control groups in achieving all the therapy outcomes, despite differences in the doses, frequency, route of administration, timepoints of outcomes assessment used in different clinical trials. Efficacy outcomes evaluated were the proportion of patients achieving the following endpoints at different times, including week 12, 16, 20 and 48. In terms of route of administration in our study, four RCTs were administered subcutaneously and two RCTs were administered intravenously. According to reports, due to its high bioavailability, subcutaneous injection proved to be the most commonly used and most convenient method (Citation19). The frequency of dosing was every 4 weeks in all except two study, in which they were every 3 weeks and only once (Citation11–16). As indicated by sensitivity analysis in our study, the research results can be deemed reliable. Most of the RCTs reported efficacy over and above placebo groups but few reportedly showed significantly better results as compared to active control groups in our study. Therefore, bimekizumab has undergone several phase II and III studies and was more effective in treating moderate-to-severe plaque psoriasis than ustekinumab in the phase III BE VIVID trial (Citation16), adalimumab in the phase III BE SURE trial (Citation20), and secukinumab in the phase III BE RADIANT trial (Citation21).
The greater efficacy of bimekizumab may be due to its unique affinity maturation to selectively inhibit IL-17A and IL-17F (Citation22). IL-17 inhibitors are important in the treatment of psoriasis because IL-17 is an important cytokine in the pathogenesis of psoriatic plaques. The role of IL-17 in the pathogenesis of plaque psoriasis is diverse, as it causes the release of several psoriasis-causing proteins, activates other proteins to drive plaque formation, and activates epidermal hyperplasia factor cells (Citation23). Bimekizumab can inhibit the inflammatory response of psoriasis by neutralizing IL-17A and IL-17F at the same time. Compared with the inhibition of IL-17A alone, the dual inhibition of IL-17A and IL-17F significantly reduces the migration of inflammatory cells, the production of pro-inflammatory cytokines and the expression of pro-inflammatory genes (especially those related to psoriasis) in vitro. Dual inhibition of IL-17A and IL-17F is a highly effective therapeutic option for the treatment of psoriasis, both for new patients and for those resistant to previous biologic treatments (Citation24). Early clinical data in psoriasis suggest that dual inhibition of IL-17A and IL-17F with bimekizumab provides a new therapeutic approach for patients with immune-mediated inflammatory diseases (Citation23).
There were no significant differences in tolerability or safety between bimekizumab group and placebo group in our study, which have shown that bimekizumab has favorable safety records for psoriasis. There was a network meta-Analysis that aimed at comparing the clinical efficacy, tolerability, and safety of seven kinds of biologic drugs in the treatment of ankylosing spondylitis(AS) (Citation25). Based on the cluster-rank analysis, the best tolerated and most effective biologic drug is bimekizumab, which have shown that bimekizumab, may be an ideal future treatment choice for AS while IL-23 and IL-6 inhibitors demonstrate little potential in the treatment of AS. There was a meta-analysis that assessed the efficacy and safety of interleukin-17 inhibitors (ixekizumab, secukinumab, bimekizumab, netakimab and brodalumab) in chronic inflammatory rheumatic diseases, including AS and PsA. In this meta-analysis, their findings found no increased risk of any adverse events was reported in PsA patients (Citation26).
This meta-analysis had some limitations. First, a small number of studies were included, which limited the sample size and the comparison with other biologicals. Second, the follow-up periods are inconsistent across the studies; there is a need for more well-designed multicenter RCTs with bigger sample sizes and long-term follow-up. Finally, the doses of bimekizumab are inconsistent across the studies.
Conclusion
Current studies have shown that bimekizumab has promising efficacy for psoriasis with favorable safety records.
Ethics approval and consent to participate
Not applicable.
Author contribution
YY.Q. wrote the manuscript. Y.Z., YY.Q. and QL.L. extracted and analyzed the data. Y.L. and QL.L. provided professional comments to the manuscript. All authors have read and approved the final manuscript.
Supplemental Material
Download PDF (591.6 KB)Acknowledgments
The authors thank AiMi Academic Services (www.aimieditor.com) for English language editing and review services.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Additional information
Funding
References
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(2):1–11.
- Kivelevitch DN, Hebeler KR, Patel M, et al. Emerging topical treatments for psoriasis. Expert Opin Emerg Drugs. 2013;18(4):523–532.
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. Jama. 2020;323(19):1945–1960.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385.
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149(10):1173–1179.
- Gooderham M, Posso-De Los Rios CJ, Rubio-Gomez GA, et al. Interleukin-17 (IL-17) inhibitors in the treatment of plaque psoriasis: a review. Skin Therapy Lett. 2015;20(1):1–5.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71(1):141–150.
- Johansen C, Usher PA, Kjellerup RB, et al. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160(2):319–324.
- Hot A, Miossec P. Effects of interleukin (IL)-17A and IL-17F in human rheumatoid arthritis synoviocytes. Ann Rheum Dis. 2011 May;70(5):727–732.
- van Baarsen LG, Lebre MC, van der Coelen D, et al. Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther. 2014;16(4):426.
- Ritchlin CT, Kavanaugh A, Merola JF, et al. Bimekizumab in patients with active psoriatic arthritis: results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2020;395(10222):427–440.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77(4):523–532.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83(5):991–1001.
- Papp KA, Merola JF, Gottlieb AB, et al. Dual neutralization of both interleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. J Am Acad Dermatol. 2018;79(2):277–286.e10.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397(10273):475–486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397(10273):487–498.
- Higgins JP, Altman DG, Gøtzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560.
- Singh S, Singh S, Thangaswamy A, et al. Efficacy and safety of risankizumab in moderate to severe psoriasis: a systematic review and meta-analysis. Dermatol Ther. 2021;34(1):e14487.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385(2):130–141.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385(2):142–152.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- Hawkes JE, Yan BY, Chan TC, et al. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201(6):1605–1613.
- Freitas E, Blauvelt A, Torres T. Bimekizumab for the treatment of psoriasis. Drugs. 2021;81(15):1751–1762.
- Cao Z, Guo J, Li Q, et al. Optimal biologic drugs for the treatment of ankylosing spondylitis: results from a network Meta-Analysis and network metaregression. Biomed Res Int. 2022;2022:8316106. 2022: published 2022 Jul 6.
- He C, Xue C, Zhu G, et al. Efficacy and safety of interleukin-17 inhibitors in the treatment of chronic rheumatic diseases: a combined and updated meta-analysis. J Clin Pharm Ther. 2021;46(4):895–906.