Keywords:
Dear Editor,
Plaque psoriasis is an immune-mediated chronic disease that affects up to 3% of children and adults worldwide (Citation1). Palmoplantar psoriasis is a subtype of psoriasis that involves palms or soles and occurs in 12–26% of patients during the natural course of the disease (Citation2). Like plaque psoriasis, moderate-to-severe cases require treatment with conventional immunosuppressive drugs (i.e. methotrexate or cyclosporine), phototherapy, or acitretin. According to the Italian Guidelines, biological drugs are indicated when these therapies are ineffective or contraindicated (Citation3). Despite the continued approval of new biological treatments for plaque psoriasis with brilliant results also in very severe cases (Citation4), limited data are available on the role of these drugs in palmoplantar psoriasis. This is due mostly because these patients are usually excluded from randomized clinical trials for their lower Psoriasis Area and Severity Index (PASI) at baseline.
We conducted a retrospective study to evaluate the differences in terms of effectiveness between anti-interleukin (IL)-23 drugs and anti-IL-17 in psoriatic patients with palmar or plantar involvement over 104 weeks of treatment. At each dermatological visit, palmoplantar Psoriasis Global Assessment (ppPGA), PASI and the occurrence of any adverse events (AEs) were recorded. The primary endpoint was the proportion of patients achieving ppPGA of 0 or 1 (clear or almost clear) at each time point. The difference between the two subgroups was analyzed using the Chi-square test or Exact Fisher’s test if the distribution was not normal. A p-value < .05 was considered statistically significant. We enrolled 119 patients from the Dermatology Department of Humanitas Clinical Research Hospital, all affected by palmoplantar psoriasis with a ppPGA of at least 3 out of 5. Sixty of them were treated with anti-IL-23, and 59 with anti-IL-17. The most prescribed anti-IL-23 drug was risankizumab (30 patients), followed by guselkumab (25) and tildrakizumab (5). Among anti-IL-17, the most prescribed drug was ixekizumab (40 patients), followed by secukinumab (14) and brodalumab (5). All patients reached at least 16 weeks of treatment, with 97 patients reaching week 28 and 79 reaching week 52. In addition, 38 patients were followed up for more than 104 weeks. Demographic and clinical characteristics of patients treated with anti-IL-23 and anti-IL-17 were comparable at baseline and are shown in . Patients treated with an anti-IL-23 had a mean age of 53.27 ± 15.80 with a mean BMI of 26.68 ± 5.53. Eighteen patients (28.3%) had previously received another biological treatment. Fifteen patients had a ppPGA ≥ 4 at baseline, while the mean PASI was 8.47 ± 5.67. Among the patients treated with an anti-IL-17, the mean age was 58.59 ± 14.37 years. Sixteen patients were bio-experienced (27.1%) with a mean Body Mass Index (BMI) of 25.95 ± 4.14. At baseline, the mean PASI was 8.67 ± 6.77, and 18 patients had a ppPGA of at least 4 (severe).
Table 1. Demographic and clinical characteristics at baseline of the two subgroups of patients.
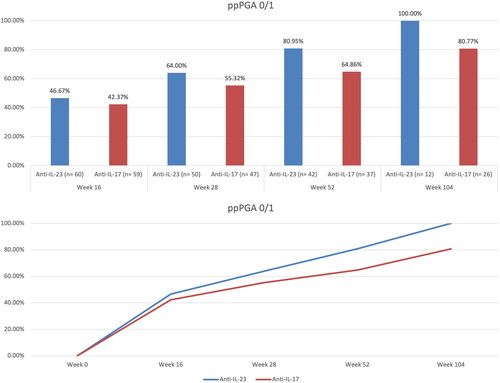
At week 16, 46.67% of patients treated with anti-IL-23 and 42.37% of those receiving an inhibitor of IL-17 achieved a ppPGA of 0/1. After 28 weeks, 64% and 55.32% of patients treated with anti-IL-23 and anti-IL-17 respectively, experienced complete or almost complete skin clearance at palmoplantar regions. After one year of treatment, ppPGA of 0/1 was reached by 80.95% and 64.86% of patients receiving anti-IL-23 and anti-IL-17, respectively. At week 104, the effectiveness endpoint was achieved by all patients under treatment with IL-23 inhibitors and by 80.77% of those receiving anti-IL-17 drugs (). No statistically significant differences were observed throughout the study period. No serious AEs or AEs leading to discontinuation were observed in our experience. Despite the small proportion of surface area affected in palmoplantar psoriasis, there is a disproportion between the clinical picture and the impact on the patient’s well-being (Citation2). Few data are available regarding the use of biologics in palmoplantar psoriasis. In a few clinical trials, different biologics (ustekinumab, secukinumab and ixekizumab) have shown efficacy compared with placebo, with ixekizumab also demonstrating superiority compared to etanercept (Citation5–7). Data regarding IL-23 inhibitors for treating palmoplantar psoriasis are currently limited to case reports (Citation8,Citation9) and real-world experiences. However, real-life studies have not specifically focused on palmar and plantar involvement (Citation10–12). Our experience supports the effectiveness of interleukin inhibitors for the treatment of palmoplantar psoriasis without significant differences between anti-IL-17 and anti-IL-23 drugs in both the short- and long-term. However, larger and longer studies are needed to further investigate these treatments’ role in severe palmoplantar psoriasis.
Compliance with ethics Guidelines
Institutional review board approval was exempted as the study protocol did not deviate from standard clinical practice. The patient received biologics as in good clinical practice, in accordance with European guidelines. The patient and her parents had provided written consent for retrospective study of data collected during routine clinical practice (demographics, clinical scores) and for the publication of clinical pictures. The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Data collection and handling complied with applicable laws, regulations, and guidance regarding patient protection, including patient privacy.
Disclosure statement
L. Gargiulo has been a consultant for Almirall. A. Narcisi has served on advisory boards, received honoraria for lectures and research grants from Almirall, Abbvie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi‐Genzyme, Amgen and Boehringer Ingelheim. A. Costanzo has served as an advisory board member, consultant and has received fees and speaker’s honoraria or has participated in clinical trials for Abbvie, Almirall, Biogen, LEO Pharma, Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB-Pharma. M. Valenti has been a consultant and/or speaker for Sanofi, Leo Pharma, Eli Lilly, Novartis, Janssen, AbbVie and Boehringer Ingelheim. The other authors have nothing to disclose.
Data availability statement
Data available on request from the authors.
Additional information
Funding
References
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64 Suppl 2(Suppl 2):1–3.
- Merola JF, Qureshi A, Husni ME. Underdiagnosed and undertreated psoriasis: nuances of treating psoriasis affecting the scalp, face, intertriginous areas, genitals, hands, feet, and nails. Dermatol Ther. 2018;31(3):e12589.
- Gisondi P, Altomare G, Ayala F, et al. Italian guidelines on the systemic treatments of moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2017;31(5):774–790.
- Valenti M, Gargiulo L, Ibba L, et al. Sub-erythrodermic psoriasis successfully treated with bimekizumab: a case report. Dermatol Ther. 2022;35(12):e15952.
- Menter A, Warren RB, Langley RG, et al. Efficacy of ixekizumab compared to etanercept and placebo in patients with moderate-to-severe plaque psoriasis and non-pustular palmoplantar involvement: results from three phase 3 trials (UNCOVER-1, UNCOVER-2 and UNCOVER-3). J Eur Acad Dermatol Venereol. 2017;31(10):1686–1692.
- Au SC, Goldminz AM, Kim N, et al. Investigator-initiated, open-label trial of ustekinumab for the treatment of moderate-to-severe palmoplantar psoriasis. J Dermatolog Treat. 2013;24(3):179–187.
- Paul C, Reich K, Gottlieb AB, et al. Secukinumab improves hand, foot and nail lesions in moderate-to-severe plaque psoriasis: subanalysis of a randomized, double-blind, placebo-controlled, regimen-finding phase 2 trial. J Eur Acad Dermatol Venereol. 2014;28(12):1670–1675.
- Al Muqrin AM, Alghamdi AA, AlShaalan ZM. Rapid response of palmoplantar psoriasis to risankizumab: a case report. Clin Cosmet Investig Dermatol. 2022;15:2129–2132.
- Gambardella A, Licata G, Tancredi V, et al. A case of refractory palmoplantar psoriasis treated with tildrakizumab. Ital J Dermatol Venerol. 2022;157(4):379–380.
- Gargiulo L, Ibba L, Malagoli P, et al. Real-life effectiveness and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: a 104-week multicenter retrospective study - IL PSO (ITALIAN LANDSCAPE PSORIASIS). Acad Dermatol Venereol. 2023;00:1–11. doi:10.1111/jdv.18913.
- Megna M, Tommasino N, Potestio L, et al. Real-world practice indirect comparison between guselkumab, risankizumab, and tildrakizumab: results from an italian 28-week retrospective study. J Dermatolog Treat. 2022;33(6):2813–2820.
- Narcisi A, Valenti M, Gargiulo L, et al. Real-life effectiveness of tildrakizumab in chronic plaque psoriasis: a 52-week multicentre retrospective study-IL PSO (italian landscape psoriasis). J Eur Acad Dermatol Venereol. 2023;37(1):93–103.